Abstract
Animal models of fetal alcohol spectrum disorder (FASD) have been instrumental in isolating alcohol as a teratogen and demonstrating behavioral and neural effects. There are a number of different models for rodents with various strengths and weaknesses. A three-trimester model of FASD is described here; the model uses intragastric intubation of both pregnant dams and pups to mimic alcohol exposure across all three trimesters in humans. The model does not use expensive equipment and is relatively easy to accomplish. The model allows excellent control of alcohol dose and uses an oral route of administration. There are no undernutrition effects with the doses used here. A drawback of the model is the stress of the intubation procedures and ways in which to minimize this stress are discussed. In addition, a method to measure blood alcohol levels is described.
Keywords: Ethanol alcohol, fetal alcohol spectrum disorder, fetal alcohol syndrome, postnatal alcohol, prenatal alcohol
1 Introduction
Fetal alcohol spectrum disorder (FASD) is the leading known cause of preventable mental retardation in the Western world (1), and animal models have been instrumental in isolating alcohol as a teratogen and describing behavioral and neural deficits induced by alcohol (2,3). Animal models of FASD are currently being used to conduct translational research directed towards delineating possible behavioral (e.g. [4,5]) and/or pharmacological treatments (e.g. [6–8]) of FASD and mechanisms of alcohol-induced damage (e.g. [9,10]). Alcohol administration during development in rodents (predominantly mice and rats but also including guinea pigs and ferrets) has been accomplished using a variety of different methods, and each of these methods has different strengths and weaknesses (11). The most common models use rats or mice. The gestational period in rats and mice is only equivalent to the first two trimesters in the human with respect to brain growth (12) and so, to target a period equivalent to all three trimesters, alcohol administration in both the prenatal and postnatal period must occur.
An ideal method of alcohol administration in rodents would allow complete control over the actual alcohol exposure, entail no stress or handling of the animal, mimic the pharmacological time course of blood alcohol concentrations in the human fetus, be technically easy to do, and control for nutritional effects resulting from intoxication from alcohol. Such a method does not exist, but it is useful to go through the different methods and evaluate them along the different criteria (Table 1) (13–22). The most commonly used methods include vapor inhalation, liquid diets, artificial rearing, and intragastric intubation. All of these methods can be considered stressful to the animal although the nature of the stressor varies across methods. Vapor inhalation and artificial rearing require the purchase of expensive equipment, whereas the other methods do not. In contrast to the other methods, the liquid diet procedure does not allow close experimental control of the dose given to the animal.
Table 1.
Evaluation of Alcohol Administration Methods for Rats During Development
| Methoda | Period of exposure |
Experimenter control of dose |
Nutritional control | Stress | Pharmacological time course |
Technical difficulty and equipment |
|---|---|---|---|---|---|---|
| Vapor inhalation (15,16) | Prenatal | Yes | No, but no observed body weight differences | Yes, restricted to breathing ethanol fumes | Very swift increase phase compared with oral ingestion | Easy, requires inhalation chambers |
| Vapor inhalation (15,16) | Postnatal | Yes | No, but no observed body weight differences | Yes, restricted to breathing ethanol fumes | Very swift increase phase compared with oral ingestion | Easy, requires inhalation chambers |
| Liquid diet (17,18) | Prenatal | No | Yes, observed effects | Yes, diet restriction | Results from oral ingestion | Easy, requires graduated liquid containers |
| Artificial rearing (19,20) | Postnatal | Yes | Yes, observed effects | Yes, handling and maternal and sibling deprivation | Results from oral ingestion by the pup | Difficult, requires infusion pumps |
| Intragastric intubation (13,14,21,22) | Prenatal | Yes | Yes, no observed body weight differences in dams | Yes, intragastric intubation and handling | Results from oral ingestion by dam | Easy, requires feeding tubes |
| Intragastric intubation (13,14,21,22) | Postnatal | Yes | No, but no observed body weight differences in pups | Yes, intragastric intubation and handling | Results from oral ingestion by the pup | Moderate, requires PE tubing |
The references given for each method are representative and not exhaustive.
This chapter will focus on a three trimester model of FASD that uses intragastric intubation during the prenatal and postnatal period (13,14,23). This procedure is relatively easy to do, gives excellent control over the dose, and allows administration across both the prenatal and postnatal period. The alcohol is administered orally so that the time course of blood alcohol levels is appropriate and there are no detectable nutritional effects using the doses described here (see Note 1) (14). However, the administration procedure of intubation is stressful to the animals. As a result, it is important to include a control for the stress and allow a comparison with a nontreated control group. It is also very important to make efforts to minimize the stress; these efforts are emphasized in the Methods section. This alcohol administration method does not require expensive equipment and is not technically demanding. In addition, a method to evaluate blood alcohol concentrations is also included since this is typically done in many alcohol studies (24). The procedure described here uses doses in dams and pups that, when given in a single bolus, results in equivalent peak blood alcohol concentrations between 300 and 400 mg/dL in both dams and pups.
2 Materials
2.1 Alcohol Administration During the Prenatal Period
Feeding needles, curved, 18 gage (7.62 cm, 2.25-mm ball; VWR).
10-mL syringes, slip tip connection.
Maltose-dextrin (Bio-Serv; 3.89 kcal/g) is dissolved in water to give a solution that is isocaloric with the ethanol solution. This solution must be heated and stirred to get the maltose-dextrin into solution.
Ethanol (Acros) (100%, 0.7893 g/mL or 95%, 0.7498 g/mL, 7 kcal/g) is dissolved in distilled water to give a solution of 0.225 g of ethanol per millilter. When injected in a volume of 20 mL/kg, this will give a dose of 4.5 g/kg.
2.2 Alcohol Administration During the Postnatal Period
Intramedic tubing, PE 10 Clay Adams and PE 50 Clay Adam (VWR).
1-mL syringes and 23-gage needles.
A milk solution that is similar in composition to rat milk is made (25). This solution is made under sterile conditions. A mineral mix is first made combining 1.2 g of ZnSO4, 1.2 g of CuSO4, 1.2 g of FeSO4, 20 g of MgCl, and 20 g of KCl in 500 mL of sterile H2O. It is a slurry mix that must be stirred before adding to the milk solution. A specially formulated vitamin mix is ordered from BioServ; it is listed as the custom mix for the University of Iowa. The milk solution is made by homogenizing 1500 mL of evaporated milk (purchased at a grocery store), 450 mL of sterile H2O, 70 g of Supro 710 protein power (see Note 2), 130 mL of corn oil, 2 g of methionine, 1 g of tryptophan, 10 g of vitamin mix, 11 g of calcium phosphate dibasic, 0.2 g of deoxycholic acid, and 50 mL of the mineral mix. After homogenizing, the solution is put in bottles of a measured amount (usually 50 mL), and the bottle is sealed a rubber topper. The milk is then pasteurized by putting the bottles into an oven at 60 to 65°C for 30 min. The milk is then cooled rapidly and stored at −60°C, where it is stable indefinitely. When milk solutions are needed, a bottle of milk is thawed and used alone for the second injection for the pups. A separate bottle is used as the base of the ethanol intubation and is made such that 3.0 g/kg of ethanol is given when 0.0278 mL/g is given to the pup. The milk solutions that are being used are stored in the refrigerator (4°C) and will be stable for approx2 wk.
2.3 Measurement of Blood Alcohol Concentrations (BACs)
Heparinized capillary tubes (10 µL, Drummond Scientific special ordered through VWR, vendor part 1-000-0100-H).
0.53 N perchloric acid (Sigma).
0.30 M potassium carbonate (Sigma).
Alcohol dehydrogenase (Sigma) is dissolved in distilled water to get a solution of 89.25 units/mL. The ADH solution is frozen at −4°C in 5-mL aliquots and thawed before the assay (see Note 3).
β-Nicotinamide adenine dinuculeotide (NAD) (Grade III, Sigma) is dissolved in 0.5 M TRIS buffer (Sigma 7–9) to give a solution of 1.875 mM NAD. This solution is kept refrigerated (4°C) until time of assay.
Ethanol standards are made such that there are solutions of 0, 50, 100, 200, 300, 400, 500, and 50 mg/dL of ethanol dissolved in water. These are kept refrigerated (4°C) until time of assay.
3 Methods
Subjects in our experiments are Long-Evans rats purchased from Harlan and housed in an animal colony with a 12-h light -dark cycle (7:00 am lights on), temperature at 22°C, and humidity at 20%. Female animals are bred before alcohol administration in this model. The female rats are at least 90 d of age and are immediately put on breeder blocks (Purina, a diet specifically designed for breeding rats) and allowed a week of recovery after shipment from Harlan. They are housed in groups of two or three during this period. For breeding, they are put in groups of four or five overnight (from 5 pm on) with a proven male breeder. The next morning at 08:00 am (see Note 4), vaginal smears are taken and examined for the presence of sperm. If sperm is detected, that day is designated gestational day (GD), 1 and the female rat is assigned to one of three experimental groups, which are ET (Ethanol), IC (intubated control), or NC (nontreated control; see Note 5). The experimental dams are singly housed in polypropylene cages on GD 1.
3.1 Alcohol Administration During the Prenatal Period
Each ET dam is allowed free access to rat chow (breeder blocks) and water; the amount of food intake is measured daily in order to provide data for pair-feeding of the IC dams.
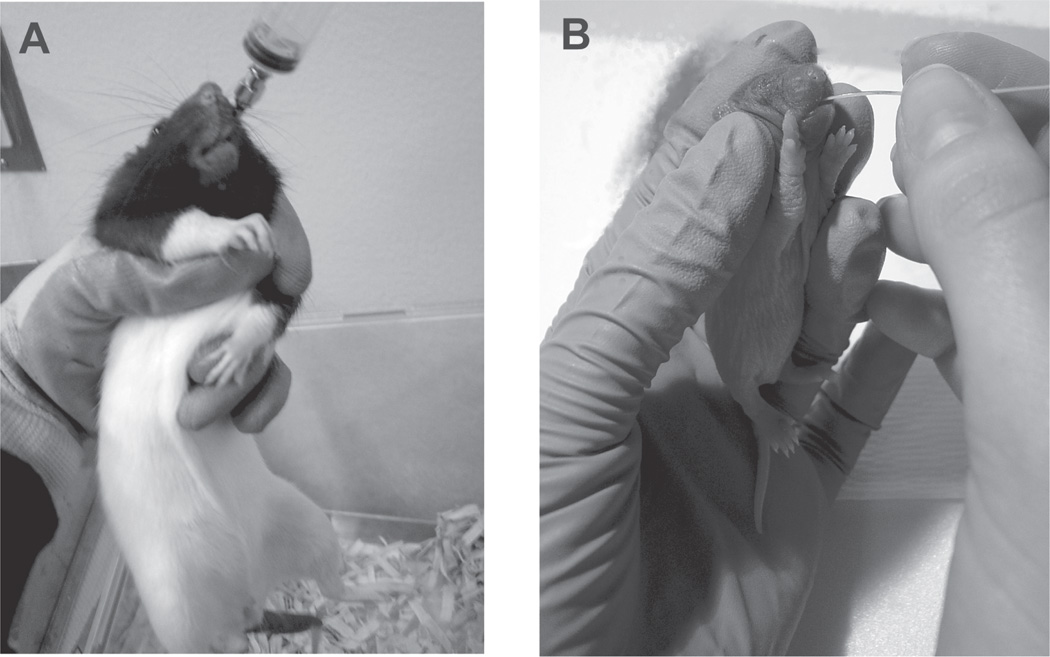
From GD 1 through GD 22, the ET dams is weighed and then intubated with 4.5 g/kg of ethanol in a volume of 20 mL/kg via intragastric intubation. The ethanol solution is drawn up into a 10-mL syringe and then attached to an intubation tube. Intragastric intubations are given by first dipping the stainless steel feeding tube in corn oil to provide lubrication; the tube is then inserted down the esophagus of the rat (see Note 6 and Fig. 1A). Handling time should be minimized. Intubation of ethanol is done in the late afternoon in order to minimize effects on circadian rhythms (26).
For IC dams, the treatment is similar except that food is restricted and the intubation is of isocaloric maltose-dextrin solution. On GD 1, the IC dam is matched by body weight to an ET dam of similar weight that has successfully given birth to a litter. The IC dam is then only given the amount of rat chow consumed by the matched ET dam on that particular gestational day. The IC dam is weighed daily and immediately intubated with a maltose-dextrin solution that is isocaloric with the ethanol solution in a volume of 20 mL/kg. The intubation process is the same as for the ET dams.
The NC dams are weighed on GD 1 and GD 22 to minimize handling during gestation. In earlier studies, NC dams were weighed on a daily basis in order to conclusively show that there were no dam weight differences, but it is felt that there are enough data showing this finding to warrant fully controlling handling to allow maximal detection of handling and stress effects.
Fig. 1.
(A) Intubation of a dam. Dams are held in a grip such that one of their forelimbs is over the thumb and the other is between the middle and ring fingers. The grip is firm such that the rat cannot slide down in the grip. This gavage tube is dipped in corn oil and inserted over the tongue. It should go down the esophagus without resistance and should feel as if it could be dropped into the rat’s stomach. Note that the gavage tube is inserted all of the way down to the joint between the tube and syringe. (B) Intubation of a rat pup. Rat pups are held so that the esophagus is in a straight line. The PE 10 tubing is dipped in corn oil and inserted so that it slides over the tongue and follows the roof of the mouth to the esophagus. The tubing should slide with little resistance down the esophagus. The resistance that may sometimes be felt on rats on PD 2 is because the esophagus is still very narrow; the resistance is not that of the tubing feeling as if it should be forced into the rat
3.2 Alcohol Administration During the Postnatal Period
The day of birth (typically GD 23) is designated postnatal day 1 (PD1) and the dams and pups do not receive any intubations on this day (see Note 7). Litters are culled to 10 pups with as close to an even number of males and females as possible. On PD 2 through PD 10, pups from the ET and IC groups are removed from their litter, one at a time and weighed. They are then given their first intragastric intubation and then 2 h later, they are given their second intragastric intubation. This is done on PD 2 through PD 10.
All intubations given to the pups are administered using PE10 Intramedic tubing attached to a 1-mL syringe via a short piece of PE 50 Intramedic tubing. A small amount of waterproof glue from a hot glue gun makes the connection between the PE 10 and PE 50 tubing tight. The PE 10 tubing is dipped in corn oil prior to the intubation in order to facilitate the procedure (see Note 8 and Fig. 1B). ET pups receive a 3.0 g/kg dose of ethanol in a volume of 0.0278 mL/g milk solution (PD 2–10). Two hours after the first intubation, ET pups are intubated a second time with the milk solution only (0.0278 mL/g). This procedure does not result in deficits in body weight when this dose of alcohol of alcohol is used (see Note 1). The IC pups receive the same procedure (two intubations) as the ET pups except that solutions are not given (see Note 9). NC pups are weighed on PD 2 and PD 10 but not treated in any other way.
On PD 2, pups can be permanently paw-marked with India ink for identification purposes (28). India ink is injected subcutaneously using a 1-mL syringe and 26-gage needle. The coding system resulting from this injection is such that the two forepaws represent the numbers 1 and 2 and the two hindpaws represent 4 and 8. With this numbering system, addition of the numbers represented by the different paws can number pups from 1 through 15. Alternatively, a rat tattooing system (Animal Identification and Marking systems, Inc., Hornell, NY) can be used to tattoo the paws of the rats.
3.3 BACs
On GD 20, 10 µL of blood is taken up using a heparinized capillary tube from a nick to the tail from the ET and IC dams 3 h after the intubation procedure. On PD 10, 10 µL of blood is taken using a heparinized capillary tube from a nick to the tail from the ET and IC pups 2 h after the alcohol intubation and just before the second intubation of milk only. The blood from the pups can be encourage to flow by holding the pup so that its tail is hanging and then slowly moving your fingers along the tail to encourage the blood drops to come out. It is important not to put too much pressure on the tail. The blood from the ET dams and pups is used to measure BACs via a colorimetric enzymatic assay as described below. The alcohol doses are chosen because they produce similar peak BACs prenatally and postnatally and the time points are chosen to assay peak BACs (29), which has been shown to be a critical determinant of the teratogenic effects of ethanol (30, 31).
The blood alcohol concentrations are measured as described in Dudek and Abbott (24). The 10 µL of blood of the experimental animal and 10 µL of distilled water are added to 190 µL of 0.53 N perchloric acid. Then, 200 µL of 0.30 M potassium carbonate is added. The solution is vortexed and then centrifuged in a refrigerated (4°C) centrifuge for 15 min at 12,000 rpm. On the same day, standards are made. Ethanol standards of 0, 50, 100, 200, 300, 400, 500, and 600 mg/dL (ethanol in distilled water) are made and stored at 4°C. Ten microliters of the standard and 10 µL of blood from a nontreated, nonexperimental animal are added to 190 µL of 0.53 N perchloric acid. The standards are then treated as the blood samples from the experimental animals. At this stage, the samples and standards can be frozen together at −80°C until the time of assay or the assay can be conducted immediately.
If the samples and standards are frozen, they should be thoroughly thawed and centrifuged for 15 min in a refrigerated (4°C) centrifuge at 12,000 rpm. The ADH solution should also be thoroughly thawed. Into a glass culture tube, 400 µL of the NAD-Tris solution and 50 µL of ADH should be combined. Then, 50 µL of the samples and standards should be added to each tube. The tubes should be vortexed and then incubated for one hour at room temperature. Each solution should then be read for absorbance of a light of 340 nm wavelength in a spectrophotometer. The standards should be used to construct a linear standard curve (with a correlation of greater than 0.95) and then the unknown samples are calculated from that curve.
Acknowledgments
This work was supported by National Institute of Alcoholism and Alcohol Abuse grant RO1 11566 to S. J. K. A special thanks to Dr. Tuan D. Tran who was instrumental in the development of the three trimester model.
Footnotes
This procedure uses 4.5 g/kg and 3.0 g/kg of ethanol in dams and pups respectively, delivered in one bolus. Others have used higher doses and found that loss of body weight in the pups occurs. If higher doses or two ethanol intubations of a higher dose are used, careful pilot work and consideration of more than one supplemental milk injection should be considered. In addition, it is likely that the peak blood alcohol concentration will occur at a different point with two intubations.
The Supro 710 protein powder can be purchased from Purina only in bulk (40 kg). It is possible to get free samples from some of the sales representatives.
A common problem with this assay will appear as a flat standard curve. This occurs because the aliquots of ADH have been thawed and frozen too many times, resulting in a degradation of the enzyme and a failure of the reaction.
In general, the earlier the smears are taken, the better the detection of sperm.
Animals are assigned in cohorts such that all three groups are represented. Because survival of the litter tends to be less from ET dams because of neglect of the pups or spontaneous resorptions of the litters, more ET dams are designated than IC or NC dams.
Learning to do intragastric intubations in dams can be tricky, and it is highly recommended that extensive training take place prior to being allowed to intubate experimental animals. Training can be facilitated by first training with lightly anesthetized rats. Protective gloves should be thin enough to enable a good touch; we use gardening gloves. A good intubation should take less than 1 min.
Very rarely a litter is born either early or late. If this occurs, postnatal day is determined from the point of conception; postnatal day 2 then is always gestational day 24.
Intubating rat pups is the most demanding part of this technique and requires practice. Marking the PE 10 intubation tube to indicate the length that would reach the stomach helps gauge how deep the tube should be inserted. The tube should slide down the esophagus with little resistance although it is sometimes a tight fit in rats at PD 2. If resistance occurs, the tube should be removed and the procedure tried again. For training, starting with rat pups at PD 4 or 5 tends to result in more success.
Initially with this model, it was though that the IC control animals should receive intubations of milk solution in attempt to match the ET groups. However, pups do not regulate their food intake well, and the intubation of milk resulted in IC control animals weighing considerably more that NC control animals; this has been shown in pilot studies in our laboratory and in a published study (27).
References
- 1.May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome: A summary. Alcohol Res. Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol. Teratol. 2000;22:143–149. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driscoll CD, Streissguth AP, Riley EP. Prenatal alcohol exposure: comparability of effects in humans and animal models. Neurotoxicol. Teratol. 1990;12:231–237. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- 4.Hannigan JH, Berman RF. Amelioration of fetal alcohol-related neurodevelopmental disorders in rats: Exploring pharmacological and environmental treatments. Neurotoxicol. Teratol. 2000;22:103–111. doi: 10.1016/s0892-0362(99)00050-1. [DOI] [PubMed] [Google Scholar]
- 5.Hannigan JH, O’leary-Moore SK, Berman RF. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neurosci. Biobehav. Rev. 2006 doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Thomas JD, Weinert SP, Sharif S, Riley EP. MK-801 administration during ethanol withdrawal in neonatal rat pups attenuates ethanol-induced behavioral deficits. Alcoholism: Clin. Exp. Res. 1997;21:1218–1225. [PubMed] [Google Scholar]
- 7.Thomas JD, Garcia GG, Dominguez HD, Riley EP. Administration of eliprodil during ethanol withdrawal in the neonatal rat attenuates ethanol-induced learning deficits. Psychopharmacology (Berl) 2004;175:189–195. doi: 10.1007/s00213-004-1806-x. [DOI] [PubMed] [Google Scholar]
- 8.Thomas JD, Garrison M, O’Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol. Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Heaton MB, Paiva M, Madorsky I, Shaw G. Ethanol effects on neonatal rat cortex: comparative analyses of neurotrophic factors, apoptosis-related proteins, and oxidative processes during vulnerable and resistant periods. Dev. Brain Res. 2003;145:249–262. doi: 10.1016/j.devbrainres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Siler-Marsiglio KI, Shaw G, Heaton MB. Pycnogenol (R) and vitamin E inhibit ethanol-induced apoptosis in rat cerebellar granule cells. J. Neurobiol. 2004;59:261–271. doi: 10.1002/neu.10311. [DOI] [PubMed] [Google Scholar]
- 11.Riley EP, Meyer LS. Considerations for the design, implementation, and interpretation of animal models of fetal alcohol effects. Neurobehav. Toxicol. Teratol. 1984;6:97–101. [PubMed] [Google Scholar]
- 12.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 13.Cronise K, Marino MD, Tran TD, Kelly SJ. Critical periods for the effects of alcohol exposure on learning in rats. Behav. Neurosci. 2001;115:138–145. doi: 10.1037/0735-7044.115.1.138. [DOI] [PubMed] [Google Scholar]
- 14.Tran TD, Cronise K, Marino MD, Jenkins WJ, Kelly SJ. Critical periods for the effects of alcohol exposure on brain weight, body weight, activity, and investigation. Behav. Brain Res. 2000;116:99–110. doi: 10.1016/s0166-4328(00)00263-1. [DOI] [PubMed] [Google Scholar]
- 15.Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav. Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- 16.Ryabinin AE, Cole M, Bloom FE, Wilson MC. Exposure of neonatal rats to alcohol by vapor inhalation demonstrates specificity of microcephaly and Purkinje cell loss but not astrogliosis. Alcoholism: Clin. Exp. Res. 1995;19:784–791. doi: 10.1111/j.1530-0277.1995.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg J. Nutritional issues in perinatal alcohol exposure. Neurobehav. Toxicol. Teratol. 1984;6:261–269. [PubMed] [Google Scholar]
- 18.Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcoholism: Clin. Exp. Res. 1982;6:523–531. doi: 10.1111/j.1530-0277.1982.tb05017.x. [DOI] [PubMed] [Google Scholar]
- 19.Samson HH, Diaz J. Effects of neonatal ethanol exposure on brain development in rodents. In: Abel EL, editor. Fetal alcohol syndrome. Boca Raton, FL: CRC Press; 1982. pp. 131–150. [Google Scholar]
- 20.Kelly SJ, Mahoney JC, Randich A, West JR. Indices of stress in rats: effects of sex, perinatal alcohol and artificial rearing. Physiol. Behav. 1991;49:751–756. doi: 10.1016/0031-9384(91)90314-e. [DOI] [PubMed] [Google Scholar]
- 21.Kelly SJ, Tran TD. Alcohol exposure during development alters social recognition and social communication in rats. Neurotoxicol. Teratol. 1997;19:383–389. doi: 10.1016/s0892-0362(97)00064-0. [DOI] [PubMed] [Google Scholar]
- 22.Light KE, Kane CJ, Pierce DR, Jenkins D, Ge Y, Brown G, Yang H, Nyamweya N. Intragastric intubation: Important aspects of the model for administration of ethanol to rat pups during the postnatal period. Alcoholism: Clin. Exp. Res. 1998;22:1600–1606. doi: 10.1111/j.1530-0277.1998.tb03954.x. [DOI] [PubMed] [Google Scholar]
- 23.Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol. Teratol. 2003;25:519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 24.Dudek BC, Abbott ME. A biometrical genetic analysis of ethanol response in selectively bred long-sleep and short-sleep mice. Behav. Genet. 1984;14:1–19. doi: 10.1007/BF01066065. [DOI] [PubMed] [Google Scholar]
- 25.West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rats. Alcohol. 1984;1:83–95. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]
- 26.Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J. Nutr. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- 27.Goodlett CR, Johnson RB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol. Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- 28.Geller LM, Geller EH. A simple technique for the permanent marking of newborn rats. Psychol.l Rep. 1966;18:221–222. doi: 10.2466/pr0.1966.18.1.221. [DOI] [PubMed] [Google Scholar]
- 29.Marino MD, Cronise K, Lugo JN, Jr, Kelly SJ. Ultrasonic vocalizations and maternal-infant interactions in a rat model of fetal alcohol syndrome. Devel. Psychobiol. 2002;41:341–351. doi: 10.1002/dev.10077. [DOI] [PubMed] [Google Scholar]
- 30.Pierce DR, West JR. Blood alcohol concentration: a critical factor for producing fetal alcohol effects. Alcohol. 1986;3:269–272. doi: 10.1016/0741-8329(86)90036-4. [DOI] [PubMed] [Google Scholar]
- 31.Pierce DR, West JR. Alcohol-induced microencephaly during the third trimester equivalent: relationship to dose and blood alcohol concentration. Alcohol. 1986;3:185–191. doi: 10.1016/0741-8329(86)90043-1. [DOI] [PubMed] [Google Scholar]