Abstract
Objectives
The aim of this study was to investigate the frequency of CYP2B6 polymorphisms (according to ethnicity) and the influence of heterozygosity and homozygosity on plasma concentrations of efavirenz and nevirapine.
Methods
Following written informed consent, 225 Caucasians and 146 Blacks were recruited from the German Competence Network for HIV/AIDS. Plasma concentrations of efavirenz and nevirapine were assessed by HPLC, and genotyping for 516G>T, 983T>C and 1459T>C polymorphisms in CYP2B6 was conducted by real-time PCR-based allelic discrimination.
Results
The minor allele frequency for 516G>T, 983T>C and 1459T>C was 0.29, 0 and 0.08 in Caucasians and 0.34, 0.07 and 0.02 in Blacks, respectively. Two Black patients with the 983C allele receiving efavirenz were identified and both were withdrawn from therapy within 1 week of sampling due to toxicity. In multivariate analyses, efavirenz and nevirapine plasma concentrations were significantly associated with 983T>C (P < 0.0001 and P = 0.02, respectively), 516G>T (P < 0.0001 and P = 0.002, respectively) and time of drug analysis post-dose (P < 0.0001 for both). Body mass index was independently related to efavirenz (P = 0.04) but not nevirapine concentrations, and age was related to nevirapine (P = 0.05) but not efavirenz concentrations. Consistent with other studies, 1459C>T was not associated with plasma concentrations of either drug (P > 0.05 for both drugs).
Conclusions
This is the first report that the 983T>C genotype (part of the CYP2B6*18 haplotype) impacts on nevirapine plasma concentrations and the first study to assess the impact of 983C homozygosity on efavirenz concentrations. These data have implications for administration of non-nucleoside reverse transcriptase inhibitors to Black patients.
Keywords: NNRTIs, pharmacogenetics, pharmacokinetics, metabolism, drug disposition
Introduction
Drug treatment in HIV disease is characterized by variable responses, in terms of both efficacy and toxicity. Genetic and environmental factors are important determinants of this variability, although the relative contributions are unclear and likely to vary with different drugs (see Owen et al.1 for review).
Efavirenz and nevirapine are metabolized by CYP2B6 (although CYP3A also contributes to nevirapine metabolism). Single nucleotide polymorphisms (SNPs) and haplotype organization of CYP2B6 in Caucasians were originally described by Lang et al.,2 who showed a reduced hepatic CYP2B6 protein expression and activity in carriers of the 516G>T (rs3745274) and 1459T>C (rs3211371) polymorphisms. However, the association with protein expression and activity was not statistically significant for 516G>T, although the authors acknowledged the limitation of the sample size. With respect to HIV, the role of the 516G>T SNP in disposition and treatment response to non-nucleoside reverse transcriptase inhibitors (NNRTIs) is now well established.3-9 Moreover, a recent study indicated that efavirenz dose reduction according to 516G>T genotype was a feasible strategy.10
Recently, the 983T>C SNP (rs28399499) was described in Black populations. This polymorphism results in an amino acid change in the CYP2B6 protein (Ile328Thr), and heterozygosity has been shown to impact upon efavirenz pharmacokinetics.11,12 Furthermore, a laboratory-based assessment of this polymorphism indicated that it may represent a null allele.11 Investigation of the 983T>C polymorphism in various populations has shown that the allele is absent in Caucasian populations, yet its frequency is as high as 7.5% in African-Americans and Ghanaians.13 However, to date, no homozygotes for this polymorphism have been described.
The aim of this study was to investigate the frequency of CYP2B6 polymorphisms (516G>T, 983T>C and 1459T>C) and the influence of heterozygosity and homozygosity for these polymorphisms on plasma concentrations of efavirenz and nevirapine in a cohort of Caucasian and Black patients from the German Competence Network for HIV/AIDS. In addition, the association with gender, ethnicity, weight, height, alcohol consumption, smoking status, glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT) was also determined. Finally, a multivariate analysis was conducted using best subset analysis.
Patients and methods
Patients
Whole blood and plasma were provided by the German Competence Network for HIV/AIDS. Three hundred and seventy-one patients (225 Caucasians and 146 Blacks), with age range from 21 to 82 (median = 43) years, who were on stable efavirenz- (n = 186) or nevirapine- (n = 185) containing HAART for at least 3 months, were included in the study. Median (range) CD4 counts were 487 (24–1690) cells/mm3, and 88% had undetectable plasma viral RNA. Ethics approval was granted by the Ethics Committee of the Ruhr-Universität Bochum, Germany, and local Ethics Committee approval was obtained at each site. Written informed consent was obtained.
Genotyping
Total genomic DNA was isolated using the QIAamp DNA mini kit according to the manufacturer’s instructions. Following extraction, purity was assessed by comparing the A260 and A280 ratio. DNA was quantified using PicoGreen® (PG) dsDNA Quantitation Reagent (Molecular Probes, CA, USA) and normalized to 20 ng/μL. Pre-amplification for exon 4, exon 9 and exons 7 and 8 (combined) was first conducted to discriminate from the CYP2B6 pseudogene (CYP2B7) by modification of previously reported methods.2 Genotyping for 516G>T, 983T>C and 1459C>T was then performed on the resultant amplicons by real-time PCR allelic discrimination using standard methodology (95°C for 15 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min) in a DNA Engine Opticon® 2 system (MJ Research Inc., USA). Full PCR conditions as well as primer and probe sequences are available on request.
Quantification of drug levels
Plasma obtained from blood samples was heat-inactivated, and efavirenz and nevirapine concentrations were determined (median time post-dose was 10 h) using HPLC with UV-Detection using previously validated assays as described elsewhere.14,15 The Liverpool Laboratory participates in an external quality assurance scheme (KKGT, The Netherlands).
Statistical analysis
All data are given as median (range), unless otherwise stated. Genotypes were tested for Hardy–Weinberg equilibrium by χ2 test of observed versus predicted (from allele frequency) genotype frequencies. Normality of the data was assessed using a Shapiro–Wilk test. Subsequently, univariate statistical analysis was conducted by simple linear regression (continuous data) or one-way analysis of variance (categorical data). Missing values were imputed by regression against efavirenz concentrations, and multivariate analysis was conducted by multiple linear regressions using best subset selection.
Results
Genotype frequencies
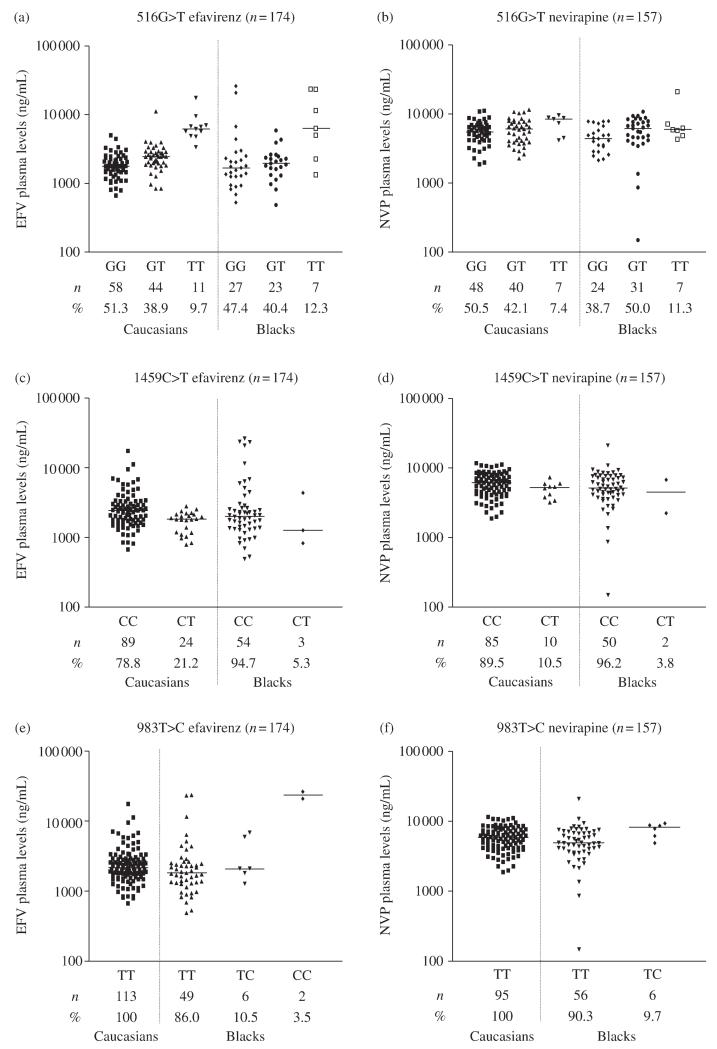
When χ2 test of observed versus predicted genotype frequencies was conducted, all polymorphisms were found to be in Hardy–Weinberg equilibrium. The frequencies of 516G>T, 983T>C and 1459C>T are given in Figure 1.
Figure 1.
The impact of 516G>T (a and b), 1459C>T (c and d), and 983T>C (e and f) polymorphisms in CYP2B6 on efavirenz (a, c and e) and nevirapine (b, d and f) plasma concentrations according to ethnicity (see text for experimental procedures). The genotype frequencies within each group are also given.
Efavirenz plasma concentrations
In the entire cohort, efavirenz plasma concentrations were 2077 (487–21 486) ng/mL. In the univariate analysis, 516G>T [1779 (530–26 018), 2299 (487–11 198) and 6248 (1345–23 592) ng/mL for GG, GT and TT, respectively; P < 0.0001], 983T>C [2068 (530–11 198), 2076 (1269–6835) and 23 418 (20 818–26 018) ng/mL for TT, TC and CC, respectively; P < 0.0001], body mass index (BMI) (R2 = 0.02; P = 0.05), alcohol consumption (R2 = 0.03; P = 0.04) and time post-dose of drug analysis (R2 = 0.22; P < 0.0001) were all significantly associated with efavirenz plasma concentrations (Table 1). Following multivariate analysis, only 516G>T (P < 0.0001), 983T>C (P < 0.0001), BMI (P = 0.04), and time post-dose (P < 0.0001) remained statistically significant (Table 1).
Table 1.
Multivariate analysis
| Covariate | R 2 | Univariate P | Multivariate P |
|---|---|---|---|
| Efavirenz | |||
| gender | N/A | 0.21 | N/A |
| ethnicity | N/A | 0.15 | 0.23 |
| 516G>T | N/A | <0.0001 | <0.0001 |
| 983T>C | N/A | <0.0001 | <0.0001 |
| 1459C>T | N/A | 0.06 | 0.78 |
| BMI | 0.02 | 0.05 | 0.04 |
| GOT | 0.01 | 0.15 | 0.52 |
| GPT | 0.002 | 0.55 | N/A |
| alcohol status | 0.03 | 0.04 | 0.25 |
| smoking status | 0.02 | 0.09 | 0.35 |
| age | 0.001 | 0.66 | N/A |
| time post-dose | 0.22 | <0.0001 | <0.0001 |
| Nevirapine | |||
| gender | N/A | 0.62 | N/A |
| ethnicity | N/A | 0.50 | N/A |
| 516G>T | N/A | 0.007 | 0.002 |
| 983T>C | N/A | 0.02 | 0.02 |
| 1459C>T | N/A | 0.18 | N/A |
| BMI | 0.01 | 0.20 | N/A |
| GOT | 0.004 | 0.42 | N/A |
| GPT | 0.006 | 0.33 | N/A |
| alcohol status | 0.002 | 0.55 | N/A |
| smoking status | 0.005 | 0.34 | N/A |
| age | 0.03 | 0.03 | 0.05 |
| time post-dose | 0.09 | 0.0001 | 0.0004 |
Associations with a P value less than 0.15 in the univariate analysis and therefore included in the multivariate analysis are shown in bold, as are those associations with a P value less than 0.05 in the multivariate analysis.
Neither 1459C>T [2241 (530–26 018) and 1816 (792–4357) ng/mL for CC and CT, respectively], gender [2168 (487–26 018) and 1974 (688–23 592) ng/mL for male and female, respectively], ethnicity [2143 (669–11 198) and 1963 (487–26 018) ng/mL for Caucasian and Black, respectively], GOT (R2 = 0.01), GPT (R2 = 0.002), smoking status (R2 = 0.02), nor age (R2 = 0.001) was significantly associated with efavirenz plasma concentrations (Table 1).
Nevirapine plasma concentrations
In the entire cohort, nevirapine plasma concentrations were 5589 (149–21 026) ng/mL. In the univariate analysis, 516G>T [5184 (1894–11 158), 6132 (149–11 689) and 6699 (4164–21 026) ng/mL for GG, GT and TT, respectively; P = 0.007], 983T>C [5483 (864–21 026) and 8685 (4890–10 294) for TT and TC, respectively; P = 0.02], age (R2 = 0.03; P = 0.03), and time post-dose of drug analysis (R2 = 0.09; P = 0.0001) were all significantly associated with nevirapine plasma concentrations (Table 1). Following multivariate analysis, 516G>T (P = 0.002), 983T>C (P = 0.02), age (P = 0.05), and time post-dose (P = 0.0004) all remained statistically significant (Table 1).
Neither 1459C>T [5670 (864–21 026) and 5243 (2230–7436) ng/mL for CC and CT, respectively], gender [5893 (149–11 689) and 5483 (864–21 026) ng/mL for male and female, respectively], ethnicity [5893 (1894–11 689) and 5138 (149–21 026) ng/mL for Caucasian and Black, respectively], BMI (R2 = 0.01), GOT (R2 = 0.004), GPT (R2 = 0.006), alcohol consumption (R2 = 0.002), nor smoking status (R2 = 0.005) was significantly associated with nevirapine plasma concentrations (Table 1).
Discussion
The CYP2B6 gene is highly polymorphic with numerous SNPs and associated haplotypes. The association of the 516G>T SNP with efavirenz and nevirapine pharmacokinetics is well established, but a recent study indicated that the 516TT genotype was not associated with the time to failure of efavirenz-containing regimens or increases in the CD4 cell count.16 This polymorphism did predict CNS adverse effects at week 1 of therapy, but tolerance developed despite the higher plasma efavirenz exposure.16 Nonetheless, a recent manuscript described the use of 516G>T (along with 499C>G and 785A>G) in successful dose reduction of efavirenz in a Japanese cohort.10 The cost-effectiveness of this approach now requires further investigation.
Genetic variability has been assessed in different ethnicities and a number of novel functional variants discovered,11,17 among these, the 983T>C polymorphism is a suspected null allele. With the increasing use of NNRTIs in developing countries, it is imperative that the functional significance of these alleles on NNRTI therapy in different sub-populations be assessed. The 983T>C polymorphism has only previously been identified in Hispanic and African populations,11,12 with allele frequencies of 1.1% in Hispanic Americans and up to 7.5% in some African countries.13 We observed this allele with a frequency of 2.6% in Blacks residing in Germany.
In agreement with previous studies, we observed a significant gene dose effect between 516G>T and efavirenz and nevirapine plasma concentrations. In addition, 983T>C genotype was also associated with plasma concentrations of both drugs. Importantly, homozygote patients in the efavirenz arm discontinued therapy 1 week after blood was collected for this study (but before drug analysis) due to CNS toxicity. Previous studies have illustrated that heterozygosity of this polymorphism (present in the CYP2B6*18 allele) is associated with efavirenz concentrations.12 However, no homozygotes for this polymorphism have been previously reported. Therefore, this is the first study to illustrate the phenotypic consequence of homozygosity for efavirenz and to show an association with nevirapine plasma concentrations. Further studies are now required to determine the impact of 983T>C on long-term efficacy and toxicity of this important class of drug.
Acknowledgements
We would like to thank Doris Reichelt, Münster, Hartmut Klinker, Würzburg, Matthias Stoll and Reinhold Schmidt, Hannover Practice Adam/Schewe/Weitner, Hamburg, Practice Gölz/Moll, Berlin, Hans-Jürgen Stellbrink, Hamburg, Practice Dupke/Carganico/Baumgarten, Berlin, Ansgar Rieke, Koblenz and Mark Oette, Düsseldorf for enrolling patients into the study. Furthermore, we would like to thank Gislea Kremer and Ellen Rund, Köln for administrative assistance and Winfried Siffert, Essen for collecting and providing the blood samples of the German Competence Network for HIV/AIDS. We are extremely grateful to all patients who contributed to the study.
Funding
This work was funded by the UK Department of Health Biomedical Research Centre for Microbial Diseases, the German Competence Network for HIV/AIDS and the BMBF (Bundesministerium für Bildung und Forschung; grant 01 KI 0771). H. H. was supported by a scholarship of the Else Kroener Foundation.
Transparency declarations
A. O., S. H. K. and D. J. B. have received research funding from Boehringer Ingelheim, GlaxoSmithKline, Abbott Laboratories, Pfizer, AstraZeneca, Tibotec, Merck and Roche Pharmaceuticals. G. F. has received honoraria for lectures and advisory boards from Abbott, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, GlaxoSmithKline, Novartis, Pfizer, Roche, Schering Plough and Tibotec. The other authors have none to declare.
References
- 1.Owen A, Pirmohamed M, Khoo SH, et al. Pharmacogenetics of HIV therapy. Pharmacogenet Genomics. 2006;16:693–703. doi: 10.1097/01.fpc.0000236338.41799.57. [DOI] [PubMed] [Google Scholar]
- 2.Lang T, Klein K, Fischer J, et al. Extensive genetic polymorphism in the human CYP2B6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11:399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Haas DW, Bartlett JA, Andersen JW, et al. Pharmacogenetics of nevirapine-associated hepatotoxicity: an Adult AIDS Clinical Trials Group collaboration. Clin Infect Dis. 2006;43:783–6. doi: 10.1086/507097. [DOI] [PubMed] [Google Scholar]
- 4.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–400. [PubMed] [Google Scholar]
- 5.Ribaudo HJ, Haas DW, Tierney C, et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group Study. Clin Infect Dis. 2006;42:401–7. doi: 10.1086/499364. [DOI] [PubMed] [Google Scholar]
- 6.Ritchie MD, Haas DW, Motsinger AA, et al. Drug transporter and metabolizing enzyme gene variants and nonnucleoside reverse-transcriptase inhibitor hepatotoxicity. Clin Infect Dis. 2006;43:779–82. doi: 10.1086/507101. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Novoa S, Barreiro P, Rendon A, et al. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin Infect Dis. 2005;40:1358–61. doi: 10.1086/429327. [DOI] [PubMed] [Google Scholar]
- 8.Rotger M, Colombo S, Furrer H, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchiya K, Gatanaga H, Tachikawa N, et al. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem Biophys Res Commun. 2004;319:1322–6. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 10.Gatanaga H, Hayashida T, Tsuchiya K, et al. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin Infect Dis. 2007;45:1230–7. doi: 10.1086/522175. [DOI] [PubMed] [Google Scholar]
- 11.Klein K, Lang T, Saussele T, et al. Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics. 2005;15:861–73. doi: 10.1097/01213011-200512000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Rotger M, Tegude H, Colombo S, et al. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin Pharmacol Ther. 2007;81:557–66. doi: 10.1038/sj.clpt.6100072. [DOI] [PubMed] [Google Scholar]
- 13.Mehlotra RK, Bockarie MJ, Zimmerman PA. CYP2B6 983T>C polymorphism is prevalent in West Africa but absent in Papua New Guinea: implications for HIV/AIDS treatment. Br J Clin Pharmacol. 2007;64:391–5. doi: 10.1111/j.1365-2125.2007.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almond LM, Hoggard PG, Edirisinghe D, et al. Intracellular plasma pharmacokinetics of efavirenz in HIV-infected individuals. J Antimicrob Chemother. 2005;56:738–44. doi: 10.1093/jac/dki308. [DOI] [PubMed] [Google Scholar]
- 15.Clarke SM, Mulcahy FM, Tjia J, et al. Pharmacokinetic interactions of nevirapine and methadone and guidelines for use of nevirapine to treat injection drug users. Clin Infect Dis. 2001;33:1595–7. doi: 10.1086/322519. [DOI] [PubMed] [Google Scholar]
- 16.Haas DW, Smeaton LM, Shafer RW, et al. Pharmacogenetics of long-term responses to antiretroviral regimens containing efavirenz and/or nelfinavir: an Adult AIDS Clinical Trials Group study. J Infect Dis. 2005;192:1931–42. doi: 10.1086/497610. [DOI] [PubMed] [Google Scholar]
- 17.Lang T, Klein K, Richter T, et al. Multiple novel nonsynonymous CYP2B6 gene polymorphisms in Caucasians: demonstration of phenotypic null alleles. J Pharmacol Exp Ther. 2004;311:34–43. doi: 10.1124/jpet.104.068973. [DOI] [PubMed] [Google Scholar]