Abstract
BACKGROUND
More than 500,000 deaths are attributed to rotavirus gastroenteritis annually worldwide, with the highest mortality in India. Two successive, naturally occurring rotavirus infections have been shown to confer complete protection against moderate or severe gastroenteritis during subsequent infections in a birth cohort in Mexico. We studied the protective effect of rotavirus infection on subsequent infection and disease in a birth cohort in India (where the efficacy of oral vaccines in general has been lower than expected).
METHODS
We recruited children at birth in urban slums in Vellore; they were followed for 3 years after birth, with home visits twice weekly. Stool samples were collected every 2 weeks, as well as on alternate days during diarrheal episodes, and were tested by means of enzyme-linked immunosorbent assay and polymerase-chain-reaction assay. Serum samples were obtained every 6 months and evaluated for seroconversion, defined as an increase in the IgG antibody level by a factor of 4 or in the IgA antibody level by a factor of 3.
RESULTS
Of 452 recruited children, 373 completed 3 years of follow-up. Rotavirus infection generally occurred early in life, with 56% of children infected by 6 months of age. Levels of reinfection were high, with only approximately 30% of all infections identified being primary. Protection against moderate or severe disease increased with the order of infection but was only 79% after three infections. With G1P[8], the most common viral strain, there was no evidence of homotypic protection.
CONCLUSIONS
Early infection and frequent reinfection in a locale with high viral diversity resulted in lower protection than has been reported elsewhere, providing a possible explanation why rotavirus vaccines have had lower-than-expected efficacy in Asia and Africa. (Funded by the Wellcome Trust.)
Group a rotaviruses are the leading cause of dehydrating gastroenteritis in young children worldwide, and rotavirus gastroenteritis results in more than half a million deaths annually.1 Two rotavirus vaccines, Rotarix and RotaTeq, are licensed for use in the United States, Europe, and Latin America,2-4 and the World Health Organization has recommended their inclusion in national immunization programs in Africa and Asia on the basis of trials showing efficacy there.5,6
Naturally occurring rotavirus infection has been shown to confer protection against subsequent infection and disease in birth cohorts in Mexico and Guinea-Bissau, with each new infection reducing the severity of subsequent diarrhea.7,8 Despite these findings, the efficacy of the Rotarix and RotaTeq vaccines against subsequent severe disease in developing countries of Asia, Africa, and Central America does not appear to be as high as that seen in developed countries.6,9-12
In India, the low efficacy of oral vaccines, particularly the oral polio vaccine, has been recognized for decades.13-15 Given this and reports of reduced efficacy of rotavirus vaccines in Asia and Africa, there is a need to consider how well these vaccines may perform in India, where one fourth of worldwide deaths associated with rotavirus disease occur.1 We evaluated the protective effect of natural rotavirus infection against subsequent infection and disease in a birth cohort in India.
METHODS
STUDY CONDUCT, RECRUITMENT, AND SAMPLE COLLECTION
The study was approved by the institutional review boards of Christian Medical College, Vellore; London School of Hygiene and Tropical Medicine, London; and Baylor College of Medicine, Houston. Written informed consent was obtained from each child’s parent or guardian. The enrollment criteria and methods of follow-up have been published previously.16,17 All authors vouch for the completeness and accuracy of the data and analyses presented.
Our study was conducted from 2002 through 2006 in Chinnallapuram, Ramanaickanpalayam, and Kasba, three contiguous slums in Vellore, India, with a total population of approximately 35,000. A cohort of 452 newborns was recruited at birth between March 2002 and August 2003. Field workers visited each child’s house twice weekly and obtained a surveillance stool sample every 2 weeks. At each visit, the mother or care-giver was asked about any illness after the previous visit; any respiratory symptoms, fever, diarrhea, or other signs or symptoms in the child or other members of the household were recorded. If diarrhea was reported, the family was encouraged to take the child to the clinic for assessment of severity and management; the family was instructed to collect samples during every diarrheal episode, and the field worker visited the child daily until the end of the diarrheal episode, recording the frequency, consistency, and color of the stool. In addition, stool samples were collected when the diarrhea was first reported and every other day until the episode ended.
A blood sample was collected at birth (cord blood) or during the first week of life and at least every 6 months for the 3 years of follow-up. One aliquot of stool specimen was tested on the day of stool collection; other aliquots were stored either with or without protease inhibitor at −70°C. Serum samples were stored at −20°C.
DEFINITIONS
An episode of diarrhea was defined as three or more watery stools in a 24-hour period or, in breast-fed children, an increased number of daily stools considered to be diarrhea by the mother. The episode ended on the day after the day the child’s bowel movements returned to normal. An interval of at least 48 hours during which bowel movements were normal separated two episodes of diarrhea. Rotavirus infection was defined as rotavirus detected in stool or an increase in the antirotavirus IgG antibody level by a factor of 4 or an increase in the IgA antibody level by a factor of 3 in sequential serum samples.7,18,19 A rotavirus infection was considered asymptomatic if the child did not have diarrhea during the week before and the week after detection of rotavirus in the stool or if a child had seroconversion with no diarrhea in the interval between the two serum-sample collections. An infection was defined as symptomatic when rotavirus was identified in a stool sample during the week before or after the diarrheal episode. Since there could be several diarrheal episodes in the interval between two serum-sample collections, an infection detected only on the basis of serologic testing and associated diarrhea, without detection of rotavirus, was considered an infection of unknown status.
ASSESSMENT OF SEVERITY OF DIARRHEA
During diarrheal episodes, diarrhea was assessed daily for severity with the use of the Vesikari scale.20 An episode was classified as asymptomatic if the score was 0, mild if the score was between 1 and 10, moderate if the score was between 11 and 15, and severe if the score was between 16 and 20. The scores were compared with scores on a modified Vesikari scale that were reported previously for a Mexican birth cohort.7
TESTING FOR ROTAVIRUS
All surveillance and diarrheal stool samples were screened for rotavirus antigen by means of enzyme-linked immunosorbent assay (ELISA) (Rotavirus IDEIA, Dako). All rotavirus-positive surveillance stool samples were retested with the use of ELISA, and if these results were also positive, RNA was extracted and the strain was genotyped by means of a reverse-transcriptase—polymerase-chain-reaction (RT-PCR) assay.21-24 This was done because a lack of specificity of ELISA testing was noted for surveillance samples. All diarrheal stool samples were screened by means of ELISA but underwent RNA extraction and RT-PCR assay even if the screening ELISA was negative. A positive result was defined as a sample that was positive for rotavirus either on two ELISA tests or an RT-PCR assay. PCR positivity alone was not used to define a positive result because 31 PCR-negative infections were associated with an immune response; in these cases, the negative PCR result may have been due to the tim-ing or quality of stool collection.
TESTING FOR ANTIROTAVIRUS IgG AND IgA ANTIBODIES
For each child, the serum specimens obtained at birth and at 6-month intervals thereafter were analyzed for antirotavirus IgA and IgG antibodies by means of an antibody-sandwich enzyme immunoassay. The IgA or IgG titer was determined by comparing the optical density values from sample wells with a standard curve based on pooled human serum samples, as previously described.25
STATISTICAL ANALYSIS
We restricted our primary analyses to the 373 children who completed 3 years of follow-up, for two reasons. First, a survival analysis showed that the 79 children who did not complete the study did not differ significantly from the 373 who did, in terms of either their baseline demographic characteristics (Table 1) or the pattern of infection until they dropped out. Second, 31 of the 79 children who did not complete the study dropped out within 3 months after recruitment, because the mothers had delivered at their natal homes, after which they returned to their own homes outside the study area. We also performed an analysis in which we included data for the 44 children who did not complete the study but for whom follow-up data were available for at least 3 months and at least one follow-up serum sample was obtained (see Table 3 in the Supplementary Appendix, available with the full text of this article at NEJM.org).
Table 1.
Baseline Household Characteristics of the 373 Children Who Completed 3 Years of Follow-up and the 79 Who Did Not.*
| Characteristic | 3 Yr of Follow-up (N = 373) |
<3 Yr of Follow-up (N = 79) |
P Value | |
|---|---|---|---|---|
| number | ||||
| Religion | 0.18 | |||
| Hindu | 176 | 30 | ||
| Muslim | 180 | 47 | ||
| Christian | 17 | 2 | ||
| Type of family† | 0.25 | |||
| Joint | 73 | 21 | ||
| Extended | 107 | 17 | ||
| Nuclear | 193 | 41 | ||
| No. of household members | 0.52 | |||
| ≤5 | 250 | 50 | ||
| >5 | 123 | 29 | ||
| Socioeconomic status‡ | 0.46 | |||
| Class I | 229 | 52 | ||
| Class II | 144 | 27 | ||
| Bidi worker in household | 0.46 | |||
| Yes | 173 | 33 | ||
| No | 200 | 46 | ||
| Animals owned by household | 0.11 | |||
| Yes | 53 | 6 | ||
| No | 320 | 73 | ||
| Birth weight | 0.76 | |||
| Missing | 8 | 3 | ||
| <2.5 kg | 43 | 8 | ||
| ≥2.5 kg | 322 | 68 | ||
| Sex of child | 0.50 | |||
| Male | 186 | 41 | ||
| Female | 187 | 38 | ||
| No. of siblings | 0.38 | |||
| 0 | 118 | 21 | ||
| ≥1 | 255 | 58 | ||
| Maternal age | 0.97 | |||
| ≤23 yr | 188 | 40 | ||
| >23 yr | 185 | 39 | ||
| Maternal education | 0.06 | |||
| None | 106 | 33 | ||
| Primary, middle, or high school | 199 | 33 | ||
| College | 68 | 13 | ||
| Mode of delivery | 0.50 | |||
| Normal vaginal | 339 | 75 | ||
| Instrument-aided | 9 | 1 | ||
| Cesarean | 25 | 3 | ||
| Place of birth | 0.39 | |||
| Hospital or health center | 365 | 76 | ||
| Home | 8 | 3 | ||
Water sources are not listed because all drinking water in the area is from the same main overhead tank with intermittent supply either to street standpipes or domestic taps.
Joint families include the study child, siblings, parents, aunts or uncles and their families, and grandparents. Extended families include the study child, siblings, parents, and grandparents.
For socioeconomic status, class I is defined as lower lower class, and class II is defined as upper lower class, according to a modified Kuppuswamy scale, which takes into account educational level, occupation, and possessions.17
The protective effects of rotavirus infection, both in general and for specific strains of the major rotavirus genotypes, were studied. Shared gamma frailty survival models were used to obtain relative risks and confidence intervals adjusted for repeat infection in the same child. Parametric-regression survival modeling26 was performed with the use of Stata software, version 8.0 (Stata). Our previously reported analysis for all illnesses showed that male sex, low level of personal and household hygiene, and working in the manufacture of bidis (indigenous cigarettes) in the household were associated with an increased risk of respiratory and gastrointestinal disease.16,17 We retained these variables in the final model to calculate the adjusted relative risks and protective efficacy of prior rotavirus infections. Details of the analysis and of adjustment for potential confounders are provided in the Supplementary Appendix.
RESULTS
COHORT MONITORING
The cohort was monitored for a total of 13,937 child-months, with 121,005 home visits. A total of 452 children were recruited for the study; 391, 380, and 373 children remained in the study at the end of the first, second, and third years of follow-up, respectively. The baseline demographic characteristics did not differ significantly between the 373 children who completed 3 years of follow-up and the 79 children who did not (Table 1). Nearly half the children who did not complete the study with-drew early, in most cases because the family left the area or did not comply with sample collection. Five children died, three from gastroenteritis; two of the three cases of fatal gastroenteritis were associated with rotavirus infection.
Nearly all the data specified by the protocol for collection during the study were obtained (99.4%, representing 13,340.7 child-months) for the 373 children completing follow-up: 26,902 surveillance stool samples were collected (91.8% of the scheduled collections), and 4759 diarrheal samples during 1829 diarrheal episodes (98.6% of the episodes that occurred) were collected and tested for rotavirus. Of the serum specimens planned for collection, 4246 (94.9%) were collected, and of the 2598 specimens (99.5% of the planned number) obtained at least every 6 months after birth, 2565 (98.7%) were tested for either IgA or IgG antibody and 2468 (95.0%) were tested for both antibodies. In most cases, the reason for a missed sample collection was that the child was away at the time of the scheduled collection or the mother or care-giver did not obtain the sample despite reminders.
IDENTIFICATION OF ROTAVIRUS INFECTION
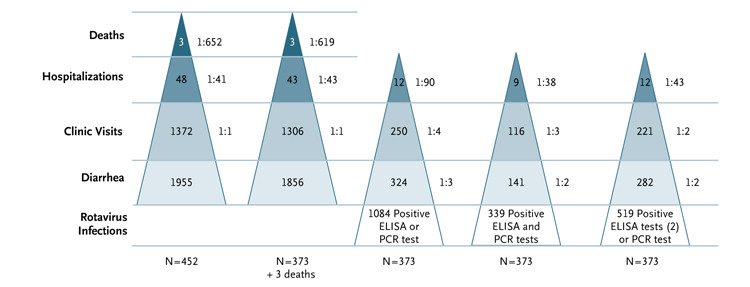
Figure 1 shows the frequencies of diarrhea and related events in the 452 recruited children and in the 373 who completed follow-up, as well as the frequencies according to whether the diagnosis of rotavirus infection was based on stool testing alone, ELISA or PCR positivity (the most sensitive definition), ELISA and PCR positivity (the most specific definition), or two positive findings on ELISA or one positive finding on PCR (the definition used here). Inclusion of a repeat positive ELISA assay in the definition of a rotavirus-positive stool reduced false positives, as shown in Figure 1, by eliminating samples that were positive on only one ELISA test.
Figure 1. Incidence of Diarrhea and Rotavirus Infection Detected by Stool Testing Alone in the Birth Cohort.
The two pyramids at the left show the numbers of deaths, hospitalizations, clinic visits, and diarrheal episodes in the recruited cohort of 452 children and in the cohort of 373 children who completed the 3-year follow-up, respectively; the values to the right of each pyramid are the ratios of the frequencies shown within the pyramid to the total number of diarrheal episodes. Two deaths that were not associated with diarrheal disease are not shown. The three pyramids at the right show the numbers of hospitalizations, clinic visits, diarrheal episodes, and rotavirus infections — and the corresponding ratios of these frequencies to the total number of rotavirus infections — according to the means of detection of rotavirus in stool specimens: positive finding on enzyme-linked immunosorbent assay (ELISA) or polymerase-chain-reaction (PCR) assay, the most sensitive definition; positive findings on ELISA and PCR assay, the most specific definition; or two positive findings on ELISA or one positive finding on PCR assay, the definition used here. These data do not include infections detected by means of serologic testing.
Using the definition of two positive ELISA assays or one positive PCR assay of a stool sample — or a positive serologic test for rotavirus — we identified a total of 1103 rotavirus infections in the 373 children, with 255 infections (23.1%) identified in both stool and serum samples, 264 (23.9%) detected only in stool, and 584 (52.9%) only in serum; the 584 infections detected only in serum included 244 of unknown status. Of the 1103 infections, 371 (33.6%) were primary and the remainder were repeat infections. Rotavirus was detected in stool specimens in 48.5% of primary infections and in 46.3% of later infections.
The overall incidence of rotavirus infection was 0.99 (95% confidence interval [CI], 0.94 to 1.05) per child-year, and the overall incidence of rotaviral diarrhea was 0.25 (95% CI, 0.22 to 0.29) per child-year. In the first year of life, the incidences of infection and diarrhea were 1.20 (95% CI, 1.14 to 1.37) per child-year and 0.49 (95% CI, 0.42 to 0.58) per child-year, respectively. The cumulative proportions of children infected were 56%, 81%, 96%, and 99% at 6, 12, 24, and 36 months of age, respectively, with rotaviral diarrhea occurring in 20%, 36%, 43%, and 48%, respectively. Rates of reinfection were high: 33 of the 373 children (8.8%) had only one documented infection, where-as 102 (27.3%) had two infections, 136 (36.5%) had three, 58 (15.5%) had four, and 42 (11.3%) had five or more.
Baseline characteristics, malnutrition status, and history (vs. no history) of frequent illness were compared between children with five or more infections and children with fewer than five infections. No significant differences in baseline characteristics or nutritional status were identified, but the children with five or more infections had greater overall morbidity (Table 2 in the Supplementary Appendix).
Two children were not found to have had a rotavirus infection during the 3 years of follow-up. This finding was consistent among all the tests used in the study.
MORBIDITY FROM DIARRHEA AND ROTAVIRUS INFECTION
Of all 1856 diarrheal episodes, 1047 (56.4%) had a Vesikari score of 1 to 5, 598 (32.2%) had a score of 6 to 10, 139 (7.5%) had a score of 11 to 15, and 9 (0.5%) had a score of 16 to 20. A pathogen was identified in 36.0% of all episodes; rotavirus was the most common (present in 15.2% of episodes), followed by a norovirus (8.5%), giardia (8.0%), aeromonas (3.8%), cryptosporidium (3.1%), and shigella (2.2%). Mixed infection was seen in 29 of 282 cases of rotaviral diarrhea (10.3%), with the additional pathogen most often being a norovirus (in 8 of the 29 cases) or giardia (in 10 of the 29 cases). Diarrhea was seen in association with 29.9% of primary rotavirus infections, with similar frequencies in subsequent infections: 28.1% of second infections, 18.2% of third infections, 18.0% of fourth infections, and 25.8% of fifth or subsequent infections (P=0.08 for trend, by the chi-square test). Rotavirus infection was associated with more severe disease than were infections with other diarrheal agents, with this pathogen found in higher proportions as the Vesikari score increased: in 11.5% of diarrheal episodes with a score of 1 to 5, 15.5% of episodes with a score of 6 to 10, 34.5% of episodes with a score of 11 to 15, and 67.4% of episodes with a score of 16 to 20. Overall, 2.3% of all diarrheal episodes required hospitalization, as compared with 4.3% of rotaviral diarrheal episodes. The incidence of rotavirus-associated gastroenteritis with a Vesikari score above 10 was 0.05 per child-year.
PROTECTION CONFERRED BY NATURALLY OCCURRING ROTAVIRUS INFECTION
Table 2 shows the protective efficacy of prior infections on subsequent rotavirus infection and disease. The incidence of infection and disease decreased as the number of infections increased. The adjusted efficacy after three prior infections was 67% against infection and 79% against moderate or severe diarrhea, with 81% efficacy against any diarrhea, 79% against mild diarrhea, and 84% against diarrhea of unknown status, but the efficacy against asymptomatic infection was lower (46%).
Table 2.
Relative Risk of Subsequent Rotavirus Infection and Diarrhea among the 373 Children Who Completed 3 Years of Follow-up, According to the Number of Previous Infections and Diarrheal Episodes.
| No. of Infections | No. of Diarrheal Episodes |
Incidence* | Relative Risk of Subsequent Event (95% CI) |
Adjusted Efficacy (95% CI)‡ |
|
|---|---|---|---|---|---|
| Unadjusted | Adjusted† | ||||
| per 100 child-mo | percent | ||||
| Total§ | |||||
| 0 | 371 | 13.81 | |||
| 1 | 338 | 8.50 | 0.62 (0.53 to 0.71) | 0.61 (0.53 to 0.71) | 39 (29 to 47) |
| 2 | 236 | 6.70 | 0.49 (0.41 to 0.57) | 0.48 (0.41 to 0.57) | 52 (43 to 59) |
| 3 | 100 | 4.68 | 0.34 (0.27 to 0.42) | 0.33 (0.26 to 0.41) | 67 (59 to 74) |
| Infection with any diarrhea | |||||
| 0 | 111 | 4.13 | |||
| 1 | 95 | 2.39 | 0.58 (0.44 to 0.76) | 0.57 (0.44 to 0.76) | 43 (24 to 56) |
| 2 | 43 | 1.22 | 0.30 (0.21 to 0.42) | 0.29 (0.20 to 0.41) | 71 (59 to 80) |
| 3 | 18 | 0.84 | 0.20 (0.12 to 0.34) | 0.19 (0.12 to 0.31) | 81 (69 to 88) |
| Infection with moderate or severe diarrhea | |||||
| 0 | 17 | 0.63 | |||
| 1 | 21 | 0.53 | 0.83 (0.44 to 1.58) | 0.82 (0.43 to 1.57) | 18 (−57 to 57) |
| 2 | 10 | 0.28 | 0.45 (0.21 to 0.98) | 0.43 (0.20 to 0.94) | 57 (6 to 80) |
| 3 | 3 | 0.14 | 0.22 (0.07 to 0.76) | 0.21 (0.06 to 0.71) | 79 (29 to 94) |
| Infection with mild diarrhea | |||||
| 0 | 84 | 3.13 | |||
| 1 | 70 | 1.76 | 0.56 (0.41 to 0.77) | 0.56 (0.41 to 0.77) | 44 (23 to 59) |
| 2 | 32 | 0.91 | 0.29 (0.19 to 0.44) | 0.28 (0.19 to 0.42) | 72 (58 to 81) |
| 3 | 15 | 0.70 | 0.23 (0.13 to 0.39) | 0.21 (0.12 to 0.36) | 79 (64 to 88) |
| Infection with unknown diarrheal status | |||||
| 0 | 102 | 3.80 | |||
| 1 | 66 | 1.66 | 0.44 (0.32 to 0.60) | 0.44 (0.32 to 0.60) | 56 (40 to 68) |
| 2 | 53 | 1.50 | 0.40 (0.28 to 0.55) | 0.39 (0.28 to 0.55) | 61 (45 to 72) |
| 3 | 13 | 0.61 | 0.16 (0.09 to 0.29) | 0.16 (0.09 to 0.29) | 84 (71 to 91) |
| Asymptomatic infection | |||||
| 0 | 158 | 5.88 | |||
| 1 | 177 | 4.45 | 0.76 (0.61 to 0.94) | 0.76 (0.61 to 0.94) | 24 (6 to 39) |
| 2 | 140 | 3.97 | 0.68 (0.54 to 0.85) | 0.67 (0.54 to 0.84) | 33 (16 to 46) |
| 3 | 69 | 2.23 | 0.55 (0.41 to 0.73) | 0.54 (0.41 to 0.72) | 46 (28 to 59) |
The group with no previous infections was monitored for 2686 child-months, the group with one previous infection for 3978 child-months, the group with two previous infections for 3524 child-months, and the group with three previous infections for 2137 child-months.
The relative risk was adjusted for sex, level of personal and household hygiene, and involvement in making bidis (yes vs. no).
The adjusted efficacy was calculated as the percent reduction in the risk of an outcome as compared with the risk for children who were not yet infected.
The category of total infections includes symptomatic infections, asymptomatic infections, and infections for which the symptom status was unknown.
Similar results were obtained after repeating the analysis with the inclusion of 44 children who had at least 3 months of follow-up data and one serum sample collected (Table 3 in the Supplementary Appendix). Censoring the data at 24 months and analyzing the data separately for children infected after 6 months of age showed reduced protective efficacy across all categories. Using the most specific definition of rotavirus infection (i.e., a positive test on both ELISA and PCR assay), we found that efficacy after three prior infections against moderate or severe diarrhea and asymptomatic infection increased to 86% and 82%, respectively, but efficacy against infections of unknown status decreased to 40%. Among the 364 children who had an increase in the IgG or IgA level by a factor of 4 or 3, respectively, rotavirus gastroenteritis developed in 80 (22.0%) after seroconversion.
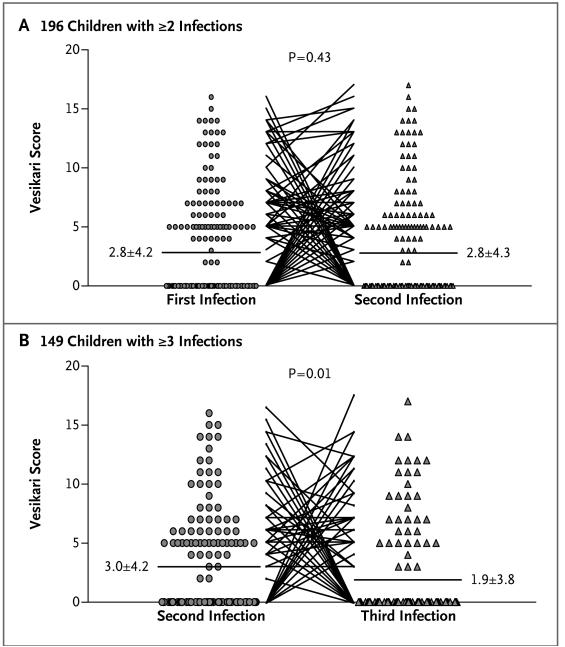
Surprisingly, in children with multiple infections and diarrhea, the severity of diarrhea did not tend to decrease between the first and second infections but did between the second and third (P=0.01) (Fig. 2). In children in whom two or three infections were symptomatic, 5 of 39 second episodes were more severe than the first episode, and 4 of 24 third episodes were more severe than the second episode.
Figure 2. Vesikari Scores for Diarrheal Disease Severity in Consecutive Rotavirus Infections.
Panel A shows scores between the first and second rotavirus infections in 196 children with at least two infections. Panel B shows scores between the second and third rotavirus infections in 149 children with at least three infections. An episode was classified as asymptomatic if the score was 0, mild if the score was between 1 and 10, moderate if the score was between 11 and 15, and severe if the score was between 16 and 20. The horizontal bars represent the mean score for each group (with the mean ±SD shown next to the bar).
ROTAVIRUS GENOTYPES AND REINFECTION
A total of 472 rotavirus strains were genotyped. The G and P types could not be identified in 12.1% of infections. The most common types were G1P[8] (in 15.9% of infections), G2P[4] (in 13.6%), G10P[11] (in 8.7%), G9P[8] (in 7.2%), G1P[4] (in 4.4%), G10P[4] (in 1.7%), G9P[4] (in 1.5%), G12P[6] (in 1.1%), and G1P[6] (in 0.6%). The G10P[11] infections were primarily neonatal and asymptomatic, with a median age of 0.3 months (interquartile range, 0.2 to 0.5) at the time of the primary asymptomatic infection and 0.4 months (interquartile range, 0.2 to 1.5) at the time of the primary symptomatic infection; 26 of these 41 infections (63.4%) were asymptomatic.
To assess protection against subsequent infection with a particular rotavirus strain, we evaluated the risk of primary and subsequent infections with the same G-type or P-type rotavirus strain; the data for G1, G2, and P[8] are shown in Table 3. No decrease in the risk of overall or homotypic rotavirus infection or diarrhea was found in later infections, indicating a lack of clear protection on the basis of genotype.
Table 3.
Rate Ratios for Subsequent Diarrhea or Infection from G1, G2, or P[8] Rotavirus, According to Strain of Primary Infection.*
| Value | G1 Primary Infection (N = 44) |
Non-G1 Primary Infection (N = 327) |
Rate Ratio (95% CI) |
P Value | G2 Primary Infection (N = 25) |
Non-G2 Primary Infection (N = 346) |
Rate Ratio (95% CI) |
P Value | P[8] Primary Infection (N = 41) |
Non-P[8] Primary Infection (N = 330) |
Rate Ratio (95% CI) |
P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up — child-mo | 1167.8 | 7917.4 | 594 | 8491 | 1005.3 | 8079.9 | ||||||
| Any diarrhea — % (95% CI) | 11.4 (8.1–16.0) |
7.9 (6.9–9.0) |
1.4 (1.0–2.1) |
0.05 | 12.4 (7.9–19.5) |
8.0 (7.0–9.1) |
1.55 (0.97–2.49) |
0.07 | 8.3 (5.7–12.1) |
8.3 (7.3–9.5) |
1.0 (0.67–1.50) |
1.00 |
| Any rotavirus infection — % (95% CI) |
8.7 (7.2–10.6) |
8.0 (7.4–8.6) |
1.1 (0.89–1.35) |
0.38 | 8.3 (6.2–10.9) |
8.0 (7.5–8.7) |
1.03 (0.77–1.37) |
0.87 | 8.0 (6.5–10.0) |
8.1 (7.5–8.7) |
1.00 (0.79–1.26) |
1.00 |
| Asymptomatic rotavirus infection — % (95% CI) |
4.5 (3.5–5.9) |
4.6 (4.2–5.1) |
0.98 (0.74–1.31) |
0.90 | 3.2 (2.0–5.0) |
4.7 (4.3–5.2) |
0.68 (0.43–1.08) |
0.10 | 5.1 (3.9–6.7) |
4.6 (4.1–5.1) |
1.11 (0.83–1.49) |
0.47 |
| Rotaviral diarrhea — % (95% CI) |
2.2 (1.4–3.5) |
1.8 (1.5–2.2) |
1.22 (0.75–1.99) |
0.42 | 3.3 (1.9–5.6) |
1.8 (1.5–2.1) |
1.89 (1.08–3.30) |
0.07 | 1.7 (1.0–2.9) |
1.9 (1.6–2.3) |
0.89 (0.50–1.56) |
0.68 |
| Moderate orsevere rotaviral diarrhea — % (95% CI) |
0.4 (0.2–1.0) |
0.4 (0.3–0.6) |
1.06 (0.41–2.72) |
0.90 | 0.5 (0.2–1.6) |
0.5 (0.3–0.6) |
1.26 (0.39–4.11) |
0.70 | 0.3 (0.1–0.9) |
0.4 (0.3–0.6) |
0.71 (0.22–2.31) |
0.57 |
| Homotypic rotavirus infection — % (95% CI) |
1.2 (0.7–2.1) |
0.3 (0.1–1.4) |
0.7 (0.3–1.5) |
|||||||||
| Any rotavirus infection in chil dren with nonhomotypic primary infection — % (95% CI) |
1.0 (0.7–1.2) |
1.26 (0.79–2.27) |
0.44 | 0.7 (0.5–0.9) |
0.49 (0.12–2.04) |
0.33 | 1.1 (0.9–1.4) |
0.62 (0.28–1.35) |
0.23 | |||
| Homotypic rotaviral diarrhea — % (95% CI) |
0.8 (0.4–1.6) |
0.3 (0.1–1.4) |
0.4 (0.1–1.1) |
|||||||||
| Any rotaviral diarrhea in chil dren with nonhomotypic primary infection — % (95% CI) |
0.5 (0.3–0.7) |
1.58 (0.71–3.52) |
0.26 | 0.3 (0.2–0.5) |
1.01 (0.22–4.59) |
0.99 | 0.5 (0.4–0.7) |
0.74 (0.25–2.15) |
0.58 |
Data for a subgroup of rotavirus strains (G1, G2, and P[8]) are shown. Data are based on stool testing alone, since analysis of homotypic and heterotypic protection requires genotyping of the infective virus. Percentages and 95% confidence intervals refer to incidence per 100 child-months of follow-up.
DISCUSSION
In previous cohort studies, reported rates of protective efficacy of a natural rotavirus infection against subsequent infection or disease have ranged from 46 to 100%.7,8,27-32 Our results are consistent with this broad range but show much less protection than was shown in birth cohorts in Mexico7 and Guinea-Bissau8 (Table 1 in the Supplementary Appendix). In the Mexican cohort, there was complete protection against severe gastroenteritis after two prior infections; whereas in our cohort, after adjustment for possible confounders, the rate of protection was 79% after three prior infections (Table 2).
Although rotavirus was the single most common cause of diarrhea and accounted for a higher proportion of severe cases of diarrheal disease than any other cause, the rates of reinfection and asymptomatic infection were much higher than expected; only 30% of all identified infections were primary, as compared with 52% and 81% in Mexico and Guinea-Bissau, respectively, and only 30% of the primary infections were symptomatic (Table 1 in the Supplementary Appendix). Our high rate of identified infection was only partially due to the use of PCR assay for detection, because the majority of infections were identified, as in Mexico, on the basis of an immune response to rotaviral proteins.
Another key difference is the early occurrence of infection in our study, with 53% of children infected by 6 months of age, whereas in Mexico and Guinea-Bissau, 34% and 26% of children, respectively, were infected by that age. For vaccination strategies, this raises concern about the balance between the maturity of the immune system and the age at which vaccines should be delivered to cover the period of highest risk.
It has generally been believed that protection from infections is initially homotypic and then broadens to heterotypic protection. Our data (Table 3) show no evidence of initial homotypic protection; the rates of subsequent homotypic rotavirus infection and diarrhea were similar regardless of whether the primary infection was with G1, G2, or P[8] virus. Extending our previous findings of a lack of protection with G10 primary infection in our cohort,33 further testing showed that G2 primary infection also did not provide protection against the same or different strains.
In summary, our data show that in India, rotavirus infections occur early, reinfection is more common than previously believed, and the rate of protection against diarrhea of any severity is lower than has been reported in previous studies. Taken together, these data indicate that rotavirus-vaccination strategies for India and similar settings may need to be modified by increasing the dose or number of doses of vaccine and considering earlier vaccination, such as neonatal or maternal immunization.
Supplementary Material
Acknowledgments
Supported by the Wellcome Trust.
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Parashar UD, Burton A, Lanata C, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9–S15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 2.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 4.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 5.Meeting of the Immunization Strategic Advisory Group of Experts, April 2009 — conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:220–36. [PubMed] [Google Scholar]
- 6.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–98. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 7.Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–8. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 8.Fischer TK, Valentiner-Branth P, Steinsland H, et al. Protective immunity after natural rotavirus infection: a community cohort study of newborn children in Guinea-Bissau, west Africa. J Infect Dis. 2002;186:593–7. doi: 10.1086/342294. [DOI] [PubMed] [Google Scholar]
- 9.O’Ryan ML, Hermosilla G, Osorio G. Rotavirus vaccines for the developing world. Curr Opin Infect Dis. 2009;22:483–9. doi: 10.1097/QCO.0b013e32833040a9. [DOI] [PubMed] [Google Scholar]
- 10.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301:2243–51. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 11.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 12.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 13.John TJ. Problems with oral poliovaccine in India. Indian Pediatr. 1972;9:252–6. [PubMed] [Google Scholar]
- 14.John TJ, Jayabal P. Oral polio vaccination of children in the tropics: the poor seroconversion rates and the absence of viral interference. Am J Epidemiol. 1972;96:263–9. doi: 10.1093/oxfordjournals.aje.a121457. [DOI] [PubMed] [Google Scholar]
- 15.Richie EE, Punjabi NH, Sidharta YY, et al. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000;18:2399–410. doi: 10.1016/s0264-410x(00)00006-2. [DOI] [PubMed] [Google Scholar]
- 16.Gladstone BP, Das AR, Rehman AM, et al. Burden of illness in the first 3 years of life in an Indian slum. J Trop Pediatr. 2010;56:221–6. doi: 10.1093/tropej/fmp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladstone BP, Muliyil JP, Jaffar S, et al. Infant morbidity in an Indian slum birth cohort. Arch Dis Child. 2008;93:479–84. doi: 10.1136/adc.2006.114546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein DI, Smith VE, Sander DS, Pax KA, Schiff GM, Ward RL. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis. 1990;162:1055–62. doi: 10.1093/infdis/162.5.1055. [DOI] [PubMed] [Google Scholar]
- 19.Velázquez FR, Matson DO, Guerrero ML, et al. Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis. 2000;182:1602–9. doi: 10.1086/317619. [DOI] [PubMed] [Google Scholar]
- 20.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–67. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 21.Boom R, Sol CJ, Salimans MM, Jansen CL, Wertheim-van Dillen PM, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentsch JR, Glass RI, Woods P, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–73. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gouvea V, Glass RI, Woods P, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–82. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iturriza-Gómara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31:259–65. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Ward RL, Bernstein DI, Smith VE, et al. Rotavirus immunoglobulin a responses stimulated by each of 3 doses of a quadrivalent human/bovine reassortant rotavirus vaccine. J Infect Dis. 2004;189:2290–3. doi: 10.1086/421248. [DOI] [PubMed] [Google Scholar]
- 26.Hosmer DW, Lemeshow S. Applied survival analysis: regression modeling of time to event data. John Wiley; New York: 1999. [Google Scholar]
- 27.Bernstein DI, Sander DS, Smith VE, Schiff GM, Ward RL. Protection from rotavirus reinfection: 2-year prospective study. J Infect Dis. 1991;164:277–83. doi: 10.1093/infdis/164.2.277. [DOI] [PubMed] [Google Scholar]
- 28.Bhan MK, Lew JF, Sazawal S, Das BK, Gentsch JR, Glass RI. Protection conferred by neonatal rotavirus infection against subsequent rotavirus diarrhea. J Infect Dis. 1993;168:282–7. doi: 10.1093/infdis/168.2.282. [DOI] [PubMed] [Google Scholar]
- 29.Bishop RF, Barnes GL, Cipriani E, Lund JS. Clinical immunity after neonatal rotavirus infection — a prospective longitudinal study in young children. N Engl J Med. 1983;309:72–6. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- 30.Georges-Courbot MC, Monges J, Beraud-Cassel AM, Gouandjika I, Georges AJ. Prospective longitudinal study of rotavirus infections in children from birth to two years of age in Central Africa. Ann Inst Pasteur Virol. 1988;139:421–8. doi: 10.1016/s0769-2617(88)80077-7. [DOI] [PubMed] [Google Scholar]
- 31.Reves RR, Hossain MM, Midthun K, et al. An observational study of naturally acquired immunity to rotaviral diarrhea in a cohort of 363 Egyptian children: calculation of risk for second episodes using age-specific person-years of observation. Am J Epidemiol. 1989;130:981–8. doi: 10.1093/oxfordjournals.aje.a115431. [DOI] [PubMed] [Google Scholar]
- 32.Ward RL, Bernstein DI. Protection against rotavirus disease after natural rotavirus infection. J Infect Dis. 1994;169:900–4. doi: 10.1093/infdis/169.4.900. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee I, Gladstone BP, Le Fevre AM, et al. Neonatal infection with G10P[11] rotavirus did not confer protection against subsequent rotavirus infection in a community cohort in Vellore, South India. J Infect Dis. 2007;195:625–32. doi: 10.1086/510853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.