Abstract
Chymase, a serine protease found in mast cell granules, is released into the interstitium following injury or inflammation. Chymase is the primary ACE-independent pathway of angiotensin II formation, and also functions to activate TGF-beta and other promoters of extracellular matrix degradation, thereby playing a role in tissue remodeling. In the diseased kidney, chymase-containing mast cells markedly increase and their density correlates with tubulointerstitial fibrosis severity. Studies in humans support the pathologic role of chymase in diabetic nephropathy, while animal studies form the basis for the importance of increased chymase-dependent angiotensin II formation in progressive hypertensive, diabetic and inflammatory nephropathies. Moreover, humans with kidney disease express chymase in diseased blood vessels in concordance with significantly elevated plasma chymase levels. Conversely, specific chymase inhibitors attenuate angiotensin II production and renal fibrosis in animal models, suggesting their potential therapeutic benefit in human nephropathy, where chymase-containing mast cells accumulate and contribute to progressive disease.
Keywords: Chymase, mast cells, kidney disease, angiotensin II, transforming growth factor-β
INTRODUCTION
Chymase is synthesized as a zymogen, but is activated by dipeptidyl peptidase I in the mast cell granule [1]. Mast cell granules mainly store the active form of chymase in complex with granular heparin proteoglycans, which occur in abundance in the granule [2]. Macromolecular interactions between anionic heparin and cationic chymase molecules mask the substrate–binding site of chymase [3]. However, substrate access is expected to increase greatly as mast cells degranulate and the chymase–heparin complex disperses in the interstitial spaces between cells. Importantly, also, its association with heparin reduces diffusional loss of chymase from the interstitial space, which allows the enzyme to remain in the tissue compartment, in an active state, for several weeks [2, 4, 5]. Chymase is present in mast cell granules and angiotensinogen is mainly extracellular, originating from the liver. To date, there is no evidence to suggest that chymase-dependent Ang II formation occurs within mast cell granules. It is unlikely that chymase-dependent Ang II formation can occur within the mast cell cytosol because the redox potential in the cytosol would prevent disulfide bond formation that is critical to proper chymase folding. By contrast, there is evidence that chymase dependent Ang II formation occurs extra-cellularly in the interstitium [6].
Chymase functions as the primary angiotensin I-converting enzyme (ACE)-independent pathway of angiotensin II (Ang II) formation and, in mice, has been shown to be involved in blood pressure regulation in the setting of ACE inhibitor therapy [7]. Chymase is released primarily in the setting of injury or inflammation, and is believed to be involved in the promotion of tissue remodeling by activating latent transforming growth factor-β (TGF-β), pro-matrix metalloproteinases (MMP) and pro-endothelins, and thrombin and plasmin degradation [8, 9]. Chymase activity is linked to renal interstitial fibrosis and glomerulosclerosis in animal models. Animal studies suggest that chymase inhibition might be beneficial for the treatment of diabetic, hypertensive and inflammatory nephropathies.
In this review, we discuss the biology of chymase, consider its role and actions in the kidney, and review the potential benefits of chymase inhibition as a novel therapy in the treatment of progressive kidney disease. Our review underlies the belief that while the mechanisms that underlie the pathological effects of chymase in the kidney are not yet well defined, studies to date support the notion that chymase inhibitors may have significant therapeutic benefits in humans.
ONLY CERTAIN CHYMASES ARE ANG II-FORMING
Chymases occur in two isoenzyme groups; α and β, based on their primary structure [10]. A single α-chymase gene is expressed in all mammals studied, including humans, dogs and mice. A β-chymase does not occur in humans but a variable number of β-chymases are expressed in rodents [10]. Members of both chymase groups convert Ang I to Ang II. However due to remarkable species differences in substrate specificity, some β-chymases are net Ang II-degrading enzymes11 . Interestingly, also, some α-chymases (e.g. mouse mast cell protease 5 [MMCP-5]) neither form nor degrade Ang I [6, 12]. Human chymase is a highly efficient Ang II-forming enzyme [13].
The distribution of human chymase varies; it is present in blood vessels and the heart14, and high levels are found in the gut and uterus. However, it is nearly undetectable in the normal human kidney [14].
MAST CELL DENSITY INCREASED IN DISEASED KIDNEYS
As early as the 1920’s it was noted that mast cells were rare in normal human kidney parenchyma, but increased in cases of renal tuberculosis, renal sclerosis and chronic pyelonephritis [15]. By 1960 it was observed that mast cell number increased in a variety of human nephropathies, localized to the renal interstitium, and were nearly always observed in conjunction with fibroblast hyperplasia [15]. Despite these early observations, little attention was paid to the role of mast cells in kidney disease until the 1990’s, when it was observed that a significant correlation existed between interstitial fibrosis and mast cell proliferation, regardless of the underlying renal disease [16-21].
Mast cells originate from CD34-positive bone marrow progenitor cells, and migrate from the circulation through vascularized tissue where they mature [22] and exist on mucosal surfaces and within connective tissues [23]. Their location around blood vessels, nerves and epithelia facilitates their participation in homeostatic functions and rapid response to abnormalities at immune barrier locations, where their presence is increased [24].
Although known for their role in asthma and anaphylaxis, mast cells have intra-cytoplasmic granules containing a wide array of mediators with diverse functional capabilities [25]. When activated, they can secrete inflammatory mediators, including the proteases chymase and tryptase, and pro-fibrotic cytokines such as tumor necrosis factor α (TNFα) and TGF-β1, which can attract inflammatory cells and promote tissue remodeling [2, 26-28].
Mast cells achieve their functional maturity and phenotype based on their anatomic location and subtype, and vary by their cytoplasmic granule structure, quantity of stored mediators, and response to various secretagogues [2]. The mast cell’s cytoplasmic granule protease content characterizes it, and in humans, serves to differentiate it into its two subtypes, the density of which differ by tissue type [2]. Human mast cells containing chymase and tryptase are designated as MCTC. Mast cells containing only tryptase are designated as MCT [29]. The two subtypes can be distinguished from one another using immunohistochemical techniques, allowing accurate quantification when both are present in the same tissue section. MCTC is the predominant mast cell in the skin, tonsils and intestinal submucosa, while MCT predominate in lung alveolar tissue, nasal mucosa and small intestine mucosa [2].
MAST CELL DENSITY CORRELATES WITH RENAL TUBULOINTERSTITIAL DISEASE SEVERITY
Within the diseased kidney, mast cells predominate within the interstitium, can infiltrate renal tubules [30], are rare in the glomeruli, but are present within the fibrotic intima of intra-renal arteries in chronic allograft vasculopathy. The number of mast cells closely correlates with the severity of tubulointerstitial disease in renal diseases, including IgA nephropathy [31, 32], primary and secondary glomerulonephritis [18, 19, 33, 34], diabetic nephropathy [35,36], hypertensive nephropathy [26] and allograft rejection [16, 20, 37, 38]. Mast cell accumulation is also inversely correlated with a decline in glomerular filtration rate and disease progression [31, 34, 39-41].
How are mast cells recruited to the kidney? Mast cells are rarely found in the normal kidney. A key element to mast cell recruitment to the renal interstitium may be stem-cell factor (SCF), which is increased in CKD [42]. SCF, which localizes to areas of interstitial expansion and fibrosis, induces differentiation, chemoattraction and activation of mast cells in humans [41]. Since pulmonary fibroblasts produce SCF, it is suggested that activated renal interstitial fibroblasts produce SCF following injury, and attract mast cells to sites of injury [41]. SCF level significantly correlates with the number of interstitial mast cells and degree of interstitial fibrosis, suggesting that it plays a role in to renal mast cell recruitment [39].
CHYMASE-DEPENDENT ANG II FORMATION
The identification of chymase as the major Ang II-forming enzyme in the human heart [14,43] provided a biochemical basis for the study of non-ACE pathway. In pharmacological studies in conscious baboons, intravenous infusion of [Pro11, DAla12]Ang I—a chymase selective substrate that is converted to Ang II by chymase but not ACE [44] resulted in increased left ventricular systolic and diastolic pressures consistent with arterial vasoconstriction [44]. An Ang II receptor antagonist but not an ACE inhibitor inhibited these effects, showing that chymase is a functional Ang II-forming enzyme in vivo. Similar findings were reported in the marmoset [45]. The potency of [Pro11, DAla12]Ang I was much lower than that anticipated from the kinetic studies of its conversion to Ang II and suggested that [Pro11, DAla12]Ang I delivery to the adventitial and medial compartments of the blood vessel, where chymase is localized, is limited when this chymase selective substrate is administered intravascularly.
Chymase is found in human arteries and veins [46], and [Pro11, DAla12]Ang I administration into the dorsal hand vein of patients with coronary artery disease produces an ACE inhibitor-resistant vasoconstrictor response [47], showing in vivo the presence of a chymase pathway for Ang II production in humans. The predominance of chymase-dependent Ang II-forming activity in primate tissues over other non-ACE Ang II-forming activities and the in vivo findings with the chymase selective substrate suggest a role for chymase in tissue Ang II formation.
CHYMASE-POSITIVE MAST CELLS LOCALIZE TO SITES OF KIDNEY FIBROSIS
Chymase-positive mast cells are increased in human renal allografts with chronic rejection 37 and in the setting of IgA nephropathy [32], and correlate with the severity of interstitial fibrosis and intra-renal vascular resistance. In children with crescentic glomerulonephritis, chymase-positive mast cells also correlate with tubolointerstitial fibrosis and with loss of renal function [33]. Interestingly, IgA nephropathy patients treated with valsartan and prednisolone for two months, compared with prednisolone alone, were observed to experience a significant reduction in the number of chymase-positive mast cells on repeat renal biopsy accompanied by improved renal parenchymal circulation [32], suggesting that blocking Ang II actions by inhibiting its AT1 receptor could possibly attenuate recruitment of mast cells to the kidney. This hypothesis, however, needs to be tested.
ROLE OF CHYMASE-DEPENDENT TGF- AND MMP EXPRESSION IN THE PROMOTION OF RENAL FIBROSIS
TGF-β
Renal fibrosis is commonly found in progressive chronic kidney disease (CKD) that leads to end-stage renal disease (ESRD), and is characterized by tubulointerstitial fibrosis and gomerulosclerosis. Multiple cell types participate in renal fibrogenesis, resulting in activation of inflammatory cells within the glomerulus or renal interstitium, to produce fibrogenic and inflammatory cytokines, induce cellular phenotypic change, and produce extracellular matrix components [48]. With prolonged renal injury comes a fibrous scar and loss of kidney function.
TGF-β is an established cytokine in renal fibrosis [49-51], and its upregulation is found in nearly every etiology of CKD. TGF-β can cause myofibroblastic activation of mesangial cells, interstitial fibroblasts and tubular epithelial cells or transform them into matrix-producing fibrogenic cells [48]. Ang II and high glucose induce TGF-β synthesis in renal cells [52]. Further, TGF-β reduces collagenase production and stimulates tissue inhibitor of metalloproteinases, resulting in the overall inhibition extracellular matrix turnover [53]. Conversely, TGF-β inhibition attenuates renal fibrosis and progression of renal disease. Thus, research focusing on TGF-β downstream effectors or signaling may offer a therapeutic advantage [48].
An underlying mechanism for the correlation of chymase with interstitial fibrosis in the diseased kidney may be because chymase is shown to increase the expression of TGF-β indirectly via promotion of Ang II formation and directly by activating pro-TGF-α1 [54-56]. In fact, in human veins of CKD and ESRD patients with pre-existing intimal hyperplasia taken at the time of vascular access surgery, TGF-β co-localizes with chymase, and is most prominent in the intima and media layers of the blood vessel [57].
Direct evidence for the effect of chymase on renal fibrosis and TGF-β expression comes from comparing kidney tissue sections from MMCP-4-deficient and - sufficient mice with anti-GBM glomerulonephritis [58]. While anti-GBM mice had a significant increase in interstitial type 1 collagen and fibrin 14 days after injury compared with control mice, MMCP-4 null mice had significantly lower amounts of type 1 collagen and local Ang II generation, and trended toward lower expression of TGF-β [58]. These findings show that MMCP-4 promotes renal fibrosis. MMCP-4 also plays a prominent role in regulating Ang II in vivo in the interstitium of the ACE inhibitor-treated mouse heart [6]6.
MMP’s
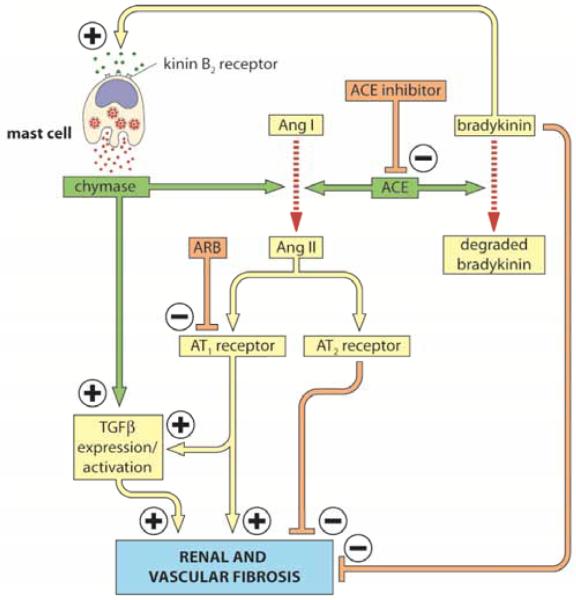
Renal fibrosis is characterized by the accumulation of extracellular matrix (ECM), made up primarily of collagen. MMP’s are produced in multiple tissues, including the kidney, and function to degrade collagen and ECM proteins. It is believed that MMP’s have both anti- and pro-fibrotic roles. Most MMP’s are secreted as pro-MMP’s, and require cleavage for activation [59]. MMP activity is also regulated through inhibition by specific tissue inhibitors of MMPs (TIMP’s). Chymase can potentially regulate MMP activity by increasing the levels of pro-MMP and by direct enzymatic activation of pro-MMP to its active form. This mechanism is illustrated in Fig. (1). Increase in pro-MMP likely occurs via Ang II forming activity of chymase.
Fig. (1). Regulation of Ang II and TGF-β by mast cell chymase and ACE.
Mast cell activation by both immune and non-immune mechanisms may result in advancing fibrosis in tissues. Several receptor-binding activators cause mast cells to degranulate [84]. These include, IgE, complement, peptide hormones, cytokines and chemokines. In this scheme we illustrate the potential interaction between the two main tissue Ang II-forming enzymes, ACE and chymase, in regulating tissue Ang II and TGF-β levels; factors that promote tissue fibrosis. Based on the mechanistic studies in the heart [6], the effect of chronic inhibition of ACE on Ang I to Ang II conversion in the kidney could be counteracted via bradykinin/B2 receptor–dependent chymase release from mast cells. Thus the antifibrotic effects of bradykinin/B2 receptor activation on the kidney [85] could be limited by chymase release and subsequent activation of Ang II-formation and TGF-β expression/activation. In addition, Ang II via its effects on its AT2 receptor can limit its profibrotic effects that are mediated by AT1 receptor activation [86]. Chymase release also has a myriad of effects on factors that promote the inflammatory response [84].
MMP’s play an important role in tissue fibrosis, although their function may depend of the etiology and stage of disease. In the renal fibrotic model of unilateral ureteral obstruction in which there is an increase in collagen types I and IV, MMP-2 expression and activity increase early, with a subsequent biphasic increase in TIMP-1 expression, suggesting impaired matrix degradation in the setting of interstitial fibrosis in the obstructed kidney [60]. MMP’s are thought to function as matrix degrading enzymes. In keeping with this, decreased MMP-9 expression is correlated with the development of renal fibrosis in mice [61].
MMP-2 and -9 are gelatinases. Chymase is shown to regulate MMP-2 and -9 activity in experimental models [62, 63], and human chymase can cleave pro-MMP-9 to its active form, which suggests a beneficial role of chymase in minimizing renal fibrosis. However, chymase-dependent pro-MMP activation could also produce unexpected consequences. For example, Cheng et al. [64] show that MMP-2 overexpression in transgenic mice using a renal proximal tubule-specific type I γ GT promoter leads to structural alterations in the tubular basement membrane, which triggers tubular epithelial-mesenchymal transition, with resultant tubular atrophy, fibrosis and renal failure. Thus, chymase-dependent regulation of MMP-2 and -9 activity could have complex effects on renal fibrosis, which remain to be studied in more detail.
ROLE OF CHYMASE IN ISCHEMIC, DIABETIC AND INFLAMMATORY KIDNEY DISEASE
Although studies are limited in humans, experimental evidence suggests that a primary mechanism by which chymase may promote kidney disease progression is via chymase-dependent Ang II formation (Fig. 1).
Ischemic Nephropathy
In the two-kidney, one-clip rat model, Sadjadi et al. [65] have reported that chymase activity is upregulated in the ischemic kidney compared with the non-ischemic control kidney [65], but that renal Ang II is increased in both ischemic and non-ischemic controls. In a similar experimental model in dogs, Tokuyama et al. [66] found that an ACE inhibitor increased renal plasma flow in the non-ischemic kidney. By contrast, a chymase inhibitor (chymostatin), but not an ACE inhibitor, increased renal plasma flow in the ischemic kidney. Furthermore, in clipped kidneys, a chymase inhibitor suppressed renal Ang II whereas an ACE inhibitor was effective in reducing renal Ang II levels in the non-clipped kidney. Together, these studies suggest that suppression of the Ang II production through chymase inhibition may be useful for the correction of hypoperfusion to the kidneys, which is a characteristic of ischemic nephropathy.
Diabetic Nephropathy
The importance of Ang II in the progression of diabetic nephropathy has been well established [67], and several intra-renal cell types, including mesangial cells, podocytes, and epithelial cells, possess all renin–angiotensin system components and can synthesize Ang II. Increased intra-renal Ang II is believed to play a key role in mesangial expansion and the development of glomerulosclerosis in diabetes. Under normal glucose conditions the non-specific chymase inhibitor chymostatin reduced Ang II levels in human mesangial cell lysates and in the culture medium, but the ACE inhibitor captopril had no effect [68]. This suggests a role for chymase in Ang II generation by these cells. However, from these studies it is not possible to distinguish between cell-bound Ang II (resulting from extracellular conversion of Ang I to Ang II by chymase in the culture media) and intracellular Ang II and therefore, no firm conclusions can be made about the role of chymase in intracellular Ang II formation. It is noteworthy that high glucose caused human mesangial cells to increase chymase mRNA and protein expression and it increased intracellular ACE activity 10-fold. Concomitantly, intracellular Ang II was increased by high glucose; but this was not inhibited by extracellular administration of chymostatin and captopril [68]. More detailed studies looking at subcellular compartments that contain ACE, chymase and Ang II are required to make definitive statements about their role in regulating intracellular Ang II levels.
In diabetes, Ang II up-regulation also plays a key role in podocyte cell injury. The podocyte is present in the glomerular capillary wall and functions as a barrier to negatively charged proteins. High glucose increases Ang II synthesis in cultured podocytes, which is significantly reduced by chymostatin, and not with ACE inhibition [69], indicating chymase-independent Ang II formation in podocytes exposed to high glucose.
A further link between chymase-dependent Ang II formation and diabetic nephropathy comes from examining the association of chymase gene polymorphism with diabetic CKD. Genes and genetic loci associated with excess Ang II production were compared among Asian Indian patients with type 2 diabetes and CKD, and patients with diabetes and no diabetic CKD70. Investigators found a significant association between chymase gene polymorphism (GA genotype of G>A promoter SNP) and diabetic CKD in Asian Indian patients [70].
Anti-Glomerular Basement Membrane Nephritis
The most compelling evidence to date for the role of chymase in progressive kidney disease is from a recent study of inflammatory kidney disease. In a model of anti-glomerular basement membrane (anti-GBM) glomerulonephritis, mice deficient in MMCP-4, the functional homolog of human chymase, developed significantly lower late-phase (days 10– 14) proteinuria and had a significantly lower decline in renal function compared with WT mice [58]. This was accompanied by a significant reduction in glomerular infiltration and subendothelial deposits, and interstitial infiltration and tubular necrosis compared with WT mice. To discern whether these benefits occurred specifically as a result of MMCP-4 deficiency rather than mast-cell deficiency, mast cell deficient KitW/KitW-v mice were reconstituted with bone marrow derived mast cells from either WT or MMCP-4 deficient mice prior to induction of anti-GBM glomerulonephritis [58]. Those reconstituted with MMCP-4 deficient mast cells showed a trend to lower proteinuria and serum creatinine concentrations, significantly lower BUN, and a reduction in interstitial infiltration and fibrosis and tubular necrosis, suggesting that MMCP-4 is responsible for the observed benefits. MMCP-4 deficient mice with anti-GBM glomerulonephritis also had less renal expression of pro-inflammatory cytokine and chemokine mRNA, significantly lower type 1 collagen deposition in the glomerulus and interstitium, and nearly absent interstitial Ang II expression, indicating the key role MMCP-4 has in mediating two established contributors to renal fibrosis [58, 71]. These data are supported by recent findings that chymase promotes glomerular albumin permeability in vitro [72], and progressive human aortic aneurysm formation by multiple mechanisms, including elastin degradation [73].
In contrast, another investigation has shown that mast-cell deficient mice with anti-GBM glomerulonephritis have greater proteinuria and glomerular damage compared with control mice, and increased numbers of infiltrating T cells and macrophages [74]. Differences in these finding may result from global genetic c-kit inactivation that not only makes the mouse mast cell deficient but also has a number of defects other than mast cell deficiency. More detailed studies involving adoptive transfer of wild-type mast cells into mast-cell deficient mice should help clarify the role of mast cells in glomerulonephritis. It is also possible that MMCP-4 has deleterious effects on the kidney in anti-GBM glomerulonephritis, while other mast cell mediators may be protective. If this is the case, a chymase inhibitor could be expected to be more effective in treating anti-GBM glomerulonephritis than a mast cell stabilizer.
CIRCULATING CHYMASE IS MARKEDLY INCREASED IN CKD AND ESRD PATIENTS
Measurement of plasma chymase has recently been used to explore whether elevated tissue chymase expression is reflected in the systemic circulation. Recently Sun et al. [73] reported a significant correlation between the rates of expanding human abdominal aortic aneurysms (AAA), which have high intra-lesion chymase-positive mast cell density, to serum chymase levels. These findings, combined with experimental data showing that AAA formation was significantly reduced in chymase (MMCP-4)-null mice, suggest that mast cell chymase plays a role in the progression of human and mice AAA [73]. Increased plasma chymase activity has also been shown in women with preeclampsia, which markedly declines postpartum [75], and is expressed in maternal vessel endothelium, suggesting a state of inflammation and a mechanism for increased vascular sensitivity to Ang II in preeclampsia.
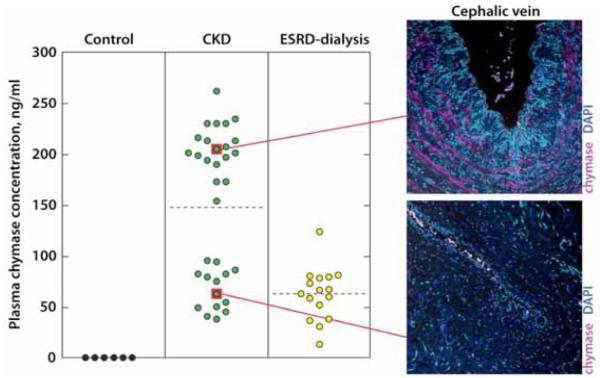
Similar studies have now been conducted in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD). We recently showed that venous tissue taken at the time of surgical arteriovenous fistula creation, which exhibited pre-existing venous neointimal hyperplasia, highly expressed chymase [57]. Plasma chymase concentrations in these patients were significantly elevated. Circulating chymase concentrations were increased in all CKD patients 4–33-fold more than in non-CKD control subjects, who had undetectable plasma chymase concentrations (< 8 ng/ml), which was associated with a virtual lack of vascular chymase (Fig. 2).
Fig. (2). Plasma chymase concentrations in CKD and ESRD patients and corresponding vascular chymase expression.
Plasma chymase concentration by patient cohort. Note bimodal distribution of plasma chymase in CKD patients. Elevated plasma chymase is shown with corresponding mast cell chymase expression in veins of same patients with intimal hyperplasia. ESRD patients have a different plasma chymase concentration, suggesting that creatinine clearance may influence plasma chymase. From Wasse et al. [57].
Interestingly, chymase distribution was bimodal in CKD patients, yet there were no significant differences in co-morbidities between the groups except BMI. The significance of the bimodal plasma chymase distribution in CKD patients needs to be confirmed using a larger sample size. It is tempting to speculate that circulating chymase levels may be a predictor of extant intimal hyperplasia in patients with CKD. In these CKD patients, plasma chymase levels were much greater than in patients with mastocytosis or aortic aneurysm [73, 76]. Although there are several endogenous inhibitors of chymase, such as alpha-1 antitrypsin, Raymond et al. [76] have shown that circulating chymase exists in a protected state in complex with alpha-2 macroglobulin such that it is capable of converting Ang I to Ang II in the circulation. Further, angiotensin receptor blockers (ARB’s) but not ACE inhibitors have been shown to attenuate intimal hyperplasia after vascular injury [77-80]. It is therefore possible that elevated chymase levels underlie the therapeutic advantage of ARB’s versus ACE inhibitors in this clinical setting.
Finally, ESRD subjects had a different plasma chymase distribution than CKD subjects, suggesting that reduced creatinine clearance may be responsible for elevated plasma chymase levels in CKD subjects. Clearly, dialysis had an effect on plasma chymase levels, however, it is unlikely that this was due to direct removal of chymase from the bloodstream by dialysis, because chymase exists as a 200 kDa complex with alpha-2 macroglobulin in plasma [76]. This chymase-alpha-2 macroglobulin complex is too large to be cleared by the dialyzer membrane. This led the investigators to speculate that plasma markers of systemic inflammation may be elevated among CKD patients with the greatest plasma chymase concentrations. However, an association between plasma C-reactive protein and plasma chymase concentration levels was not observed. Moreover, the plasma chymase bi-modal distribution was not associated with statin use. However, interleukin-6 (IL-6), a local marker of inflammation, was present in vascular intimal hyperplasia lesions that express high levels of chymase.
CHYMASE INHIBITION ATTENUATES RENAL DISEASE IN ANIMAL MODELS
Intra-renal Ang II and AT1 receptor expression increase in unilateral ureteral obstruction-induced interstitial fibrosis. Yet ACE inhibition is not effective in reducing renal injury in this setting [81], which could suggest that chymase-dependent but not ACE-dependent intra-renal Ang II formation promotes renal fibrosis. In support of this notion, in hamsters with ureteral obstruction-induced renal injury, AT1 receptor blocker olmesartan significantly lowered interstitial fibrosis relative to vehicle controls. Treatment with either olmesartan or the specific chymase inhibitor 4-[1-(4-methyl-benzo[b]thiophen-3-ylmethyl)-1H-benzimidazol-2-ylsulfanyl]-butyric acid resulted in similar and significantly lower renal tissue expression of α-smooth muscle actin, type 1 collagen and TGF-β mRNA compared with vehicle-treated animals [82].
The importance of chymase has also been studied in a hamster model of streptozotocin (STZ)-induced diabetes. STZ-induced diabetic hamsters had a > 2-fold increase in renal cortical and > 5-fold increase in renal medullary chymase mRNA levels compared with controls, while ACE expression was localized to the proximal tubule brush border [83]. Following 8-week treatment with specific chymase inhibitors, TEI-E00548 or TEI-F00806, blood pressure was unchanged, yet intra-renal overproduction of Ang II was completely suppressed, while serum Ang II was not affected. The converse occurred with ramipril 8-week therapy: blood pressure dropped significantly in conjunction with serum Ang II concentrations, yet intra-renal Ang II overproduction did not change in diabetic hamsters [83]. Untreated diabetic hamsters had a > 7-fold increase in urinary protein excretion after 8 weeks compared with controls. Treatment with TEI-E00806 at week 8 significantly reduced proteinuria by 80% in diabetic hamsters, but proteinuria was not significantly affected by ramipril treatment. Improvements in renal histology were also apparent with administration of chymase inhibition. Untreated diabetic hamsters exhibited characteristic renal mesangial expansion, as reflected by a > 1.5-fold increase in mesangial matrix index (MMI), compared to controls. In contrast, both TEI-E00548 and TEI-F00806 completely attenuated the MMI to nondiabetic levels, while ramipril produced a lesser but substantive reduction in MMI compared to untreated animals [83]. The mechanism by which chymase inhibition, but not ramipril, attenuated renal mesangial expansion appears to be by suppression of glomerular TGF-β1 and fibronectin protein expression [83]. These data suggest that chymase may be an important drug target in the treatment of human diabetic nephropathy.
CONCLUSIONS
Progression of CKD involves multiple mediators and events. Of these, chymase activates two of most well established mediators of renal tubulointerstitial fibrosis, Ang II and TGF-β. Chymase can form Ang II independent of ACE and stimulates TGF-β indirectly via promotion of Ang II formation and directly by activating pro-TGF-β. Currently, observational studies in humans support the role of chymase in diabetic nephropathy, while limited animal studies form the basis for the importance of increased chymase-dependent Ang II formation in progressive hypertensive, diabetic and inflammatory nephropathies.
Data thus far suggest that that chymase inhibition would be most beneficial in settings in which Ang II has a major role, yet chymase may also promote progressive renal disease through its activation of TGF-β and MMP pathways.
Future investigations would benefit by the development of a transgenic mouse containing human chymase in order to mitigate the species differences in substrate specificity of chymases and to more directly implicate a role for human chymase in nephropathy, with the hope that chymase-specific inhibition can be tested in humans.
ACKNOWLEDGMENTS
This study was supported by United States National Institutes of Health (NIH) K23 Award (H.W), NIH grant HL079040 (A.H.) and a Public Health Service Grant (UL1 RR02008, KL2 RR025009 or TL1 RR025010) from the Clinical and Translational Science Award program, NIH, National Center for Research Resources.
Footnotes
CONFLICT OF INTEREST Declared none.
REFERENCES
- [1].Wolters PJ, Pham CT, Muilenburg DJ, Ley TJ, Caughey GH. Dipeptidyl peptidase I is essential for activation of mast cell chymases, but not tryptases, in mice. J Biol Chem. 2001;276(21):18551–18556. doi: 10.1074/jbc.M100223200. [DOI] [PubMed] [Google Scholar]
- [2].Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- [3].Yurt R, Austen KF. Preparative purification of the rat mast cell chymase: characterization and interaction with granule components. J Exp Med. 1977;146(5):1405–1419. doi: 10.1084/jem.146.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lindstedt L, Lee M, Kovanen PT. Chymase bound to heparin is resistant to its natural inhibitors and capable of proteolyzing high density lipoproteins in aortic intimal fluid. Atherosclerosis. 2001;155(1):87–97. doi: 10.1016/s0021-9150(00)00544-x. [DOI] [PubMed] [Google Scholar]
- [5].Takai S, Shiota N, Jin D, Miyazaki M. Functional role of chymase in angiotensin II formation in human vascular tissue. J Cardiovasc Pharmacol. 1998;32(5):826–833. doi: 10.1097/00005344-199811000-00020. [DOI] [PubMed] [Google Scholar]
- [6].Wei CC, Hase N, Inoue Y, et al. Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J Clin Invest. 2010;120(4):1229–1239. doi: 10.1172/JCI39345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li M, Liu K, Michalicek J, et al. Involvement of chymase-mediated angiotensin II generation in blood pressure regulation. J Clin Invest. 2004;114(1):112–120. doi: 10.1172/JCI20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lindstedt KA, Wang Y, Shiota N, et al. Activation of paracrine TGF-beta1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15(8):1377–1388. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- [9].Maurer M, Wedemeyer J, Metz M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432(7016):512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- [10].Chandrasekharan UM, Sanker S, Glynias MJ, Karnik SS, Husain A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science. 1996;271(5248):502–505. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- [11].Sanker S, Chandrasekharan UM, Wilk D, Glynias MJ, Karnik SS, Husain A. Distinct multisite synergistic interactions determine substrate specificities of human chymase and rat chymase-1 for angiotensin II formation and degradation. J Biol Chem. 1997;272(5):2963–2968. doi: 10.1074/jbc.272.5.2963. [DOI] [PubMed] [Google Scholar]
- [12].Caughey GH, Raymond WW, Wolters PJ. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim Biophys Acta. 2000;1480(1-2):245–257. doi: 10.1016/s0167-4838(00)00076-5. [DOI] [PubMed] [Google Scholar]
- [13].Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265(36):22348–22357. [PubMed] [Google Scholar]
- [14].Urata H, Boehm KD, Philip A, et al. Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart. J Clin Invest. 1993;91(4):1269–1281. doi: 10.1172/JCI116325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pavone-Macaluso M. Tissue mast cells in renal diseases. Acta Pathol Microbiol Scand. 1960;50:337–346. doi: 10.1111/j.1699-0463.1960.tb01202.x. [DOI] [PubMed] [Google Scholar]
- [16].Lajoie G, Nadasdy T, Laszik Z, Blick KE, Silva FG. Mast cells in acute cellular rejection of human renal allografts. Mod Pathol. 1996;9(12):1118–1125. [PubMed] [Google Scholar]
- [17].Ehara T, Shigematsu H. Mast cells in the kidney. Nephrology (Carlton) 2003 Jun;8(3):130–138. doi: 10.1046/j.1440-1797.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- [18].Hiromura K, Kurosawa M, Yano S, Naruse T. Tubulointerstitial mast cell infiltration in glomerulonephritis. Am J Kidney Dis. 1998;32(4):593–599. doi: 10.1016/s0272-6386(98)70022-8. [DOI] [PubMed] [Google Scholar]
- [19].Toth T, Toth-Jakatics R, Jimi S, Ihara M, Urata H, Takebayashi S. Mast cells in rapidly progressive glomerulonephritis. J Am Soc Nephrol. 1999;10(7):1498–1505. doi: 10.1681/ASN.V1071498. [DOI] [PubMed] [Google Scholar]
- [20].Pardo J, Diaz L, Errasti P, et al. Mast cells in chronic rejection of human renal allografts. Virchows Arch Aug. 2000;437(2):167–172. doi: 10.1007/s004280000211. [DOI] [PubMed] [Google Scholar]
- [21].Roberts IS, Brenchley PE. Mast cells: the forgotten cells of renal fibrosis. J Clin Pathol. 2000;53(11):858–862. doi: 10.1136/jcp.53.11.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- [23].Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271(5250):818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- [24].Holdsworth SR, Summers SA. Role of mast cells in progressive renal diseases. J Am Soc Nephrol. 2008;19(12):2254–2261. doi: 10.1681/ASN.2008010015. [DOI] [PubMed] [Google Scholar]
- [25].Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19(1):31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- [26].Welker P, Kramer S, Groneberg DA, et al. Increased mast cell number in human hypertensive nephropathy. Am J Physiol Renal Physiol. 2008;295(4):F1103–1109. doi: 10.1152/ajprenal.00374.2007. [DOI] [PubMed] [Google Scholar]
- [27].Kovacs EJ. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- [28].Thompson HL, Burbelo PD, Metcalfe DD. Regulation of adhesion of mouse bone marrow-derived mast cells to laminin. J Immunol. 1990;145(10):3425–3431. [PubMed] [Google Scholar]
- [29].Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37(10):1509–1515. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- [30].Colvin RB, Dvorak AM, Dvorak HF. Mast cells in the cortical tubular epithelium and interstitium in human renal disease. Hum Pathol. 1974;5(3):315–326. doi: 10.1016/s0046-8177(74)80114-0. [DOI] [PubMed] [Google Scholar]
- [31].Ehara T, Shigematsu H. Contribution of mast cells to the tubulointerstitial lesions in IgA nephritis. Kidney Int. 1998;54(5):1675–1683. doi: 10.1046/j.1523-1755.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- [32].Konishi Y, Morikawa T, Okada N, et al. Evidence for abundant presence of chymase-positive mast cells in the kidneys of patients with immunoglobulin A nephropathy: effect of combination therapy with prednisolone and angiotensin II receptor blocker valsartan. Hypertens Res. 2008;31(8):1517–1524. doi: 10.1291/hypres.31.1517. [DOI] [PubMed] [Google Scholar]
- [33].Togawa H, Nakanishi K, Shima Y, et al. Increased chymase-positive mast cells in children with crescentic glomerulonephritis. Pediatr Nephrol. 2009;24(5):1071–1075. doi: 10.1007/s00467-008-1044-2. [DOI] [PubMed] [Google Scholar]
- [34].Otsubo S, Nitta K, Uchida K, Yumura W, Nihei H. Mast cells and tubulointerstitial fibrosis in patients with ANCA-associated glomerulonephritis. Clin Exp Nephrol. 2003;7(1):41–47. doi: 10.1007/s101570300005. [DOI] [PubMed] [Google Scholar]
- [35].Ruger BM, Hasan Q, Greenhill NS, Davis PF, Dunbar PR, Neale TJ. Mast cells and type VIII collagen in human diabetic nephropathy. Diabetologia. 1996;39(10):1215–1222. doi: 10.1007/BF02658509. [DOI] [PubMed] [Google Scholar]
- [36].Jones SE, Gilbert RE, Kelly DJ. Tranilast reduces mesenteric vascular collagen deposition and chymase-positive mast cells in experimental diabetes. J Diabetes Complications. 2004;18(5):309–315. doi: 10.1016/j.jdiacomp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- [37].Yamada M, Ueda M, Naruko T, et al. Mast cell chymase expression and mast cell phenotypes in human rejected kidneys. Kidney Int. 2001;59(4):1374–1381. doi: 10.1046/j.1523-1755.2001.0590041374.x. [DOI] [PubMed] [Google Scholar]
- [38].Goto E, Honjo S, Yamashita H, Shomori K, Adachi H, Ito H. Mast cells in human allografted kidney: correlation with interstitial fibrosis. Clin Transplant. 2002;16(Suppl 8):7–11. doi: 10.1034/j.1399-0012.16.s8.1.x. [DOI] [PubMed] [Google Scholar]
- [39].El-Koraie AF, Baddour NM, Adam AG, El Kashef EH, El Nahas AM. Role of stem cell factor and mast cells in the progression of chronic glomerulonephritides. Kidney Int. 2001;60(1):167–172. doi: 10.1046/j.1523-1755.2001.00783.x. [DOI] [PubMed] [Google Scholar]
- [40].Kurusu A, Suzuki Y, Horikoshi S, Shirato I, Tomino Y. Relationship between mast cells in the tubulointerstitium and prognosis of patients with IgA nephropathy. Nephron. 2001;89(4):391–397. doi: 10.1159/000046109. [DOI] [PubMed] [Google Scholar]
- [41].Liu H, Liu F, Peng Y, et al. Role of mast cells, stem cell factor and protease-activated receptor-2 in tubulointerstitial lesions in IgA nephropathy. Inflamm Res. 2010;59(7):551–559. doi: 10.1007/s00011-010-0159-7. [DOI] [PubMed] [Google Scholar]
- [42].Kitoh T, Ishikawa H, Ishii T, Nakagawa S. Elevated SCF levels in the serum of patients with chronic renal failure. Br J Haematol Sep. 1998;102(5):1151–1156. doi: 10.1046/j.1365-2141.1998.00902.x. [DOI] [PubMed] [Google Scholar]
- [43].Husain AKA, Sung SS, Urata H, Bumpus FM. the Cardiac Renin-Angiotensin System. Futura Medical Publishers; Mount Kisco, NY: 1994. Human heart chymase. [Google Scholar]
- [44].Husain A. The chymase-angiotensin system in humans. J Hypertens. 1993;11(11):1155–1159. [PubMed] [Google Scholar]
- [45].Mangiapane ML, Rauch AL, MacAndrew JT, et al. Vasoconstrictor action of angiotensin I-convertase and the synthetic substrate (Pro11,D-Ala12)-angiotensin I. Hypertension. 1994 Jun;23(6 Pt 2):857–860. doi: 10.1161/01.hyp.23.6.857. [DOI] [PubMed] [Google Scholar]
- [46].Nishimoto M, Takai S, Sawada Y, et al. Chymase-dependent angiotensin II formation in the saphenous vein versus the internal thoracic artery. J Thorac Cardiovasc Surg. 2001;121(4):729–734. doi: 10.1067/mtc.2001.112467. [DOI] [PubMed] [Google Scholar]
- [47].McDonald JE, Padmanabhan N, Petrie MC, Hillier C, Connell JM, McMurray JJ. Vasoconstrictor effect of the angiotensin-converting enzyme-resistant, chymase-specific substrate [Pro(11)(D)-Ala(12)] angiotensin I in human dorsal hand veins: in vivo demonstration of non-ace production of angiotensin II in humans. Circulation. 2001;104(15):1805–1808. doi: 10.1161/hc4001.097220. [DOI] [PubMed] [Google Scholar]
- [48].Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- [49].Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990;346(6282):371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- [50].Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. J Am Soc Nephrol. 2002;13(10):2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- [51].Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol. 2003;284(2):F243–252. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- [52].Wolf G, Mueller E, Stahl RA, Ziyadeh FN. Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J Clin Invest Sep. 1993;92(3):1366–1372. doi: 10.1172/JCI116710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70(11):1914–1919. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- [54].Koibuchi Y, Lee WS, Gibbons GH, Pratt RE. Role of transforming growth factor-beta 1 in the cellular growth response to angiotensin II. Hypertension. 1993;21(6 Pt 2):1046–1050. doi: 10.1161/01.hyp.21.6.1046. [DOI] [PubMed] [Google Scholar]
- [55].Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270(9):4689–4696. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- [56].Zhao XY, Zhao LY, Zheng QS, et al. Chymase induces profibrotic response via transforming growth factor-beta 1/Smad activation in rat cardiac fibroblasts. Mol Cell Biochem. 2008;310(1-2):159–166. doi: 10.1007/s11010-007-9676-2. [DOI] [PubMed] [Google Scholar]
- [57].Wasse H, Rivera AA, Huang R, et al. Increased Plasma Chymase Concentration and Mast Cell Chymase Expression in Venous Neointimal Lesions of Patients with CKD and ESRD. Seminars in Dialysis. 2011;24(6):688–693. doi: 10.1111/j.1525-139X.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Scandiuzzi L, Beghdadi W, Daugas E, et al. Mouse mast cell protease-4 deteriorates renal function by contributing to inflammation and fibrosis in immune complex-mediated glomerulonephritis. J Immunol. 2010;185(1):624–633. doi: 10.4049/jimmunol.0902129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- [60].Sharma AK, Mauer SM, Kim Y, Michael AF. Altered expression of matrix metalloproteinase-2, TIMP, and TIMP-2 in obstructive nephropathy. J Lab Clin Med. 1995;125(6):754–761. [PubMed] [Google Scholar]
- [61].Uchio K, Manabe N, Tamura K, et al. Decreased matrix metalloproteinase activity in the kidneys of hereditary nephrotic mice (ICGN strain) Nephron. 2000;86(2):145–151. doi: 10.1159/000045733. [DOI] [PubMed] [Google Scholar]
- [62].Tchougounova E, Lundequist A, Fajardo I, Winberg JO, Abrink M, Pejler G. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280(10):9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- [63].Stewart JA, Jr., Wei CC, Brower GL, et al. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J Mol Cell Cardiol. 2003;35(3):311–319. doi: 10.1016/s0022-2828(03)00013-0. [DOI] [PubMed] [Google Scholar]
- [64].Cheng S, Pollock AS, Mahimkar R, Olson JL, Lovett DH. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J. 2006;20(11):1898–1900. doi: 10.1096/fj.06-5898fje. [DOI] [PubMed] [Google Scholar]
- [65].Sadjadi J, Kramer GL, Yu CH, et al. Angiotensin converting enzyme-independent angiotensin ii production by chymase is up-regulated in the ischemic kidney in renovascular hypertension. J Surg Res. 2005;127(2):65–69. doi: 10.1016/j.jss.2005.02.031. [DOI] [PubMed] [Google Scholar]
- [66].Tokuyama H, Hayashi K, Matsuda H, et al. Differential regulation of elevated renal angiotensin II in chronic renal ischemia. Hypertension. 2002;40(1):34–40. doi: 10.1161/01.hyp.0000022060.13995.ed. [DOI] [PubMed] [Google Scholar]
- [67].Leehey DJ, Singh AK, Alavi N, Singh R. Role of angiotensin II in diabetic nephropathy. Kidney Int Suppl. 2000;77:S93–98. doi: 10.1046/j.1523-1755.2000.07715.x. [DOI] [PubMed] [Google Scholar]
- [68].Cristovam PC, Arnoni CP, de Andrade MC, et al. ACE-dependent and chymase-dependent angiotensin II generation in normal and glucose-stimulated human mesangial cells. Exp Biol Med (Maywood) 2008;233(8):1035–1043. doi: 10.3181/0708-RM-229. [DOI] [PubMed] [Google Scholar]
- [69].Durvasula RV, Shankland SJ. Activation of a local renin angiotensin system in podocytes by glucose. Am J Physiol Renal Physiol. 2008;294(4):F830–839. doi: 10.1152/ajprenal.00266.2007. [DOI] [PubMed] [Google Scholar]
- [70].Prasad P, Tiwari AK, Kumar KM, et al. Chronic renal insufficiency among Asian Indians with type 2 diabetes: I. Role of RAAS gene polymorphisms. BMC Med Genet. 2006;7:42. doi: 10.1186/1471-2350-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Boffa JJ, Lu Y, Placier S, Stefanski A, Dussaule JC, Chatziantoniou C. Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol. 2003;14(5):1132–1144. doi: 10.1097/01.asn.0000060574.38107.3b. [DOI] [PubMed] [Google Scholar]
- [72].Sharma R, Prasad V, McCarthy ET, et al. Chymase increases glomerular albumin permeability via protease-activated receptor-2. Mol Cell Biochem. 2007;297(1-2):161–169. doi: 10.1007/s11010-006-9342-0. [DOI] [PubMed] [Google Scholar]
- [73].Sun J, Zhang J, Lindholt JS, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120(11):973–982. doi: 10.1161/CIRCULATIONAHA.109.849679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hochegger K, Siebenhaar F, Vielhauer V, et al. Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur J Immunol. 2005;35(10):3074–3082. doi: 10.1002/eji.200526250. [DOI] [PubMed] [Google Scholar]
- [75].Wang Y, Gu Y, Lewis DF, Alexander JS, Granger DN. Elevated plasma chymotrypsin-like protease (chymase) activity in women with preeclampsia. Hypertens Pregnancy. 2010;29(3):253–261. doi: 10.3109/10641950802001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Raymond WW, Su S, Makarova A, et al. Alpha 2-macroglobulin capture allows detection of mast cell chymase in serum and creates a reservoir of angiotensin II-generating activity. J Immunol. 2009;182(9):5770–5777. doi: 10.4049/jimmunol.0900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hermans WR, Rensing BJ, Foley DP, et al. Therapeutic dissection after successful coronary balloon angioplasty: no influence on restenosis or on clinical outcome in 693 patients. The MERCATOR Study Group (Multicenter European Research Trial with Cilazapril after Angioplasty to prevent Transluminal Coronary Obstruction and Restenosis) J Am Coll Cardiol. 1992;20(4):767–780. doi: 10.1016/0735-1097(92)90171-i. [DOI] [PubMed] [Google Scholar]
- [78].Faxon DP. Effect of high dose angiotensin-converting enzyme inhibition on restenosis: final results of the MARCATOR Study, a multicenter, double-blind, placebo-controlled trial of cilazapril. The Multicenter American Research Trial With Cilazapril After Angioplasty to Prevent Transluminal Coronary Obstruction and Restenosis (MARCATOR) Study Group. J Am Coll Cardiol. 1995;25(2):362–369. doi: 10.1016/0735-1097(94)00368-z. [DOI] [PubMed] [Google Scholar]
- [79].Peters S, Trummel M, Meyners W, Koehler B, Westermann K. Valsartan versus ACE inhibition after bare metal stent implantation - results of the VALVACE trial. International Journal of Cardiology. 2005;98(2):331–335. doi: 10.1016/j.ijcard.2004.05.062. [DOI] [PubMed] [Google Scholar]
- [80].Peters S, Gotting B, Trummel M, Rust H, Brattstrom A. Valsartan for prevention of restenosis after stenting of type B2/C lesions: the VAL-PREST trial. J Invasive Cardiol. 2001;13(2):93–97. [PubMed] [Google Scholar]
- [81].Turan T, van Harten JG, de Water R, Tuncay OL, Kok DJ. Is enalapril adequate for the prevention of renal tissue damage caused by unilateral ureteral obstruction and/or hyperoxaluria? Urol Res. 2003;31(3):212–217. doi: 10.1007/s00240-003-0320-7. [DOI] [PubMed] [Google Scholar]
- [82].Fan YY, Nishiyama A, Fujisawa Y, et al. Contribution of chymase-dependent angiotensin II formation to the progression of tubulointerstitial fibrosis in obstructed kidneys in hamsters. J Pharmacol Sci. 2009;111(1):82–90. doi: 10.1254/jphs.09152fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Maeda Y, Inoguchi T, Takei R, et al. Inhibition of chymase protects against diabetes-induced oxidative stress and renal dysfunction in hamsters. Am J Physiol Renal Physiol. 2010;299(6):F1328–1338. doi: 10.1152/ajprenal.00337.2010. [DOI] [PubMed] [Google Scholar]
- [84].Walker ME, Hatfield JK, Brown MA. New insights into the role of mast cells in autoimmunity: Evidence for a common mechanism of action? Biochimica et biophysica acta. 2012;1822(1):57–65. doi: 10.1016/j.bbadis.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Schanstra JP, Neau E, Drogoz P, et al. In vivo bradykinin B2 receptor activation reduces renal fibrosis. The Journal of clinical investigation. 2002;110(3):371–379. doi: 10.1172/JCI15493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Naito T, Ma LJ, Yang H, et al. Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. American journal of physiology Renal physiology. 2010;298(3):F683–691. doi: 10.1152/ajprenal.00503.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]