Abstract
Esophageal adenocarcinoma currently has one of the most rapidly increasing tumor incidences in the United States, with the vast majority of cases occurring on the backdrop of metaplastic epithelium (Barrett esophagus). The availability of appropriate cell line models is essential for maintaining the pace of esophageal cancer research and for pre-clinical validation of new therapeutic modalities. The identity of several of the widely utilized esophageal adenocarcinoma cell lines (BIC-1, SEG-1 and TE-7) have recently been called into question. Here we describe the establishment and characterization of a bona fide esophageal cancer cell line, JH-EsoAd1, from a patient with Barrett-associated adenocarcinoma. The rapid dissemination of this cancer cell line to the esophageal cancer research community should help ameliorate the current scarcity of preclinical models in this disease.
Keywords: Barrett esophagus, adenocarcinoma, cell line, JH-EsoAd1, genotyping
Introduction
Esophageal adenocarcinoma arises within the context of progressive histologic alterations, originating with the development of a metaplastic epithelium (Barrett esophagus) and extending through low- and high-grade dysplastic stages to frank invasive cancer.1 Esophageal cancer currently has one of the most rapidly increasing tumor incidences in the United States. The overwhelming majority of patients presenting with esophageal adenocarcinoma will do so at a late stage and therefore, of the estimated 16,470 new patients this year, almost 14,280 (~87%) are expected to succumb to their disease.2
Unlike for many other human cancers,3-5 there are no currently available genetically engineered mice simulating Barrett esophagus, although more tedious surgical reflux models have been developed in rodents.6,7 As a result, the advancement of preclinical research in esophageal adenocarcinoma rests, for the most part, on the availability of appropriate human cancer cell lines. Authenticated cell lines from specific cancer types are critical for preventing misinterpretation of laboratory results. Recent estimates from scientific laboratories however suggest that as many as one-third of cell lines are of a different origin or species than originally designated.8,9 This unfortunate trend has become increasingly evident with the application of high throughput DNA fingerprinting strategies to cell lines and their use has been recommended by the NIH (see notice regarding authentication of cultured cell lines; NOT-OD-08-017). Mistaken identity of cancer lines has particularly deleterious implications for malignancies like esophageal adenocarcinoma, where only a limited number of independent samples are available worldwide and is compounded if the mislabeling or cross-contamination occurs at the source. BIC-1 and SEG-1 have been two of the most commonly published esophageal adenocarcinoma cell lines in the scientific literature, with over 100 citations in PubMED and scores of additional conference proceedings (accessible via Google Scholar or ISI® Web of Science). Recently, the laboratory that created both cell lines indicated in an open letter to the scientific community that the identities of BIC-1 and SEG-1 have been called into question based on the genotyping of these lines compared to that of the original tissues from which they were purportedly derived (Dr. David Beer, personal communication). As this misattribution occurred at the source laboratory, the implications are significantly more widespread than would be expected from a downstream error. In conjunction with a report documenting the mistaken identity of TE-7, another widely utilized esophageal adenocarcinoma line,10 there has been a profound depletion in authenticated cell lines from this disease. Two cancer cell lines (OE33 and OE19) are currently available as esophageal adenocarcinoma derivatives from the European Collection of Cell Cultures (http://www.ecacc.org.uk/), first created in 1997.11 These two lines have been extensively utilized, as evidenced by over 50 citations in PubMED. Nevertheless, there is no DNA fingerprint available on either line comparing them to the parental tumor, which always raises the theoretical specter of accidental mislabeling or cross-contamination and distribution thereafter. Flo-1 is a third esophageal adenocarcinoma cell line and the only one to the best of our knowledge that has been authenticated based on genotypic comparison with the parental tumor (Dr. David Beer, personal communication).
Methods, Results and Discussion
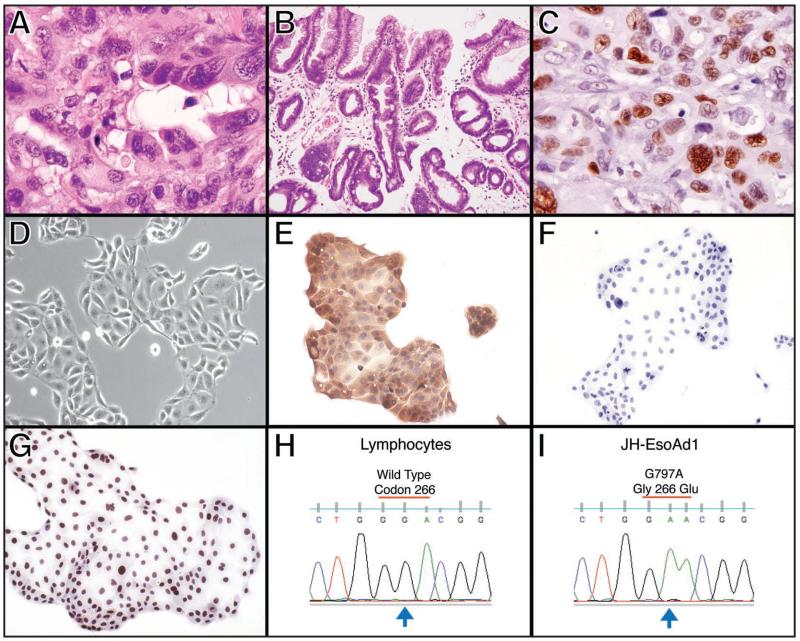
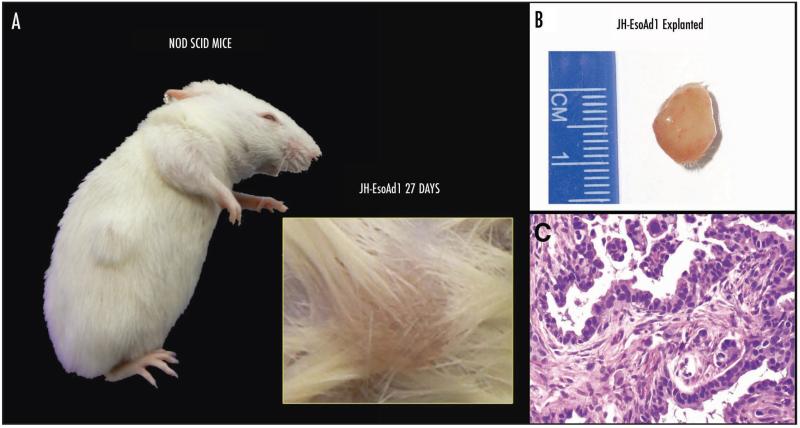
In this priority report, we briefly describe the establishment and characterization of a bona fide esophageal adenocarcinoma cell line, JH-EsoAd1. This cell line was derived from a 66-year-old caucasian male undergoing surgical resection for a distal esophageal malignancy (pT3N0M0). Histopathological examination confirmed the presence of an infiltrating, moderately to poorly differentiated adenocarcinoma (Fig. 1A) and metaplastic Barrett epithelium at the periphery of the tumor (Fig. 1B). A p53 immunostain12 demonstrated nuclear overexpression in the cancer cells (Fig. 1C), consistent with mutational inactivation and stabilization of p53 in the primary tumor. A fresh sample of the resected cancer was directly passaged in tissue culture, as previously described,13 for establishment of JH-EsoAd1. In addition, the patient’s peripheral lymphocytes were Epstein Barr virus (EBV)-immortalized, as a perpetual source of matched germline DNA. As seen in Figure 1D, the cultured epithelioid cells grew adherent in a monolayer with pleomorphic nuclei and abundant cytoplasm, with a doubling time of 20 hours in RPMI 20% FBS. In order to generate DNA fingerprinting of JH-EsoAd1 for posterity, we utilized the PowerPlex® 1.2 System (Promega Corporation) and profiled DNA obtained from the archival normal esophageal tissue, the archival microdissected Barrett adenocarcinoma and the resulting cell line. The Powerplex microsatellite results (see Suppl. Table 1) confirm the identity of JH-EsoAd1 with its parental tumor. Additional molecular characterization of JH-EsoAd1 cells included epithelial marker positivity for cytokeratin and lack of expression of the stromal marker vimentin (Fig. 1E and F respectively). Consistent with the results of the p53 immunohistochemistry in the archival tumor, JH-EsoAd1 cells express high levels of p53 expression (Fig. 1G) and also harbor a somatic G → A mutation at base 797 (Fig. 1H and I), resulting in a previously described non-synonymous Gly 266 Glu alteration in TP53 (which also confirmed in the primary tumor).14 However, KRAS2 is wild type at codons 12, 13, 61 and 146, the four most commonly mutated loci in this oncogene (data not shown). In addition, there is no genomic evidence for wnt pathway activation, as discerned by absence of mutations in APC and in the exon 3 “hot spot” of CTNNB1 (data not shown). We were also successful in establishing JH-EsoAd1 subcutaneous xenografts in 6 of 6 NOD/SCID mice (Fig. 2A and B), which generated tumors with an adenocarcinomatous morphology (Fig. 2C). Based on the xenografting data, we believe JH-EsoAd1 will be a useful preclinical tool for assessing the impact of modulating cellular pathways using genetic or pharmacological measures on the growth of esophageal adenocarcinoma.
Figure 1.
Histology and immunophenotypic marker characterization of the JH-EsoAd1 cell line. (A) Histology of the primary tissue demonstrates gland formation, consistent with an adenocarcinoma. (B) Histology of the metaplastic Barrett epithelium in the periphery of the tumor. (C) Immunohistochemical analysis of p53 (DO-1 antibody) in the primary tumor demonstrates nuclear overexpression in the neoplastic cells. (D) Phase contrast microscopy of the JH-EsoAd1 cell line growing in tissue culture. (E) Immunohistochemical analysis of cytokeratin expression on JH-EsoAd1 cells shows positivity for this epithelial marker. (F) Immunohistochemical analysis of vimentin expression on JH-EsoAd1 cells shows negativity for this mesenchymal marker. (G) Immunohistochemical analysis of p53 expression (DO-1 antibody) shows nuclear overexpression in JH-EsoAd1, as observed in the primary tissue. (H) Sequence analysis of exon 8 of the p53 gene, in the patient’s lymphocytes, shows wild type sequence in codon 266. (I) Sequence analysis of exon 8 of the p53 gene, in the cell line derived material shows a homozygous G797A somatic mutation that results in a non-synonymous Gly266Glu alteration.
Figure 2.
Subcutaneous xenografts were established with JH-EsoAd1 cells in NOD SCID mice. (A) In vivo Growth of JH-EsoAd1. (B) Explanted xenograft. (C) Histological analysis of explanted JH-EsoAd1 xenografts shows adenocarcinomatous histology, with pleomorphic cells and mitoses.
In summary, we have created an authenticated esophageal adenocarcinoma cell line that should restitute some of the current deficit resulting from mistaken identities of the most commonly used models in this disease. The JH-EsoAd1 is being deposited in a commercial cell line repository (American Type Culture Collection; www.atcc.org), in order to facilitate rapid dissemination to the scientific community.
Supplementary Material
Acknowledgements
H.A. is supported by the Jerry D’Amato Foundation.
Footnotes
References
- 1.Paulson TG, Reid BJ. Focus on Barrett’s esophagus and esophageal adenocarcinoma. Cancer Cell. 2004;6:11–6. doi: 10.1016/j.ccr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–70. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 4.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Bonde P, Sui G, Dhara S, Wang J, Broor A, Kim IF, et al. Cytogenetic characterization and gene expression profiling in the rat reflux-induced esophageal tumor model. J Thorac Cardiovasc Surg. 2007;133:763–9. doi: 10.1016/j.jtcvs.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, LoCicero J, 3rd, Macri E, Loda M, Ellis FH., Jr. Barrett’s esophagus and associated adenocarcinoma in a mouse surgical model. J Surg Res. 2000;88:120–4. doi: 10.1006/jsre.1999.5774. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee R. Cell biology. Cases of mistaken identity. Science. 2007;315:928–31. doi: 10.1126/science.315.5814.928. [DOI] [PubMed] [Google Scholar]
- 9.Masters JR. Human cancer cell lines: Fact and fantasy. Nat Rev Mol Cell Biol. 2000;1:233–6. doi: 10.1038/35043102. [DOI] [PubMed] [Google Scholar]
- 10.Boonstra JJ, van der Velden AW, Beerens EC, van Marion R, Morita-Fujimura Y, Matsui Y, et al. Mistaken identity of widely used esophageal adenocarcinoma cell line TE-7. Cancer Res. 2007;67:7996–8001. doi: 10.1158/0008-5472.CAN-07-2064. [DOI] [PubMed] [Google Scholar]
- 11.Rockett JC, Larkin K, Darnton SJ, Morris AG, Matthews HR. Five newly established oesophageal carcinoma cell lines: phenotypic and immunological characterization. Br J Cancer. 1997;75:258–63. doi: 10.1038/bjc.1997.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baas IO, Mulder JW, Offerhaus GJ, Vogelstein B, Hamilton SR. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994;172:5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- 13.McBain JA, Weese JL, Meisner LF, Wolberg WH, Willson JK. Establishment and characterization of human colorectal cancer cell lines. Cancer Res. 1984;44:5813–21. [PubMed] [Google Scholar]
- 14.Tian K, Wang Y, Xu H. WTH3 is a direct target of the p53 protein. Br J Cancer. 2007;96:1579–86. doi: 10.1038/sj.bjc.6603724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.