Abstract
Background
We aimed to compare the steady-state pharmacokinetic parameters and tolerability of Triomune 40® (stavudine 40 mg, lamivudine 150 mg and nevirapine 200 mg) and branded formulations of these drugs in HIV-infected Ugandans.
Methods
This includes a randomized, open-label, cross-over study of HIV-infected patients stable on therapy for 1 month. Patients were randomized to generic or branded formulation. Plasma pharmacokinetics were assessed after 1 month. The following day, alternate formulation was administered, and 1 month later, drug pharmacokinetics were re-assessed. Plasma pharmacokinetics were determined using HPLC–UV detection. Similarity between steady-state pharmacokinetic parameters was assessed using the US Food and Drug Administration standards for bioequivalency testing. Tolerability was assessed using questionnaires.
Results
Sixteen (10 females) patients completed the study. Median (IQR) age, weight and CD4 count were 37 (33.7–40) years, 65 (63.4–66) kg and 292 (220.7–344.5) cells/mm3, respectively. All patients received co-trimoxazole. The geometric mean ratio (90% CI) for stavudine, lamivudine and nevirapine was 0.92 (0.78–1.08), 1.11 (0.95–1.30) and 0.84 (0.64–1.11), respectively, for Cmax, and 0.83 (0.70–0.97), 1.06 (0.94–1.20) and 0.88 (0.71–1.10), respectively, for AUC. Stavudine plasma concentrations were significantly lower for the generic formulation. Pharmacokinetic parameter inter-individual variability ranged from 29% to 99%. There were no differences in tolerability for the two formulations.
Conclusions
Pharmacokinetic profiles of generic and branded drugs were similar. Differences particularly with regard to stavudine were demonstrated. Surveillance of the quality of generic antiretroviral drugs in the target populations is needed. Capacity building for pharmacokinetic research in resource-limited settings is a priority.
Keywords: antiretroviral drugs, PK, Uganda
Introduction
An estimated 1 million HIV-infected adults and children live in Uganda with an average HIV prevalence of 6.2%.1 There are 105 000 people with HIV on antiretroviral therapy (ART), constituting ~50% of those who need it.2 The major constraint for a widespread use of ART in Uganda, as in many other African countries, has been the high cost of medication. Potent ART became widely available due to initiatives including the Multicountry AIDS Programme, the President’s Emergency Plan for AIDS Relief (PEPFAR), the Global Fund to Fight AIDS, Tuberculosis and Malaria, the World Health Organization’s 3 by 5 Initiative, the Elizabeth Glaser Pediatric AIDS Foundation and Médecins sans Frontières, among others.3 Many of these programmes rely on the less expensive generic ART formulations to attain the treatment goals.3
First-line ART in Uganda consists of zidovudine or stavudine plus lamivudine with either nevirapine or efavirenz. However, the majority of the Ugandan HIV-infected patients are treated with the three-drugs-in-one fixed dose combination (FDC) of generically manufactured stavudine, lamivudine and nevirapine.4 Importantly, despite the introduction of generic FDC tablets into HIV clinical practice, data on the quality of generic drugs in HIV-infected adults in Uganda are lacking. Debate is ongoing as to whether generic antiretroviral (ARV) drug formulations are truly the equivalent of brand formulations and whether they should be approved by a regulatory agency before administration to patients in developing countries. In this context, five ARV agents have been removed from clinical use by the WHO because bioequivalence to the trade formulations was not established.5 Following new bioequivalence studies, two of these agents have been reinstated.
Although the amount of drug in the generic tablet may be similar to brand formulations, simple chemical studies conducted in vitro do not guarantee optimal drug dissolution and absorption in humans. Documentation of bioequivalence in the target population is therefore important. Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two different preparations of a drug. The US Food and Drug Administration has defined bioequivalence as ‘the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in pharmaceutical equivalents or pharmaceutical alternatives becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study’.6 However, bioequivalence is used to compare two formulations of a single dose of drug given to healthy volunteers. Intensive pharmacokinetic studies can be performed faster than expensive randomized clinical trials, which may delay ARV use where required. We performed a randomized, open-label, cross-over intensive pharmacokinetic study to compare the steady-state pharmacokinetic parameters of stavudine, lamivudine and nevirapine in Triomune 40® with the branded products using the US Food and Drug Administration standards for bioequivalency testing. In addition, the safety and tolerability of the formulations were assessed.
Methods
Study site
The study was conducted between January and September 2007 at the Infectious Diseases Institute (IDI) at Mulago Hospital in Kampala, Uganda. The IDI is a regional centre of excellence for HIV/AIDS treatment, prevention, training and research. To date, over 12 000 HIV-infected patients are registered at the IDI with over 4000 already taking ART. Over 70% of these are on Triomune therapy.7
Study design and population
This was a randomized, open-label, cross-over intensive pharmacokinetic study of generic and trade formulations of stavudine, lamivudine and nevirapine in patients with HIV-1 infection stable on ART for at least 1 month prior to pharmacokinetic sampling.
Patients were eligible to participate if they were older than 18 years of age and able to provide written informed consent. Patients weighing <60 kg, showing abnormal clinical test results and treated with known inhibitors or inducers of cytochrome P450 metabolism or herbal medications, were excluded.
Ethical considerations
The study was approved by the IDI Scientific Review Committee, and the National HIV/AIDS Research Committee (ARC 047) and was registered with Uganda National Council of Science and Technology and ClinicalTrials.gov (NCT 00455585). All participants gave written informed consent to participate, and all study procedures were conducted according to Good Clinical Practice.
Study procedures
Patients were electronically randomized to either Triomune 40; batch no. G57662 (Cipla, Goa, India) or the patented version of the drugs: Zerit®; batch no. 0059 (Bristol Myers Squibb, Princeton, NJ, USA), Epivir®; batch no. R270222 (GlaxoSmithKline, Research Triangle Park, NC, USA) and Viramune®; batch no. 506396A (Boehringer Ingelheim, Columbus, OH, USA). A regimen of stavudine 40 mg, lamivudine 150 mg and nevirapine 200 mg was taken twice daily. Participants took one tablet twice daily while on the generic formulation (Triomune 40) and one Zerit® capsule, one Epivir® tablet and one Viramune® tablet, twice daily while on the branded formulation. Participants had detailed study explanation at enrolment. On each study day, adherence to study drugs was assessed using self report and pill count by the study pharmacist. In addition, we collected information on adverse drug effects and serious adverse drug effects, and a questionnaire on quality of life was administered on each study day. All participants took their drugs for a month prior to pharmacokinetic sampling. On the evening prior to pharmacokinetic sampling, participants were reminded of their study day appointment and given detailed instructions to take their medication and food by 8.00 pm and arrive at the hospital by 7.00 am in the fasting state.
On study day 1, patients were admitted in the fasting state, an indwelling intravenous catheter was inserted following aseptic techniques and blood samples were drawn for the determination of pre-dose concentrations. The intake of a standardized breakfast and morning doses of drugs was directly observed by study staff. Pharmacokinetic sampling was performed at 2, 4, 6, 8, 10 and 12 h post-dosing. Blood (4 mL) was collected into ethylene diamine tetra-acetic acid tubes each time. Before discharge from the unit, patients were switched to brand formulations from generic and vice versa and were given the drugs to take at home. After 1 month, study subjects were readmitted, and plasma pharmacokinetic sampling was repeated. All patients resumed their pre-study treatment at the end of the second pharmacokinetic day. Blood samples were centrifuged immediately after collection; plasma was removed and stored at −20°C until shipment.
Analytical and pharmacokinetic methods
Drug concentration measurement was performed by standard HPLC with UV detection at the Department of Infectious Diseases, HIV Pharmacology Laboratory, University of Turin, Italy. The lower limit of quantification was 25, 25 and 50 ng/mL for stavudine, lamivudine and nevirapine and the limit of detection was 5, 5 and 10 ng/mL, respectively.
The calculated pharmacokinetic parameters for stavudine, lamivudine and nevirapine were the trough plasma concentration (Ctrough) defined as the 12 h concentration after the observed dose, the maximum observed plasma concentration (Cmax) and the area under the plasma concentration–time curve (AUC) from 0 to 12 h and the half-life. All pharmacokinetic parameters were calculated using actual blood sampling times and non-compartmental modelling techniques (WinNonlin Professional™ software, version 4.1; Pharsight Corp., Mountain View, CA, USA).
Statistical analysis
A sample size of 12 was calculated to have 80% power to detect a difference in means of nevirapine Cmax and AUC between branded and generic formulations based on the definition of bioequivalence using paired t-test with a significance level of 0.05. We anticipated a drop-out rate of 30%; therefore, we planned to enrol 16 subjects for 12 to complete all pharmacokinetic assessments.
Descriptive statistics, including mean and standard deviation (SD), were calculated for stavudine, lamivudine and nevirapine pharmacokinetic parameters.
Within-subject changes in drug pharmacokinetic parameters were evaluated by calculating geometric mean ratios (GMRs) and 90% confidence intervals (CIs). The concentrations measured during the branded formulation administration were used as reference. The CIs were determined using logarithms of the individual geometric mean values; the calculated values were then expressed as linear values. Steady-state pharmacokinetic parameters were considered similar if the 90% CI for the Cmax and the AUC fell within the range of 0.8–1.25.6 Inter-individual variability in drug pharmacokinetic parameters was expressed as a coefficient of variation [(SD/mean) × 100].
Results
A total of 27 HIV-infected subjects (16 females) were screened between January and March 2007; 18 were eligible and were enrolled, 1 was discontinued because she missed study appointments and 1 was excluded from the pharmacokinetic analysis because he did not complete pharmacokinetic sampling. A total of 16 (10 females) completed all pharmacokinetic phases. The clinical and demographic characteristics of the 16 subjects who completed the study are illustrated in Table 1.
Table 1.
Baseline clinical and demographic characteristics of the study patients according to the randomization arm
| Brand → generic (Arm 1) |
Generic → brand (Arm 2) |
|
|---|---|---|
| Age (years) | 37.4 (33–40) | 36.8 (34–40) |
| Weight (kg) | 64.7 (63–67) | 64.5 (63.5–66) |
| CD4 count (cells/mm3) | 324.4 (255–343) | 305.3 (220–349) |
| Females (%) | 56 | 71 |
Median (IQR) unless otherwise specified.
Both the branded and generic formulations of stavudine, lamivudine and nevirapine were well tolerated and no differences were reported. There were no adverse drug reactions, abnormal laboratory findings or drop-outs due to toxicity during the study. None of the participants who completed the study reported any missed dose during the study period. Patients preferred the FDC formulation (generic) because of dosing convenience.
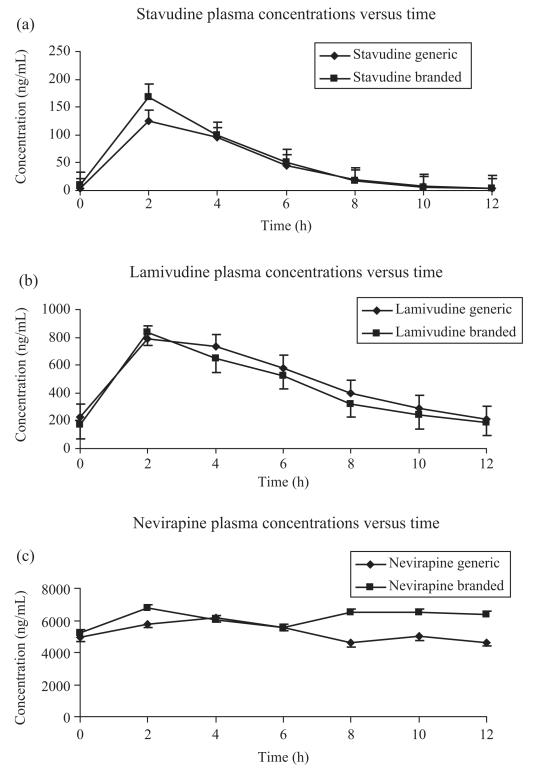
Mean [±standard error (SE)] concentration–time profile for the three drugs is shown in Figure 1. The mean (±SD) stavudine, lamivudine and nevirapine steady-state pharmacokinetic parameters measured during the administration of branded and generic formulations are reported in Table 2. Mean (SD) nevirapine exposure (AUC0–12) while on branded and generic drugs was 75 192.7 ng·h/mL (29 294.9) and 64 338.3 ng·h/mL (19 944.6), respectively. GMR of the test (generic) to reference (brand) of Cmax and AUC values and 90% CI are given for each drug in Table 2. While no significant differences in lamivudine and nevirapine Cmax and AUC were observed [GMR (90% CI): 1.11 (0.95–1.30) and 0.84 (0.64–1.11) for Cmax, and 1.06 (0.94–1.20) and 0.88 (0.71–1.10) for AUC], stavudine plasma concentrations were significantly lower for the generic formulation for Cmax 0.92 (0.78–1.08) and AUC 0.83 (0.70–0.97).
Figure 1.
Mean (±SE) plasma concentration versus time of (a) stavudine, (b) lamivudine and (c) nevirapine of 16 subjects during the intake of branded and generic formulations.
Table 2.
Pharmacokinetic parameters of branded and generic stavudine, lamivudine and nevirapine
| Parameter | Stavudine | Lamivudine | Nevirapine |
|---|---|---|---|
|
Cmax (ng/mL)a brandedb |
203.5 (±127.7) | 855.2 (±276.6) | 8594.3 (±3699.0) |
| CV (%) | 62.8 | 32.3 | 43.0 |
| genericb | 210.3 (±208.4) | 966.8 (±279.6) | 7017.3 (±2757.8) |
| CV (%) | 99.1 | 29.0 | 39.3 |
| GMR (90% CI) | 0.92 (0.78–1.08) | 1.11 (0.95–1.30) | 0.84 (0.64–1.11) |
| AUC0–12 (ng·h/mL)a branded |
685.6 (±219.6) | 5522.7 (±2009.8) | 75 192.7 (±29 294.9) |
| CV (%) | 32.0 | 36.4 | 38.9 |
| generic | 579.8 (±231.2) | 6039.0 (±2370.8) | 64 338.3 (±19 944.6) |
| CV (%) | 39.9 | 39.3 | 31.0 |
| GMR (90% CI) | 0.83 (0.70–0.97) | 1.06 (0.94–1.2) | 0.88 (0.71–1.10) |
GMR, geometric mean ratio; CI, confidence interval; Cmax, maximum plasma concentration; AUC, area under the curve for concentration–time; CV, coefficient of variation.
Mean (±SD).
Number of samples: branded drugs (stavudine, lamivudine and nevirapine) = 110 each, generic = 112.
Only the 90% CI for lamivudine AUC GMR at steady-state was within 0.80 and 1.25. All the other 90% CIs were outside the predefined limits.
Stavudine, lamivudine and nevirapine pharmacokinetic parameter inter-individual variability ranged from 29% to 99% (Table 2).
Median nevirapine Ctrough was 3615.0 ng/mL (range, 1236–6138) while on the generic formulation and 4479.5 ng/mL (range, 1070–8375) while on Viramune®. Eleven of the 16 subjects for the generic and 10 of 16 for the branded had nevirapine concentrations above the suggested minimum effective concentration (MEC) of 3000 ng/mL.8 Only one subject had Ctrough below the MEC while on both formulations.
Discussion
This study was designed to compare the steady-state pharmacokinetic parameters of stavudine, lamivudine and nevirapine in Triomune 40 and their branded products and to assess the safety and tolerability of the different formulations in HIV-infected Ugandan adults. With the exception of lamivudine, steady-state pharmacokinetic parameters of Triomune 40 did not fall within the limits of the US Food and Drug Administration standards for bioequivalency testing.6 However, pharmacokinetic profiles were similar, and there was no difference in tolerability between the two regimens. Evidence for bioequivalence of Triomune 40 to branded products was based on a single-dose study in healthy volunteers.9 Since adequate drug concentrations must be maintained for long-term suppression of HIV, it is arguable that steady-state pharmacokinetic studies are of greater clinical significance than single-dose studies.
Although the mean nevirapine pharmacokinetics were similar for the two formulations, there was considerable inter- and intrasubject variability. Steady-state Ctrough concentrations were above the alleged MEC of 3000 ng/mL8 for 11 of 16 subjects for the generic and 10 of 16 for the branded. Although it is not possible to relate plasma concentrations to adverse events,8 it is of interest to note that 3 of 16 for the generic and 9 of 16 for the branded had peak concentrations >8000 ng/mL. Nevirapine exposure in our study was lower than that reported in a recent study from Malawi.10 The metabolism of nevirapine is partly dependent on CYP2B6 activity, and there is evidence that the functional single nucleotide polymorphism (516G > T) is associated with increased levels of nevirapine. Penzak et al.11 reported a 17% prevalence of this mutation in a small Ugandan study. It is possible that a higher prevalence of this and/or other polymorphisms in patients in the Malawi study could account for the different findings and support a theory of wide variability in the distribution of polymorphisms within the African populations.
We found a statistically significant decrease in stavudine concentrations when subjects received Triomune 40. Stavudine and lamivudine are pro-drugs that require intracellular phosphorylation to the active form. Intracellular concentrations rather than plasma concentrations correlate with virological suppression.12 The 17% reductions in the plasma concentrations (AUC) of stavudine reported in this study may not be clinically significant in terms of achievement and maintenance of virological response. Our findings contrast to those of an 8 h pharmacokinetic study in Malawi reporting a 12% increase in stavudine concentrations while patients received Triomune 40.10 The differences in stavudine exposure in the two studies cannot be explained by a change in manufacturing practices arising from the reports of the Malawi study because the Triomune 40 tablets used in our study were produced before those data were known. We did not conduct in vitro analysis of the respective quantities of stavudine, lamivudine and nevirapine levels in generic and branded drugs prior to administration as this was beyond the scope of the present study. However, regulatory authorities require and routinely conduct such studies prior to drug registration in Uganda.
We conducted this study using Triomune 40 that contains 40 mg of stavudine in each tablet. Treatment guidelines for stavudine-containing regimens have been updated in an attempt to minimize toxicity possibly related to high plasma concentrations. Based on data revealing similar viral suppression rates and fewer adverse events with lower doses of stavudine, the World Health Organization recently adopted stavudine 30 mg twice daily as the standard of care irrespective of body weight in adults.13 Currently, the majority of Ugandan HIV-infected patients are treated with stavudine 30 mg-containing regimens.14 Uganda and other resource-limited countries depend on cheaper generic agents, and these have been widely utilized in the rapid scaling-up of ART. Overall, clinical outcome data with Triomune use in Africa are encouraging. Laurent et al.15 reported safety and efficacy data in a Cameroonian study in which 80% of the participants had viral loads <400 copies at 24 weeks of therapy. Data from the cohort at our centre revealed high virological suppression rates of up to 86% at 12 months of therapy with over two-thirds of the patients on Triomune.7
In conclusion, our data show that although the steady-state pharmacokinetic parameters for Triomune 40 did not fall within the limits set by the US Food and Drug Administration for bioequivalency testing, the pharmacokinetic profiles of generic and branded drugs were similar. Differences particularly with regard to stavudine were demonstrated. While the crucial role generic products have played in the progress of ART is recognized, the need for ongoing surveillance of their quality in target populations must be emphasized. Steady-state pharmacokinetic studies need to be considered as a component of regulatory procedures for ARV drug approval in developing countries. Investment in human and material resources to develop pharmacokinetic units in resource-limited settings must be considered a priority.
Acknowledgements
We are grateful to the INTERACT team for scientific advice; Chris Higgs from St Stephen’s Centre, London, UK, for training our pharmacokinetic team; and the Department of Pharmacology, University of Turin, Italy, for scientific support.
Funding Financial support was provided by the Department of Foreign Affairs, Ireland and the Infectious Diseases Network for Treatment and Research in Africa (INTERACT).
Footnotes
Transparency declarations None to declare.
References
- 1.Ministry of Health (MOH) [Uganda] and ORC Macro . Uganda HIV/AIDS Sero-behavioural Survey 2004–05. Ministry of Health and ORC Macro; Calverton, Maryland, USA: 2006. [Google Scholar]
- 2.UAC . Moving Towards Universal Access: National HIV & AIDS Strategic Plan 2007/2008-2011/2012. Uganda AIDS Commission; Republic of Uganda: 2007. [Google Scholar]
- 3.Havlir DV, Hammer SM. Patents versus patients? Antiretroviral therapy in India. N Engl J Med. 2005;353:749–51. doi: 10.1056/NEJMp058106. [DOI] [PubMed] [Google Scholar]
- 4.Songa PM, Castelnuovo B, Mugasha EB, et al. Symptomatic hyperlactatemia associated with nucleoside analogue reverse-transcriptase inhibitor use in HIV-infected patients: a report of 24 cases in a resource-limited setting (Uganda) Clin Infect Dis. 2007;45:514–7. doi: 10.1086/520023. [DOI] [PubMed] [Google Scholar]
- 5.WHO Removal of Antiretroviral Products from the WHO List of Prequalified Medicines Information and Guidance for Regulatory Bodies, National AIDS Programmes. Doctors and Patients. 2004.
- 6.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research [8 May 2008, date last accessed];Guidance for Industry Statistical Approaches to Establishing Bioequivalence. 2001 http://www.fda.gov/cder/Guidance/3616fnl.pdf.
- 7.Kamya MR, Mayanja-Kizza H, Kambugu A, et al. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46:187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 8.La Porte CJL, Back DJ, Blaschke T, et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antivir Ther. 2006;3:4–14. [Google Scholar]
- 9.Narang VS, Lulla A, Malhotra G, et al. A combined-formulation tablet of lamivudine/nevirapine/stavudine: bioequivalence compared with concurrent administration of lamivudine, nevirapine, and stavudine in healthy Indian subjects. J Clin Pharmacol. 2005;45:265–74. doi: 10.1177/0091270004273343. [DOI] [PubMed] [Google Scholar]
- 10.Hosseinipour MC, Corbett AH, Kanyama C, et al. Pharmacokinetic comparison of generic and trade formulations of lamivudine, stavudine and nevirapine in HIV-infected Malawian adults. Acquir Immune Defic Syndr. 2007;21:59–64. doi: 10.1097/QAD.0b013e3280117ca0. [DOI] [PubMed] [Google Scholar]
- 11.Penzak SR, Kabuye G, Mugyenyi P, et al. Cytochrome P450 2B6 (CYP2B6) G516T influences nevirapine plasma concentrations in HIV-infected patients in Uganda. HIV Med. 2007;8:86–91. doi: 10.1111/j.1468-1293.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoggard PG, Sales SD, Kewn S, et al. Correlation between intracellular pharmacological activation of nucleoside analogues and HIV suppression in vitro. Antivir Chem Chemother. 2000;11:353–8. doi: 10.1177/095632020001100601. [DOI] [PubMed] [Google Scholar]
- 13.WHO [6 March 2008, date last accessed]; http://www.who.int/hiv/art/ARTadultsaddendum.pdf.
- 14.Songa PM, Castelnuovo B, Mugasha EB, et al. Symptomatic hyperlactatemia associated with nucleoside analogue reverse-transcriptase inhibitor use in HIV-infected patients: a report of 24 cases in a resource-limited setting (Uganda) Clin Infect Dis. 2007;45:514–7. doi: 10.1086/520023. [DOI] [PubMed] [Google Scholar]
- 15.Laurent C, Kouanfack C, Koulla-Shiro S, et al. Effectiveness and safety of a generic fixed-dose combination of nevirapine, stavudine, and lamivudine in HIV-1-infected adults in Cameroon: open-label multicentre trial. Lancet. 2004;364:29–34. doi: 10.1016/S0140-6736(04)16586-0. [DOI] [PubMed] [Google Scholar]