Abstract
This study used a rat model of Fetal Alcohol Syndrome to investigate whether combined prenatal and postnatal ethanol exposure affects met-enkephalin levels in the brains of male and female Long–Evans adult rats. Intragastric ethanol was administered to a group of rats (ET) from gestational day (GD) 1 through 22 and from postnatal day (PD) 2 through 10. The control groups consisted of a nontreated control group (NTC) and an intubated control group (IC) that received the intragastric intubation procedure but no exposure to ethanol. We measured met-enkephalin levels in the prefrontal cortex, nucleus accumbens, hypothalamus, central and basolateral nucleus of amygdala and ventral tegmental area. Met-enkephalin levels in the hypothalamus of male and female ET animals were significantly higher than those in either the NTC or IC animals. Met-enkephalin levels in the central nucleus of the amygdala of male and female ET animals were significantly lower than the levels in the NTC animals. Met-enkephalin levels in the nucleus accumbens of ET females were significantly greater than those in the IC females. These results demonstrate that the combination of prenatal and postnatal ethanol exposure affects basal met-enkephalin levels in specific regions in a sex-specific manner. These changes in met-enkephalin levels may explain how early ethanol exposure affects opioid-regulated behaviors such as social play, sexual behavior, and other social behaviors.
Keywords: Fetal alcohol syndrome, Social behavior, Opioid, Prenatal alcohol, Sex-dependent differences, Fetal alcohol spectrum disorder
1. Introduction
Fetal Alcohol Spectrum Disorder (FASD) is a term used to classify people who were exposed to alcohol in utero and manifest mild to severe disturbances of physical, behavioral, emotional, and/or social functioning [36]. The disturbances in social functioning in FASD can be severe and include inappropriate sexual behavior and failure to understand the consequences of their actions [35,37]. The social deficits in FASD may be the result of poor judgment, planning, organizational skills, impulse control, and decision-making skills seen in these individuals [35,37]. These changes in social behavior are apparent even when IQ scores and socioeconomic backgrounds have been controlled [38].
Animal models of FASD also demonstrate that prenatal and/or early postnatal ethanol exposure affects social behavior {reviewed in Ref. [16]}. Animal models have shown that prenatal ethanol exposure decreases ultrasonic vocalizations [14], disrupts male sexual behavior [29] and pup-elicited maternal behavior [25], and increases aggression [31]. Postnatal ethanol exposure alters both social interactions and social communication in a sexually dimorphic manner [15]. A combination of prenatal and postnatal alcohol exposure increases ultrasonic vocalizations [22], alters pacing of sexual behavior in females [9], increases social reactions after isolation [18], and decreases aggression [19]. Even though it is very clear that prenatal, postnatal, and combined prenatal and postnatal ethanol exposure affect social behavior, it is not clear what ethanol-induced changes in neural structures or neural systems underlie the changes in social behavior.
One possible mediator of an ethanol-induced alteration in social behavior is met-enkephalin. Met-enkephalin is a neuropeptide that binds to mu and delta opioid receptors [20]. There have been many studies that demonstrate the role of opioid receptors and opioid peptides in various aspects of social behavior. Opioid receptor agonists suppress ultrasonic vocalizations in pups on postnatal day (PD) 10 [3]. Social interaction in adult rats increases met-enkephalin levels in the nucleus accumbens [2]. Met-enkephalin appears to play a critical role in sexual behavior. Indeed, there is a significant increase in met-enkephalin levels in hypothalamus, cortex, and midbrain twenty-four hours after ejaculation and levels remain elevated in the hypothalamus forty-eight hours after ejaculation [30]. Furthermore, a persistent inability to mate in male rats is likely due to high basal met-enkephalin levels in the hypothalamus [11,30]. In general, it appears that social behavior increases the levels of met-enkephalin in different brain areas, although it should be noted that the findings are almost exclusively in males. Given that a sex-dependent difference has been found in delta opioid receptor immunoreactivity in the amygdala of rats [43] and that human females have increased mu receptor binding in the prefrontal cortex, amygdala, thalamus, and anterior cingulate cortex compared to males [45], sexually dimorphic involvement of opioids in social behaviors might be expected.
Early ethanol exposure clearly affects social behavior across the life-span of the rat, and met-enkephalin appears to play a role in many of these behaviors. Earlier studies that have investigated the effect of early ethanol exposure on opioid peptides have either attributed any changes in the peptide to stress [34] or investigated only whole brain levels of met-enkephalin in adolescent rats [24]. Studies have not examined how ethanol exposure affects opioid peptides in specific neural regions in adults. Furthermore, no investigation has examined the effect of three trimester equivalents of exposure on met-enkephalin. Because of the deficits in social behavior induced by ethanol exposure during development and the sexually dimorphic nature of the opioid system, the current study examined the effect of a combination of prenatal and postnatal ethanol exposure on neural regions that have been shown to be involved in social behavior: prefrontal cortex, nucleus accumbens, hypothalamus, central nucleus of the amygdala, basolateral nucleus of the amygdala, and the ventral tegmental area. We hypothesize that perinatal ethanol exposure will result in a change in met-enkephalin levels in some neural areas and the effect of ethanol exposure on met-enkephalin may interact with the sex of the animal.
2. Methods
2.1. Subjects
All animals were housed in the animal colony of the Department of Psychology in the University of South Carolina. The colony temperature was maintained at 22 °C, with a 12-h light–dark cycle. Female Long–Evans rats were placed with a proven breeder male overnight for breeding. Vaginal smears were done the next morning to check for the presence of sperm. Once sperm was detected, that day was assigned as Gestational Day 1 (GD). On GD 1, the pregnant rats were housed singly in polypropylene cages with wood shavings, and the dams were assigned to one of three groups: ET (ethanol-treated group), IC (intubated control), and NTC (non-treated control).
2.2. Dam treatments
All treatments occurred in the latter half of the light cycle. Dam treatments were given from GD 1 through 22. ET dams were weighed daily and received daily intragastric (i.g.) intubations of ethanol in water (4.5 g/kg in a volume of 20 ml/kg) from GD 1 to 22. Intragastric intubations were given by first dipping the stainless-steel gavage tube in corn oil, to provide lubrication; the tube was then inserted down the esophagus of the rat. ET dams were also given unlimited access to water and rat chow and their rat chow was weighed daily to monitor food intake. A maltose–dextrin solution made isocaloric to the ethanol solution was given to IC dams every day during gestation (GD 1–22) by i.g. intubation in a volume of 20 ml/kg. IC dams were initially matched to an ET dam of similar age and weight and then given as much food as their matched ET dam during pregnancy. The NTC group was weighed daily, given free access to food and water, but did not receive any other treatments.
2.3. Pup treatments
The day of birth (GD 23) was designated postnatal day 1 (PD 1) and the dams and pups did not receive any intubations on this day. Litters were culled to 10 pups (5 males and 5 females) when-ever possible. On PD 2 through 10, pups from all groups were removed from their litter, one at a time, weighed and marked with nontoxic marker for identification. In addition, the ET and IC pups were given two intragastric intubations on PD 2 through 10.
All intubations (i.g.) given to the pups were administered using PE10 Intramedic tubing, which was dipped in corn oil to facilitate the procedure. ET pups received a 3.0 g/kg dose of ethanol in a volume of 0.0278 ml/g milk (PD 2–10). Two hours after the first intubation, ET pups were intubated a second time with the milk solution only (0.0278 ml/g). The milk solution was made to resemble rat’s milk [42]. The IC pups received the same procedure (two intubations) as the ET pups except that solutions were not given. NTC pups were weighed every day but did not receive any intubations or solutions.
On PD 10, pups were permanently paw-marked with India ink for identification purposes [10] throughout development. Pups were housed with their dam until PD 21, when the pups were separated from their dam and housed in same-sex pairs. Only one animal per sex from each litter was assigned to this study; remaining animals were assigned to other studies. In some cases we used only one animal per litter in this study, which led to the use of more total litters being used than is apparent in the experimental group size.
2.4. Blood alcohol concentrations (BACs)
On GD 20, 10 µl of blood was taken via a nick to the tail from the ET and IC dams three hours after the intubation procedure. On PD 10, 10 µl of blood was taken via a nick to the tail from each ET and IC pup two hours after the first intubation. The tail nick procedure was done quickly and every attempt was made to reduce the stress to the animals. The blood from the ET dams and pups was used to measure BACs via a colorimetric enzymatic assay as previously described [6,22]. The BAC data represent values from the blood samples collected from all the dams and pups used in this study. The alcohol doses were chosen because they produce similar peak BACs prenatally and postnatally and the time points were chosen to assay peak BACs [22].
2.5. Brain preparation
Females received daily vaginal smears for a period of five to seven days to determine the stage of estrus. There were no obvious differences in the estrus cycle across groups; cycling and cell types appeared similar across groups. Females in diestrus and males were decapitated at approximately 90–120 days of age and the brains were removed and frozen on crushed dry ice. All frozen brains were then wrapped in Parafilm® and aluminum foil and stored −80 °C until time of the assay.
2.6. Assay for met-enkephalin levels
Radioimmunoassay (RIA) kits (Peninsula Laboratories Inc.) were used to asses the levels of methionine-enkephalin [S-2119 (RIK 8602)] in ET, IC, and NTC male and female rats. The kit is very specific for met-enkephalin with a percent cross-reactivity of 100% for met-enkephalin and, 3% cross-reactivity to leu-enkephalin, 0.1% cross-reactivity to Met-enkephalin-Arg-Phe and β-endorphin and zero percent cross reactivity to other opioid peptides. The microdissections taken for radioimmunoassay were collected according to punch coordinates in a microdissection guide [27]. A microtome was used to section the brains into 1 mm frozen slices and a 1.0 mm diameter punch was used to isolate various nuclei. Bilateral sections of dissected tissue were weighed, placed in 1.5 ml microfuge tubes, and 550 µl of 2 M acetic acid was added to the tubes. The tubes were then boiled in a block heater for 15 min with cap locks and then sonicated until all tissue was homogenized. Three 30 µl aliquots of the homogenized solution were transferred into 12 × 75 mm glass tubes for analysis of protein levels using the method of Lowry et al. [17]. The remaining sample was centrifuged at 1500 ×g at 22 °C for 5 min. Following centrifugation, three 150 µl aliquots of the supernatant were placed in 12×75 mm polystyrene tubes and samples were dried using a vacuum evaporator. Each tube containing extracted peptide from the ET, IC, and NTC rat tissue was reconstituted with 100 µl of RIA buffer, vortexed, and sonicated. Tubes for determining a standard curve, non-specific binding, total binding and total counts were included in each assay. On day 1 of the RIA procedure, 100 µl of the primary antibody was added to 100 µl of the reconstituted peptide from the ET, IC, and NTC tissue. The tubes were then incubated in a cold room at 4 °C for 18 to 24 h. On day 2, 100 µl of 125I-peptide (1.0–1.5 × 104 cpm per tube) was added to each tube. On day 3 (18–24 h later), 100 µl of goat anti-rabbit IgG serum and 100 µl of normal rabbit serum were added to tubes (except for total counts tubes). Following a 90 min incubation period at room temperature, all the tubes were centrifuged at 1500 ×g for 20min at 4 °C. The supernatant was aspirated off, and the levels of 125I remaining in the pellet were counted in a gamma counter for one minute. The picograms of met-enkephalin in the samples were determined from the standard curve using log–logit transformation of the data outlined in the RIA kit. Data are expressed as pg of met-enkephalin/mg total protein in the sample.
Our initial studies included a sample size of five per sex within a group. However, based on the variance in some brain areas, we analyzed an additional set of samples for RIAs to increase the statistical power. We did not do further RIAs on some brain areas.
3. Results
3.1. Dam weight data
There were no significant differences among dam group weights during gestation (Table 1). A mixed ANOVA on dam weights throughout gestation GD 1–22 indicated that all dams gained a significant amount of weight over pregnancy (main effect of day, F(21,672) = 224.50, p < 0.001).
Table 1.
Dam variables: mean body weights (g) and SEMs and sample size
| Group | NTC | IC | ET |
|---|---|---|---|
| n = 12 | n = 13 | n = 14 | |
| GD 1 | 268 ± 6.1 | 260 ± 5.9 | 252 ± 3.8 |
| GD 5 | 268 ± 5.2 | 258 ± 5.1 | 255 ± 4.6 |
| GD 10 | 279 ± 5.3 | 267 ± 6.0 | 270 ± 4.7 |
| GD 15 | 298 ± 5.5 | 287 ± 6.3 | 290 ± 4.7 |
| GD 20 | 340 ± 5.8 | 327 ± 8.1 | 332 ± 5.2 |
3.2. Pup weight data
A mixed-design ANOVA indicated that there were no significant weight differences among the groups over days PD 2–10 (Table 2). In general, males weighed significantly more than females across PD 2–10 (main effect of sex, F(1,42) = 5.171, p < 0.05). A significant weight gain was seen in all groups over PD 2–10 (main effect of day, F(8,336) = 1500, p < 0.001). No significant interactions were found.
Table 2.
Offspring variables: mean body weights (g) and SEMs on postnatal days 2, 10, 21, 30, 60, 90 from experimental animals
| NTC | IC | ET | |
|---|---|---|---|
| PD 2 | 6.8 ± 0.1 | 6.5 ± 0.1 | 6.0 ± 0.1 |
| PD 10 | 18.9 ± 0.6 | 17.6 ± 0.3 | 17.6 ± 0.6 |
| PD 21 | 47.1 ± 3.4 | 42.0 ± 1.2 | 42.9 ± 1.4 |
| PD 60 | 256 ± 13 | 259 ± 13 | 249 ± 13 |
| PD 90 | 345 ± 22 | 339 ± 22 | 313 ± 18 |
Data are collapsed across sex.
The different groups showed no differences in weight when they reached adulthood; ANOVAs of weights on PD 60 and 90 did not reveal any significant differences among groups. Males weighed more than females on PD 60 and 90 (main effects of sex, F(1, 47) = 334.31, p < 0.001 and F(1, 47) = 353.12, p < 0.001, respectively).
3.3. BAC Data
There was no significant difference between the BACs of male and female ET pups. No significant differences were found between dam and pup BACs The means and standard errors of the mean (SEMs) of the BACs in mg/dl in male pups, female pups and dams were 311 ± 36.2, 333 ± 48.3, and 368 ± 36.6, respectively.
3.4. Neurochemical data
3.4.1. Hypothalamus
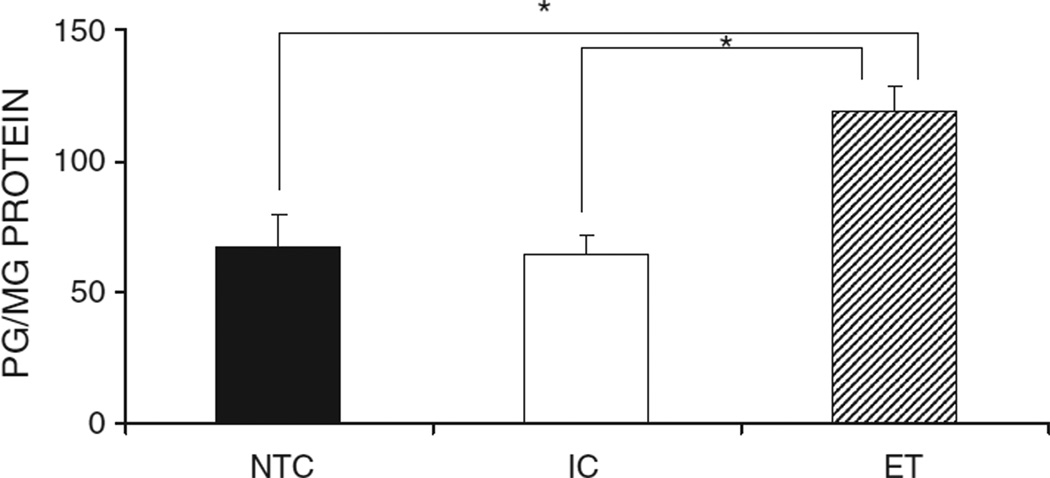
Ethanol exposed animals had a significant increase in met-enkephalin levels in the hypothalamus compared to controls (Fig. 1). There were no effects of sex or interactions so hypothalamus data from males and females was combined. A between-subjects ANOVA indicated a significant main effect of group in the met-enkephalin level of the hypothalamus F(2, 39) = 9.985, p < 0.001. Tukey’s post hoc analyses indicated that the ET animals had higher met-enkephalin levels compared to NTC and IC animals (p < 0.05), and the NTC and IC animals were not significantly different from one another.
Fig. 1.
The met-enkephalin levels for the hypothalamus for the NTC, IC, and ET groups. Ethanol exposure significantly increased basal met-enkephalin levels in the hypothalamus compared to the levels in both the IC and NTC groups. The sample sizes for NTC males, NTC females, IC males, IC females, ET males, and ET females were n = 6, 7, 7, 7, 9, 9, respectively. The asterisk indicates a significant difference. Data are collapsed across sex. Error bars depict standard errors of the mean (SEMs).
3.4.2. Nucleus accumbens
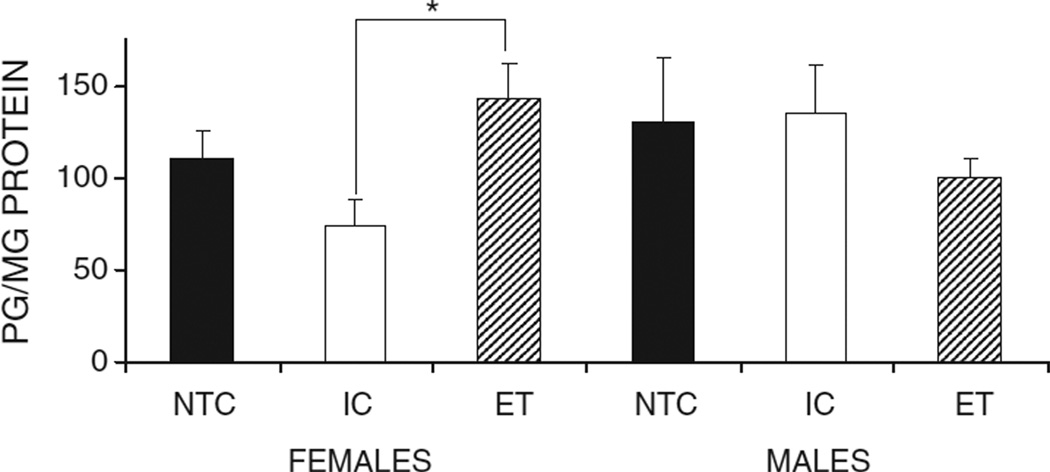
Ethanol exposed females had significantly higher levels of met-enkephalin compared to the IC group (Fig. 2). A between-subjects ANOVA indicated a significant group by sex interaction in the met-enkephalin levels of the NA, F(2, 41) = 3.256, p < 0.05. Further analyses indicated that there was a main effect of group in the female animals, F(2, 21) = 4.635, p < 0.05, and that the ET females had significantly higher levels of met-enkephalin than the IC females (p < 0.05). The control groups were not significantly different from one another. No differences between groups were seen in males.
Fig. 2.
The met-enkephalin levels for the Nucleus Accumbens for the NTC, IC, and ET groups divided by sex. Ethanol exposure significantly increased the basal met-enkephalin levels in the ET females compared to the IC females. The sample sizes for NTC males, NTC females, IC males, IC females, ET males, and ET females were n = 7, 8, 8, 8, 8, 8, respectively. The asterisk indicates a significant difference. Error bars depict SEMs.
3.4.3. Central nucleus of the amygdala
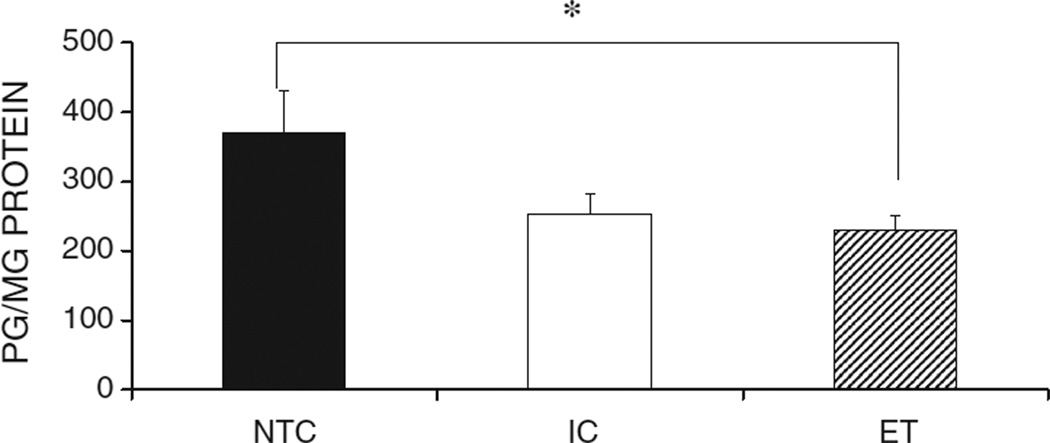
Ethanol exposed animals showed significantly lower met-enkephalin levels compared to NTC animals (Fig. 3). There was no main effect of sex and no interaction so data from males and females were combined. A between-subjects ANOVA indicated a main effect of group in the met-enkephalin levels of the CEA F(2, 37) = 3.698, p < 0.05. Tukey’s post hoc analyses indicated that the met-enkephalin levels of the ET group were significantly lower than those of the NTC group.
Fig. 3.
The met-enkephalin levels for the Central Nucleus of the Amygdala for the NTC, IC, and ET groups. Ethanol exposure significantly decreased basal met-enkephalin levels in the CEA compared to those in the NTC group. The sample sizes for NTC males, NTC females, IC males, IC females, ET males, and ET females were n = 8, 6, 6, 6, 8, 8, respectively. The asterisk indicates a significant difference. The data are collapsed across sex. Error bars depict SEMs.
3.4.4. Other brain regions, protein levels, and tissue weights
Analyses of the met-enkephalin levels in the PFC, BLA, and VTA did not show any significant differences between ET, NTC and IC groups (Table 3). The analyses of mg of protein per total tissue weight and total tissue weight in the PFC, NA, CEA, hypothalamus, and VTA indicated no significant differences or interactions between groups or sex. However, a significant difference in mg of protein per tissue weight in the BLA was found between the ET animals and the controls, F(2, 24) = 6.345, p < 0.05. Tukey’s post hoc analyses indicated that the ET group (males and females were combined) had a higher amount of tissue protein levels in BLA tissue compared to the IC and NTC groups, which did not differ from one another (means and SEMS in mg of protein/g of tissue: ET males = 0.28 ± 0.03; ET females = 0.25 ± 0.04; IC males = 0.17 ± 0.04; IC females = 0.27 ± 0.04; NTC males = 0.22 ± 0.03; NTC females = 0.18 ± 0.01). The analyses of total tissue weight in the BLA indicated no significant differences or interactions between any of the groups or sex.
Table 3.
Means, SEMs, and sample size for met-enkephalin levels (pg/mg protein) for the prefrontal cortex (PFC), basolateral amygdala (BLA), and ventral tegmental area (VTA)
| Group | Sex | PFC | BLA | VTA |
|---|---|---|---|---|
| NTC | F | 30.2 ± 5.7 | 124.1 ± 40.0 | 97.7 ± 25.1 |
| n = 5 | n = 5 | n = 8 | ||
| M | 49.1 ± 4.8 | 133.6 ± 25.4 | 90.7 ± 19.8 | |
| n = 5 | n = 5 | n = 8 | ||
| IC | F | 36.5 ± 7.9 | 113.4 ± 22.1 | 61.1 ± 10.2 |
| n = 5 | n = 4 | n = 8 | ||
| M | 35.4 ± 1.9 | 119.0 ± 32.5 | 63.0 ± 9.2 | |
| n = 5 | n = 4 | n = 8 | ||
| ET | F | 35.4 ± 6.7 | 160.6 ± 53.7 | 93.8 ± 18.6 |
| n = 5 | n = 5 | n = 8 | ||
| M | 37.4 ± 15.7 | 78.5 ± 22.8 | 85.3 ± 23.3 | |
| n = 5 | n = 4 | n = 7 |
There were no significant differences or interactions between groups or sex.
4. Discussion
The study supports the hypothesis that a combination of prenatal and postnatal ethanol exposure affects met-enkephalin levels in specific areas of the brain and can do so in a sex-specific manner. Ethanol exposure affected basal met-enkephalin levels in the hypothalamus and CEA of male and female rats, but only affected female met-enkephalin levels in the NA. The basal met-enkephalin levels in the control groups are similar to those found in other studies and the percent change induced by ethanol exposure is similar to that seen after different manipulations in other studies [30,32]. The changes in met-enkephalin levels may help explain many of the behavioral changes seen in ethanol-exposed animals.
A two fold increase in basal met-enkephalin levels was seen in the hypothalamus of both male and female ET animals. This finding could explain why ethanol exposed males show a higher persistent non-mater rate [40] since high basal levels of a met-enkephalin precursor neuropeptide in the hypothalamus are present in persistent non-maters [30].Additionally, administration of the opioid antagonist naloxone converts persistent non-maters into successful maters [11]. It is possible that the high rate of persistent non-maters in males prenatally exposed to ethanol may be reversed by the administration of naloxone. Systemic or direct injection of naloxone into the hypothalamus of ET males would address the possibility of reversing the high non-mater rate in ET males. The increase in basal met-enkephalin in the hypothalamus of ET females may be responsible for the change in pacing behavior seen in perinatal ethanol exposed females [9]. Female rats given an injection of met-enkephalin into the third ventricle have an enhanced lordosis response to males [39]. Changes in opioid peptides in the hypothalamus have a large effect on male sexual behavior and may also be very important in mediating female sexual behavior.
The increase in met-enkephalin levels in the NA of females exposed to ethanol during the perinatal period may affect motivational processes. NA is commonly known to play an important part in motivation, and opioids are believed to play a very important role in the motivational aspect of sexual behavior in females [28]. The role of NA in motivation may make it another structure that plays an important role in mediating the increased lordosis seen in female rats that received a ICV injection of met-enkephalin into the third ventricle [39]. Localized injections of met-enkephalin into the NA or hypothalamus may help to clarify which structure mediates sexual motivation in females. In addition, levels of met-enkephalin in NA can be significantly increased by social interactions. This may explain why females exposed to early ethanol exposure show an increase in social interactions compared to control females [15]. The sex-specific change in met-enkephalin levels may also explain why only ethanol-exposed female mice are less sensitive to the low-dose stimulant effect of ethanol [1]. Acute ethanol exposure increases the opioid peptide beta-endorphin in NA [21,26]. Additionally, enkephalinase inhibitor, which potentiates the action of endogenous enkephalins, increases alcohol intake [8], while an opioid antagonist administered into the NA decreases the animal’s response to alcohol [12]. It may be that ET females are more sensitive to the acute effects of ethanol and may show an increase in intake of ethanol compared to males. Future studies could investigate whether prenatal and postnatal ethanol exposure also affects drug sensitivity and intake in a sex-specific manner. However, caution should be taken in interpreting the increase of met-enkephalin levels in ET females since the increase was significantly different from IC females and not NTC females. The change may be due to a synergistic effect that can occur between early ethanol exposure and stress [33,41,44].
Another structure involved in reward and motivation that has an ethanol-induced change in met-enkephalin levels was the CEA. There is evidence that ethanol sensitivity and intake are highly regulated through opioid receptors and enkephalin. Neurons in the CEA that express pro-enkephalin are sensitive to the acute effects of ethanol [5] and direct injection of naltrexone into CEA decreases the animal’s response to ethanol [7]. There is also a lower amount of met-enkephalin found in the amygdala of an alcohol-preferring mouse strain compared to an alcohol non-preferring strain [13]. In addition, it has been found that there is a higher amount of mu-opioid receptor binding in amygdala nuclei in alcohol-preferring rats compared to alcohol non-preferring rats [23]. It is not clear what the effect of a higher basal level of met-enkephalin would have on the animals. The higher level may result in a down-regulation of mu and delta opioid receptors. This would then be the opposite of what is seen in alcohol-preferring rats and could imply that an increase in met-enkephalin in the CEA could decrease the animal’s sensitivity to ethanol [23]. Investigating the effect of a direct injection of met-enkephalin into the CEA on ethanol sensitivity and intake could help to clarify the role of met-enkephalin in the CEA. However, caution should also be taken when interpreting the decrease of met-enkephalin levels in ET animals since the decrease was significantly different from NTC animals and not IC animals. This change may be primarily due to the synergistic effect between early ethanol exposure and stress such has been reported in several studies [33,41,44].
The opioid system has to our knowledge not been investigated in the human FASD population; furthermore, little research on peptide levels has been conducted in animal models of FASD. This study shows that the opioid system is affected by early alcohol exposure and since met-enkephalin levels were examined in adulthood, the effect appears to be permanent. Investigation into mu and delta opioid receptors, beta-endorphin levels, and other aspects of the opioid system will help to provide a complete picture of what aspects of the opioid system ethanol is affecting. Many of the social behavior abnormalities seen in FASD patients may be mediated through the opioid system. This line of research may lead to possible treatments for the social deficit changes induced by ethanol because opioid receptor antagonist medications currently exist and have been shown to alleviate some of the social deficits seen in other disorders such as autism {reviewed in Ref. [4]}.
Acknowledgements
The authors would like to acknowledge the technical assistant of Kris Ford and Melissa K. Reese. J. N. Lugo, Jr. was funded by a predoctoral fellowship AA05583 from NIH. The research was funded by NIH grants RO1 AA11566 to S.J.K and RO1 MH63344 to M.A.W.
References
- 1.Becker HC, Hale RL, Boggan WO, Randall CL. Effects of prenatal ethanol exposure on later sensitivity to the low-dose stimulant actions of ethanol in mouse offspring: possible role of catecholamines. Alcohol. Clin. Exp. Res. 1993;17:1325–1336. doi: 10.1111/j.1530-0277.1993.tb05249.x. [DOI] [PubMed] [Google Scholar]
- 2.Bertrand E, Smadja C, Mauborgne A, Roques BP, Dauge V. Social interaction increases the extracellular levels of [Met]enkephalin in the nucleus accumbens of control but not of chronic mild stressed rats. Neuroscience. 1997;80:17–20. doi: 10.1016/s0306-4522(97)00136-x. [DOI] [PubMed] [Google Scholar]
- 3.Carden SE, Barr GA, Hofer MA. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Brain Res. Dev. Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- 4.Chabane N, Leboyer M, Mouren-Simeoni MC. Opiate antagonists in children and adolescents. Eur. Child Adolesc. Psychiatry. 2000;9:I44–I50. doi: 10.1007/s007870070018. [DOI] [PubMed] [Google Scholar]
- 5.Criado JR, Morales M. Acute ethanol induction of c-Fos immunoreactivity in pre-pro-enkephalin expressing neurons of the central nucleus of the amygdala. Brain Res. 2000;861:173–177. doi: 10.1016/s0006-8993(99)02468-3. [DOI] [PubMed] [Google Scholar]
- 6.Dudek BC, Abbott ME. A biometrical genetic analysis of ethanol response in selectively bred long-sleep and short-sleep mice. Behav. Genet. 1984;14:1–19. doi: 10.1007/BF01066065. [DOI] [PubMed] [Google Scholar]
- 7.Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, Cook JM, June HL. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- 8.Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology (Berl) 1991;103:467–472. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- 9.Gass JT, Marino MD, Lugo JN, Jr, Reese MK, Kelly SJ. Perinatal alcohol exposure alters sexual behavior in rats. Abstr. - Soc. Neurosci. 2002;28:207.17. [Google Scholar]
- 10.Geller LM, Geller EH. A simple technique for the permanent marking of newborn albino rats. Psychol. Rep. 1966;18:221–222. doi: 10.2466/pr0.1966.18.1.221. [DOI] [PubMed] [Google Scholar]
- 11.Gessa GL, Paglietti E, Quarantotti BP. Induction of copulatory behavior in sexually inactive rats by naloxine. Science. 1979;204:203–205. doi: 10.1126/science.432642. [DOI] [PubMed] [Google Scholar]
- 12.Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol. Clin. Exp. Res. 1999;23:1468–1476. [PubMed] [Google Scholar]
- 13.Jamensky NT, Gianoulakis C. Comparison of the proopiomelanocortin and proenkephalin opioid peptide systems in brain regions of the alcohol-preferring C57BL/6 and alcohol-avoiding DBA/2 mice. Alcohol. 1999;18:177–187. doi: 10.1016/s0741-8329(99)00002-6. [DOI] [PubMed] [Google Scholar]
- 14.Kehoe P, Shoemaker W. Opioid-dependent behaviors in infant rats: effects of prenatal exposure to ethanol. Pharmacol. Biochem. Behav. 1991;39:389–394. doi: 10.1016/0091-3057(91)90197-a. [DOI] [PubMed] [Google Scholar]
- 15.Kelly SJ, Tran TD. Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol. Teratol. 1997;19:383–389. doi: 10.1016/s0892-0362(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 16.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol. Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Lugo JN, Jr, Marino MD, Cronise K, Kelly SJ. Effects of alcohol exposure during development on social behavior in rats. Physiol. Behav. 2003;78:185–194. doi: 10.1016/s0031-9384(02)00971-x. [DOI] [PubMed] [Google Scholar]
- 19.Lugo JN, Jr, Marino MD, Gass JT, Wilson MA, Kelly SJ. Alcohol exposure during development reduces resident aggression and testosterone in rats. Physiol Behav. doi: 10.1016/j.physbeh.2005.10.005. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. doi: 10.1016/0006-8993(95)00928-j. [DOI] [PubMed] [Google Scholar]
- 21.Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl) 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- 22.Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Dev. Psychobiol. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- 23.McBride WJ, Chernet E, McKinzie DL, Lumeng L, Li TK. Quantitative autoradiography of mu-opioid receptors in the CNS of alcohol-naive alcohol-preferring P and -nonpreferring NP rats. Alcohol. 1998;16:317–323. doi: 10.1016/s0741-8329(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 24.Nelson BK, Brightwell WS, MacKenzie-Taylor DR, Burg JR, Massari VJ. Neurochemical, but not behavioral, deviations in the offspring of rats following prenatal or paternal inhalation exposure to ethanol. Neurotoxicol. Teratol. 1988;10:15–22. doi: 10.1016/0892-0362(88)90062-1. [DOI] [PubMed] [Google Scholar]
- 25.Ness JW, Franchina JJ. Effects of prenatal alcohol exposure on rat pups’ ability to elicit retrieval behavior from dams. Dev. Psychobiol. 1990;23:85–99. doi: 10.1002/dev.420230109. [DOI] [PubMed] [Google Scholar]
- 26.Olive MF, Koenig HN, Nannini MA, Hodge CW. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J. Neurosci. 2001;21:RC184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palkovits M, Brownstein MJ. Maps and Guide to Microdissection of the Rat Brain. New York: Elsevier; 1988. [Google Scholar]
- 28.Paredes RG, Martinez I. Naloxone blocks place preference conditioning after paced mating in female rats. Behav. Neurosci. 2001;115:1363–1367. [PubMed] [Google Scholar]
- 29.Parker S, Udani M, Gavaler JS, Van Thiel DH. Adverse effects of ethanol upon the adult sexual behavior of male rats exposed in utero. Neurobehav. Toxicol. Teratol. 1984;6:289–293. [PubMed] [Google Scholar]
- 30.Rodriguez-Manzo G, Asai M, Fernandez-Guasti A. Evidence for changes in brain enkephalin contents associated to male rat sexual activity. Behav. Brain Res. 2002;131:47–55. doi: 10.1016/s0166-4328(01)00371-0. [DOI] [PubMed] [Google Scholar]
- 31.Royalty J. Effects of prenatal ethanol exposure on juvenile play-fighting and postpubertal aggression in rats. Psychol. Rep. 1990;66:551–560. doi: 10.2466/pr0.1990.66.2.551. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez MD, Milanes MV, Fuente T, Laorden ML. Prenatal stress alters the hypothalamic levels of methionine-enkephalin in pup rats. Neuropeptides. 1992;23:131–135. doi: 10.1016/0143-4179(92)90090-j. [DOI] [PubMed] [Google Scholar]
- 33.Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 34.Shoemaker WJ, Kehoe P, Baker RA. Prenatal ethanol exposure: endogenous opioid systems, inappropriate emotional responsiveness, and autism. In: Miller MW, editor. Development of the Central Nervous System: Effects of Alcohol and Opiates. Wiley-Liss; 1991. [Google Scholar]
- 35.Streissguth AP. Fetal Alcohol Syndrome: a Guide for Families and Communities. Baltimore: Brooks Publishing Company; 1997. [Google Scholar]
- 36.Streissguth AP, O’Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin. Clin. Neuropsychiatry. 2000;5:177–190. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- 37.Streissguth AP, Barr HM, Kogan J, Bookstein FL. Seattle: University of Washington, Fetal Alcohol and Drug Unit; 1996. Understanding the Occurrence of Secondary Disabilities in Clients with Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Effects (FAE), Final Report to the Centers for Disease Control and Prevention (CDC) [Google Scholar]
- 38.Thomas SE, Kelly SJ, Mattson SN, Riley EP. Comparison of social abilities of children with fetal alcohol syndrome to those of children with similar IQ scores and normal controls. Alcohol. Clin. Exp. Res. 1998;22:528–533. [PubMed] [Google Scholar]
- 39.Torii M, Kubo K, Sasaki T. Differential effects of beta-endorphin and Met- and Leu-enkephalin on steroid hormone-induced lordosis in ovariectomized female rats. Pharmacol. Biochem. Behav. 1997;58:837–842. doi: 10.1016/s0091-3057(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 40.Ward IL, Ward OB, Winn RJ, Bielawski D. Male and female sexual behavior potential of male rats prenatally exposed to the influence of alcohol, stress, or both factors. Behav. Neurosci. 1994;108:1188–1195. doi: 10.1037//0735-7044.108.6.1188. [DOI] [PubMed] [Google Scholar]
- 41.Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106:306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- 42.West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1:213–222. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]
- 43.Wilson MA, Mascagni F, McDonald AJ. Sex differences in delta opioid receptor immunoreactivity in rat medial amygdala. Neurosci. Lett. 2002;328:160–164. doi: 10.1016/s0304-3940(02)00481-0. [DOI] [PubMed] [Google Scholar]
- 44.Zimmerberg B, Weston HE. Postnatal stress of early weaning exacerbates behavioral outcome in prenatal alcohol-exposed juvenile rats. Pharmacol. Biochem. Behav. 2002;73:45–52. doi: 10.1016/s0091-3057(02)00797-9. [DOI] [PubMed] [Google Scholar]
- 45.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. Mu-opioid receptor-mediated antinociceptive responses differ in men and women. J. Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]