Abstract
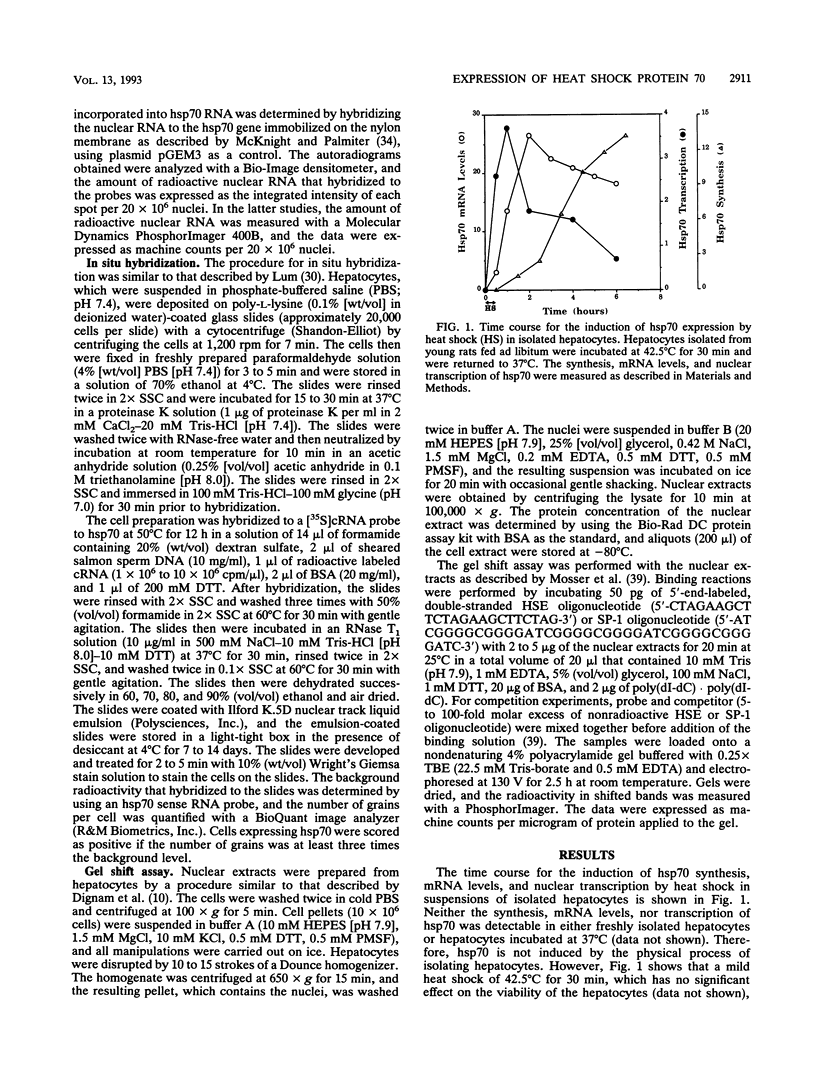
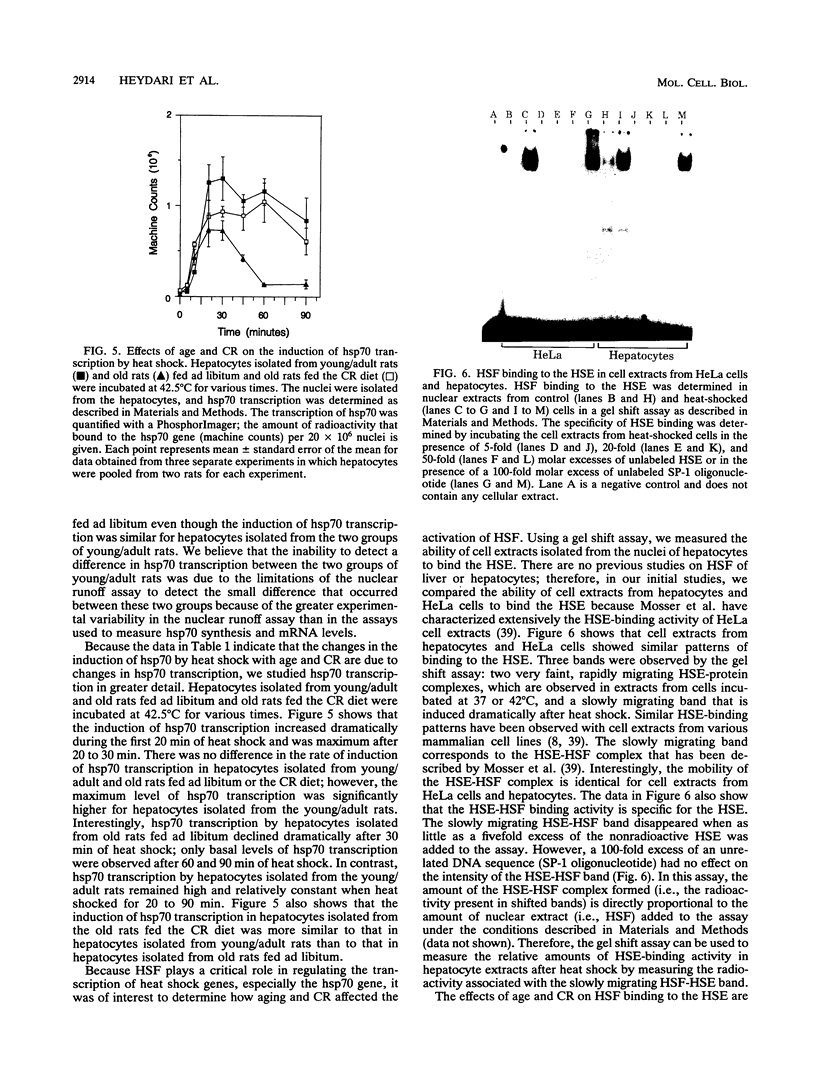
Because heat shock proteins have been shown to play a critical role in protecting cells from hyperthermia and other types of physiological stresses, it was of interest to determine what effect age and caloric restriction have on the ability of cells to regulate the expression of heat shock protein 70 (hsp70), the most prominent and most evolutionarily conserved of the heat shock proteins. Caloric restriction is the only experimental manipulation known to retard aging and increase survival of mammals. The ability of hepatocytes isolated from young/adult (4- to 7-month-old) and old (22- to 28-month-old) male Fischer F344 rats fed ad libitum or a caloric restriction diet (60% of the content of the ad libitum diet) to express hsp70 was determined after a mild heat shock (42.5 degrees C for 30 min). We found that the induction of hsp70 synthesis and mRNA levels by heat shock was 40 to 50% lower in hepatocytes isolated from old rats than in hepatocytes isolated from young rats. Using in situ hybridization, we found that essentially all hepatocytes from the young/adult and old rats expressed hsp70 in response to heat shock; therefore, the age-related decrease in the induction of hsp70 expression was not due to an age-related accumulation of cells that do not respond to heat shock. Measurements of hsp70 mRNA stability and hsp70 transcription demonstrated that the age-related decline in hsp70 expression arose from a decline in hsp70 transcription. Interestingly, the age-related decline in the induction of hsp70 expression was reversed by caloric restriction; e.g., the induction of hsp70 synthesis, mRNA levels, and nuclear transcription were significantly higher in hepatocytes isolated from old rats fed the caloric restricted diet than in hepatocytes isolated from old rats fed ad libitum. The levels of the heat shock transcription factor in nuclear extracts isolated from heat-shocked hepatocytes were measured in a gel shift assay. Binding of the heat shock transcription factor to the heat shock element decreased with age and was significantly higher in hepatocyte extracts isolated from old rats fed the caloric restriction diet than in those from old rats fed ad libitum. Thus, our study demonstrates that the ability of hepatocytes to respond to hyperthermia and express hsp70 decreases significantly with age and that this decrease occurs at the transcriptional level. In addition, caloric restriction, which retards aging, reversed the age-related decline in the induction of hsp70 transcription in hepatocytes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelidis C. E., Lazaridis I., Pagoulatos G. N. Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem. 1991 Jul 1;199(1):35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- Armbrecht H. J., Strong R., Boltz M., Rocco D., Wood W. G., Richardson A. Modulation of age-related changes in serum 1,25-dihydroxyvitamin D and parathyroid hormone by dietary restriction of Fischer 344 rats. J Nutr. 1988 Nov;118(11):1360–1365. doi: 10.1093/jn/118.11.1360. [DOI] [PubMed] [Google Scholar]
- Blake M. J., Fargnoli J., Gershon D., Holbrook N. J. Concomitant decline in heat-induced hyperthermia and HSP70 mRNA expression in aged rats. Am J Physiol. 1991 Apr;260(4 Pt 2):R663–R667. doi: 10.1152/ajpregu.1991.260.4.R663. [DOI] [PubMed] [Google Scholar]
- Butler J. A., Heydari A. R., Richardson A. Analysis of effect of age on synthesis of specific proteins by hepatocytes. J Cell Physiol. 1989 Nov;141(2):400–409. doi: 10.1002/jcp.1041410222. [DOI] [PubMed] [Google Scholar]
- Castle T., Kreamer W., Liu D. S., Richardson A. Characterization of RNA synthesis by isolated hepatocytes in suspension. Arch Biochem Biophys. 1979 Jul;195(2):423–437. doi: 10.1016/0003-9861(79)90369-2. [DOI] [PubMed] [Google Scholar]
- Celano P., Berchtold C., Casero R. A., Jr A simplification of the nuclear run-off transcription assay. Biotechniques. 1989 Oct;7(9):942–944. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choi H. S., Lin Z., Li B. S., Liu A. Y. Age-dependent decrease in the heat-inducible DNA sequence-specific binding activity in human diploid fibroblasts. J Biol Chem. 1990 Oct 15;265(29):18005–18011. [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann G. L., Richardson A., Katz A., Fierer J. A. Age-related changes in isolated rat hepatocytes. Comparison of size, morphology, binucleation, and protein content. Mech Ageing Dev. 1981;16(4):385–395. doi: 10.1016/0047-6374(81)90023-3. [DOI] [PubMed] [Google Scholar]
- Fargnoli J., Kunisada T., Fornace A. J., Jr, Schneider E. L., Holbrook N. J. Decreased expression of heat shock protein 70 mRNA and protein after heat treatment in cells of aged rats. Proc Natl Acad Sci U S A. 1990 Jan;87(2):846–850. doi: 10.1073/pnas.87.2.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T. C., Makinodan T. In situ hybridization analysis of the age-associated decline in IL-2 mRNA expressing murine T cells. Cell Immunol. 1989 Jan;118(1):199–207. doi: 10.1016/0008-8749(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Alamo I., Jr, Hollander M. C., Lamoreaux E. Induction of heat shock protein transcripts and B2 transcripts by various stresses in Chinese hamster cells. Exp Cell Res. 1989 May;182(1):61–74. doi: 10.1016/0014-4827(89)90279-6. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Changes in protein synthesis during myogenesis in a clonal cell line. Dev Biol. 1979 Nov;73(1):134–152. doi: 10.1016/0012-1606(79)90143-x. [DOI] [PubMed] [Google Scholar]
- Goldenberg C. J., Luo Y., Fenna M., Baler R., Weinmann R., Voellmy R. Purified human factor activates heat shock promoter in a HeLa cell-free transcription system. J Biol Chem. 1988 Dec 25;263(36):19734–19739. [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Harrold S., Genovese C., Kobrin B., Morrison S. L., Milcarek C. A comparison of apparent mRNA half-life using kinetic labeling techniques vs decay following administration of transcriptional inhibitors. Anal Biochem. 1991 Oct;198(1):19–29. doi: 10.1016/0003-2697(91)90500-s. [DOI] [PubMed] [Google Scholar]
- Johnston R. N., Kucey B. L. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science. 1988 Dec 16;242(4885):1551–1554. doi: 10.1126/science.3201244. [DOI] [PubMed] [Google Scholar]
- Jurivich D. A., Sistonen L., Kroes R. A., Morimoto R. I. Effect of sodium salicylate on the human heat shock response. Science. 1992 Mar 6;255(5049):1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Schuetz T. J., Larin Z. Heat-inducible human factor that binds to a human hsp70 promoter. Mol Cell Biol. 1987 Apr;7(4):1530–1534. doi: 10.1128/mcb.7.4.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreamer B. L., Staecker J. L., Sawada N., Sattler G. L., Hsia M. T., Pitot H. C. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986 Apr;22(4):201–211. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- Larson J. S., Schuetz T. J., Kingston R. E. Activation in vitro of sequence-specific DNA binding by a human regulatory factor. Nature. 1988 Sep 22;335(6188):372–375. doi: 10.1038/335372a0. [DOI] [PubMed] [Google Scholar]
- Li G. C., Li L. G., Liu Y. K., Mak J. Y., Chen L. L., Lee W. M. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Choi H. S., Lee Y. K., Chen K. Y. Molecular events involved in transcriptional activation of heat shock genes become progressively refractory to heat stimulation during aging of human diploid fibroblasts. J Cell Physiol. 1991 Dec;149(3):560–566. doi: 10.1002/jcp.1041490327. [DOI] [PubMed] [Google Scholar]
- Liu A. Y., Lin Z., Choi H. S., Sorhage F., Li B. Attenuated induction of heat shock gene expression in aging diploid fibroblasts. J Biol Chem. 1989 Jul 15;264(20):12037–12045. [PubMed] [Google Scholar]
- Luce M. C., Cristofalo V. J. Reduction in heat shock gene expression correlates with increased thermosensitivity in senescent human fibroblasts. Exp Cell Res. 1992 Sep;202(1):9–16. doi: 10.1016/0014-4827(92)90398-r. [DOI] [PubMed] [Google Scholar]
- Masoro E. J. Food restriction in rodents: an evaluation of its role in the study of aging. J Gerontol. 1988 May;43(3):B59–B64. doi: 10.1093/geronj/43.3.b59. [DOI] [PubMed] [Google Scholar]
- Masoro E. J. Nutrition and aging--a current assessment. J Nutr. 1985 Jul;115(7):842–848. doi: 10.1093/jn/115.7.842. [DOI] [PubMed] [Google Scholar]
- Masoro E. J. Retardation of aging processes by food restriction: an experimental tool. Am J Clin Nutr. 1992 Jun;55(6 Suppl):1250S–1252S. doi: 10.1093/ajcn/55.6.1250S. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. A. Age-associated decline in precursor frequency for different T cell-mediated reactions, with preservation of helper or cytotoxic effect per precursor cell. J Immunol. 1984 Jan;132(1):63–68. [PubMed] [Google Scholar]
- Mirault M. E., Southgate R., Delwart E. Regulation of heat-shock genes: a DNA sequence upstream of Drosophila hsp70 genes is essential for their induction in monkey cells. EMBO J. 1982;1(10):1279–1285. doi: 10.1002/j.1460-2075.1982.tb00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen L. A., Chang C., Garrels J. I., Welch W. J. Identification, characterization, and purification of two mammalian stress proteins present in mitochondria, grp 75, a member of the hsp 70 family and hsp 58, a homolog of the bacterial groEL protein. J Biol Chem. 1989 Dec 5;264(34):20664–20675. [PubMed] [Google Scholar]
- Mosser D. D., Kotzbauer P. T., Sarge K. D., Morimoto R. I. In vitro activation of heat shock transcription factor DNA-binding by calcium and biochemical conditions that affect protein conformation. Proc Natl Acad Sci U S A. 1990 May;87(10):3748–3752. doi: 10.1073/pnas.87.10.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechsli F. W., Buechley R. W. Excess mortality associated with three Los Angeles September hot spells. Environ Res. 1970 Nov;3(4):277–284. doi: 10.1016/0013-9351(70)90021-6. [DOI] [PubMed] [Google Scholar]
- Petersen R., Lindquist S. The Drosophila hsp70 message is rapidly degraded at normal temperatures and stabilized by heat shock. Gene. 1988 Dec 10;72(1-2):161–168. doi: 10.1016/0378-1119(88)90138-2. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Mizzen L. A., Welch W. J. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science. 1988 Oct 21;242(4877):433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- Richardson A., Butler J. A., Rutherford M. S., Semsei I., Gu M. Z., Fernandes G., Chiang W. H. Effect of age and dietary restriction on the expression of alpha 2u-globulin. J Biol Chem. 1987 Sep 15;262(26):12821–12825. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Sargent T. D., Sell S., Bonner J. alpha-Fetoprotein and albumin genes of rats: no evidence for amplification-deletion or rearrangement in rat liver carcinogenesis. Proc Natl Acad Sci U S A. 1979 Feb;76(2):695–699. doi: 10.1073/pnas.76.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Blume J. E., Nielsen D. A. Regulation of messenger RNA stability in eukaryotic cells. Bioessays. 1987 May;6(5):221–226. doi: 10.1002/bies.950060507. [DOI] [PubMed] [Google Scholar]
- Sorger P. K. Heat shock factor and the heat shock response. Cell. 1991 May 3;65(3):363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Sorger P. K., Lewis M. J., Pelham H. R. Heat shock factor is regulated differently in yeast and HeLa cells. Nature. 1987 Sep 3;329(6134):81–84. doi: 10.1038/329081a0. [DOI] [PubMed] [Google Scholar]
- Spindler S. R., Grizzle J. M., Walford R. L., Mote P. L. Aging and restriction of dietary calories increases insulin receptor mRNA, and aging increases glucocorticoid receptor mRNA in the liver of female C3B10RF1 mice. J Gerontol. 1991 Nov;46(6):B233–B237. doi: 10.1093/geronj/46.6.b233. [DOI] [PubMed] [Google Scholar]
- Wu B. J., Williams G. T., Morimoto R. I. Detection of three protein binding sites in the serum-regulated promoter of the human gene encoding the 70-kDa heat shock protein. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2203–2207. doi: 10.1073/pnas.84.8.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimarino V., Wu C. Induction of sequence-specific binding of Drosophila heat shock activator protein without protein synthesis. 1987 Jun 25-Jul 1Nature. 327(6124):727–730. doi: 10.1038/327727a0. [DOI] [PubMed] [Google Scholar]