SUMMARY
Objective
To evaluate whether T2 and T1ρ relaxation times of knee cartilage determined with 3T magnetic resonance imaging (MRI) at baseline predict longitudinal progression of cartilage degenerative changes.
Methods
Quantitative analysis of cartilage was performed using 3T MRI with both T2 and T1ρ mapping techniques in 55 subjects without evidence of severe osteoarthritis (OA) [Kellgren–Lawrence (KL) score of 0–3] at baseline. Morphological abnormalities of cartilage, menisci, ligaments and bone marrow were analyzed on sagittal fat-saturated intermediate-weighted fast spin echo (FSE) sequences. Progression of degenerative changes was analyzed over a period of 2 years. Progression was detected in 27 subjects while in 28 subjects no changes were found. Differences between T2 and T1ρ relaxation times in these two cohorts were compared using one-way analysis of variance (ANOVA) and t tests.
Results
Baseline T2 and T1ρ values were significantly higher in the progression cohort in all compartments (P < 0.05) except the lateral tibia (LT) for T2 and the medial tibia (MT) for T1ρ. Progression of cartilage degenerative disease was most pronounced at the medial femoral condyles and at the femoro-patellar joint; differences between the two cohorts for T2 and T1ρ were also most significant in these compartments.
Conclusions
T2 and T1ρ measurements were significantly higher at baseline in individuals that showed progression of cartilage abnormalities over a period of 2 years and may therefore serve as potential predictors for progression of degenerative cartilage abnormalities in knee OA.
Keywords: Osteoarthritis, T2, T1ρ, Progression, Magnetic resonance imaging
Introduction
Knee osteoarthritis (OA) is traditionally characterized using Kellgren–Lawrence (KL) scores1 on radiographs and presence of clinical symptoms2. However by the time OA is recognized on radiographs, it has progressed to a level that may force patients to alter their lifestyles. Severe OA is the leading cause of chronic disability among the elderly population in the United States2. Risk factors include genetic predisposition, obesity3 and both very low or very high levels of physical activity;4 the only preventative measures noted are measures to minimize exposure to risk factors5. Degenerative joint disease therapy of the knee includes self-management (weight loss, exercise), symptomatic medication (analgesics or intra-articular corticosteroids), and in advanced cases arthroplasty6.
Therapies such as knee braces or heel wedges have also been suggested, however, not one single therapy is known to be effective for all sufferers of OA. Guidelines from the American College of Rheumatology (ACR) recommend both drug therapy and non-pharmacologic interventions for patients with OA of the knee. ACR conditionally recommends acetaminophen, oral or topical non-steroidal antinflammatory drugs (NSAIDs), tramadol, or intra-articular corticosteroid injections for the initial treatment of knee OA5-8.
Studies have shown that quantitative MR T2 and T1ρ relaxation time measurements can show changes in cartilage before abnormalities are visualized on radiographs and morphological magnetic resonance imaging (MRI)9-13. The extracellular matrix of the knee cartilage is comprised mostly of proteoglycans and collagen and water. Loss of proteoglycan molecules is an indicator of cartilage degeneration and because T1ρ detects changes in proteoglycan content of the cartilage, T1ρ is a viable tool for detecting early degenerative disease of cartilage14. T2 has been shown to reflect the ability of free water protons to move within the cartilage matrix; changes in the collagen integrity and increase in water content of the cartilage increase the T2 value, making it a viable biomarker for early cartilage matrix degeneration11-13,15.
While T1ρ and T2 mapping techniques have been used for early detection of biochemical changes in cartilage of the knee16, there is limited information whether these measurements can predict longitudinal progression of degenerative joint disease. The purpose of this study was to evaluate whether T2 and T1ρ relaxation times of knee cartilage determined with 3T MRI at baseline predict longitudinal progression of cartilage degeneration.
Material and methods
Subjects
Subjects were recruited from the general public by advertisement, or through referral from the Orthopedic Institute of the University of California San Francisco. Subjects with self-reported clinical symptoms at the knee consistent with OA and/or radiographic evidence of OA and normal controls were studied. A total of 55 subjects (25 females and 30 males) with an age range of 25–75 years (mean age 49.9 ± 11.9) with radiographic KL scores between 0 and 3 were studied. Table I shows the demographic characteristics of our population along with KL and Western Ontario and McMasters University (WOMAC) scores. MR images of the knee were obtained in all subjects at baseline and 2-year follow-up. Exclusion factors included severe OA or KL scores of 4 and lack of follow-up MRI.
Table I.
Demographic characteristics of the 55 subjects
| Variables | N (%) or mean ± SD |
|---|---|
| Male | 30 (54.5%) |
| Female | 25 (45.4%) |
| Age (years) | 50.5 ± 10.2 |
| Race | |
| White | 36 (65.5%) |
| Black | 2 (3.6%) |
| Asian | 13 (23.6%) |
| Other | 4 (7.3%) |
| BMI (kg/m2) | 28.7 ± 4.3 |
| KL score | |
| 0 | 20 (36.4%) |
| 1 | 14 (25.5%) |
| 2 | 10 (18.2) |
| 3 | 11 (20.0%) |
| WOMAC | |
| Pain | 4.1 ± 4.3 |
| Stiffness | 2.1 ± 2.0 |
| Function | 13.2 ± 14.0 |
The study was approved by the Committee on Human Research at the University of California San Francisco. All subjects gave informed written consent prior to the study. To quantify pain, stiffness, and function in our cohort, subjects filled out the five-point scale WOMAC questionnaires17,18.
MRI
MRIs were obtained with a 3T GE Excite Signa MR Scanner (General Electric, Milwaukee, WI, USA) and an eight-channel phased-array knee coil (In vivo, Orlando, FL, USA). Using acceleration factor (AF) = 2, parallel imaging was used with an array spatial sensitivity technique (ASSET), resulting in reduced imaging times. A sagittal intermediate-weighted sequence with fat-suppression (field of view (FOV): 13–16 cm; slice thickness: 4 mm; repetition time (TR): 4000 ms; echo time (TE): 40 ms) was obtained for the clinical morphological evaluation. T2 and T1ρ (3D MAPPS-magnetization-prepared angle-modulated partitioned-k-space Spoiled Gradient Recalled (SPGR) snapshot)19,20 sequence parameters are presented in detail in Table II. Morphologic MR studies were obtained at baseline and 2 years.
Table II.
Parameters of SPGR, T2 and T1ρ MRI sequences
| Sequence | TR (ms) | TE (ms) | FOV | Matrix | Slice thickness (mm) | Bandwidth (KHz) | Parameter | Acquisition time |
|---|---|---|---|---|---|---|---|---|
| SPGR (flip angle = 12) | 15 | 6.7 | 14 | 512 × 512 | 1 | 31.25 | Cartilage volume and thickness | 6 min |
| Fast spin echo (FSE) | 4300 | 51 | 14 | 512 × 256 | 2.5 (0.5 gap) | – | WORMS | – |
| 3D T1ρ * (TSL = 0/10/40/80 ms, FSL = 500 Hz) | 9.3 | 3.7 | 14 | 256 × 192 | 3 | – | T1ρ | 8 min |
| 3D T2 | 9.3 | 3.1/13.5/23.9/44.8 | 14 | 256 × 192 | 3 | – | T2 | 8 min |
Recovery time 1200 ms.
MR image analysis
Radiological progression
Baseline and follow-up MR images were reviewed by an experienced musculoskeletal radiologist (TML) without knowledge of clinical or radiographic findings in all subjects. Cartilage abnormalities at baseline were compared with those shown in the follow-up studies on a picture archiving and communication system (PACS) workstation (Agfa, Ridgefield Park, NJ, USA). Progression was defined as an increase in depth or width of a cartilage lesion over a period of a minimum of 2 years. Presence of new lesions (incidental lesions) was also defined as disease progression. The increase in depth was defined as mild (resulting in less than 50% cartilage thickness change) or severe (resulting in more than 50% cartilage thickness change). The increase in width was defined as mild (less than 5 mm) or severe (more than 5 mm). Severe change was diagnosed if either increase in depth and/or width were graded as severe. Change in bone marrow edema pattern and joint effusion/synovitis were not used to define progression. Changes in meniscal lesions were not observed in our cohort.
Based on radiographic evidence of OA (KL > 1) at the baseline, the progression and non-progression cohorts, distinguished on MRI images, were further divided into four subcohorts: normal controls showing no progression (NN), normal controls that progress (NP), OA subjects showing no progression (OAN) and, OA subjects that progress (OAP).
Morphological analysis
One of the most frequently used classifications for scoring degenerative abnormalities is the whole-organ magnetic resonance imaging score (WORMS)21 and a modified-WORMS classification has been introduced by our research group, tailored for assessing mild to moderate abnormalities22,23. The modified-WORMS University of California, San Francisco (UCSF) classification reduces the number of the anatomical compartments from 15, evaluated by the classic WORMS to six: lateral tibia (LT), trochlea (T), medial tibia (MT), lateral femur (LF), medial femur (MF) and patella (PAT). Using this semi-quantitative scoring system, cartilage abnormalities were scored using an eight-point scale: 0 = normal thickness and signal; 1 = normal thickness but abnormal signal on fluid sensitive sequences; 2.0 = partial-thickness focal defect <1 cm in greatest width; 2.5 = full-thickness focal defect <1 cm in greatest width; 3 = multiple areas of partial-thickness (Grade 2.0) defects intermixed with areas of normal thickness, or a Grade 2.0 defect wider than 1 cm but <75% of the region; 4 = diffuse (≥75% of the region) partial-thickness loss; 5 = multiple areas of full-thickness loss (Grade 2.5) or a Grade 2.5 lesion wider than 1 cm but <75% of the region; 6 = diffuse (≥75% of the region) full-thickness loss. The WORMS max score was defined as the maximum of the WORMS scores in all compartments per patient, in addition WORMS average scores were calculated. All WORMS readings were performed by two board-certified radiologists (TML, LN).
Quantitative analysis
To determine T1ρ and T2 relaxation time measurements images were analyzed on Sun Workstations (Sun Microsystems, Palo Alto, CA, USA). Segmentation of cartilage compartments [lateral femoral condyle (LFC), medial femoral condyle (MFC), PAT, LT, MT] was performed on high resolution SPGR images using in-house software (utilizing edge detection and Bezier splines) developed in Matlab (Mathworks, Natick, MA, USA); segmentation was semi-automatic and performed by two individuals (JS, AP) with prior segmenting experience. Using the Levenberg–Marquardt mono-exponential fitting algorithm described by the equation below, T2 and T1ρ maps were generated:24
The VTK CISG Registration Toolkit (T. Hartkens, London, UK) was used to rigidly register the generated reconstructed maps to the SPGR images. Segmentations derived from SPGR images were registered to the first echo. The rotations and translations from this registration were applied to the fitted T1ρ and T2 maps, in order to extract quantitative data. For each compartment, T1 rho and T2 values were calculated on a pixel-by-pixel basis, and then averaged over the region of interest (ROI).
Statistical analysis
Statistics were calculated using JMP software, version 9.0 (SAS Institute, Cary, NC, USA). Using one-way analysis of variance (ANOVA), differences in both T2 and T1ρ relaxation times by cartilage compartment were assessed between progression and non-progression cohorts. P-values of < 0.05 were considered significant. Outliers were eliminated using Chauvenet’s criterion (values more than two standard deviations (SDs) from the mean were excluded)25.
Two outliers were found for T1ρ baseline values: one in the LFC and one in the MT and no outliers were found at baseline for T2 values based on the Chauvenet’s criteria.
Student’s t test was also performed by compartment and where t test and ANOVA results were significant, a post-hoc Tukey–Kramer Honestly Significant Differences (HSD) test was performed. In addition, nominal logistic regression models were used to assess the relationship between T2 and T1ρ vs morphologic progression.
Results
Subject characteristics
Table III shows the subject characteristics stratified by progression – there was no significant difference between age, body mass index (BMI), gender, or WOMAC scores of progressors (n = 27) and non-progressors (n = 28). Linear regression was performed with age, BMI, WOMAC scores, and gender. Although age, BMI, and WOMAC scores showed a trend (increased values were associated with progression), no significant associations were found (P > 0.05). Females tended to progress more frequently (56% of females progressed whereas only 43% of males progressed) but differences were not significant (P > 0.05). Of the progression group, there were eight subjects who had KL grade 0 on radiographs, seven had KL grade 1, five had KL grade 2 and seven subjects had KL grade 3; in the non-progressors there were 12 subjects with KL grade 0, seven had KL grade 1, five had KL grade 2 and four had KL grade 3.
Table III.
Subject characteristics stratified by progression and non-progression
| Age (years) | BMI (kg/m2) | WOMAC pain | WOMAC stiffness | WOMAC function | Number of subjects | |
|---|---|---|---|---|---|---|
| Progressors | ||||||
| Mean* | 52.55 | 26.55 | 3.96 | 2.62 | 13.37 | Males = 13 |
| SD* | 10.44 | 5.08 | 3.64 | 2.95 | 13.20 | Females = 14 |
| Non-progressors | ||||||
| Mean | 47.32 | 24.70 | 3.96 | 1.89 | 12.32 | Males = 17 |
| SD | 13.42 | 3.88 | 4.93 | 2.02 | 15.89 | Females = 11 |
| P-value | 0.1135 | 0.1356 | 0.999 | 0.2875 | 0.7908 | n/a |
Means and SDs of the two cohorts were not significantly different in this study.
Morphological findings: WORMS analysis
At baseline the non-progression cohort, stratified by WORMS maximum score was characterized by 14 subjects with a score equal to 0 or 1, four subjects had a score of 2, three subjects a score of 3 and seven had a score of 5. In the progression cohort nine subjects had a score equal to 0 or 1, six subjects had a score of 2, one of 2.5, three of 3, six of 5 and two of 6. WORMS average scores were 2.1 and 2.6 for the non-progression and progression cohorts, respectively. Though higher scores in the progression cohort appeared more frequent and average scores were higher, no statistical significant difference in WORMS was noted between the two cohorts (P = 0.07).
Progression vs non-progression criteria analysis
In total 35 progressive lesions were found as demonstrated in Table IV. Ten of the 35 lesions were new lesions characterized by partial-thickness cartilage loss and a width less than 1 cm. The other 25 lesions were characterized by increase in width and/or depth of pre-existing lesions. Two of these lesions showed severe progression in both width (more than 1 cm) and depth, while 13 out of 25 showed a mild progression in both depth (none become a full-thickness lesion) and width (less than 5 mm). Ten lesions had a mild increase in width (less than 5 mm) without any increase in depth. Figure 1 shows an example of progressive lesions after 2 years of follow-up.
Table IV.
Summary of lesion location and type within the progression cohort
| Compartments | Lesion # | New lesions (incidental lesion) | Increase of pre-existing (prevalent) lesion |
|---|---|---|---|
| MFC | 14 | 5 | 9 |
| MT | 2 | – | 2 |
| PAT | 10 | 3 | 7 |
| T | 9 | 2 | 7 |
| LFC | 0 | 0 | 0 |
| LT | 0 | 0 | 0 |
| Total | 35 | 10 | 25 |
Fig. 1.
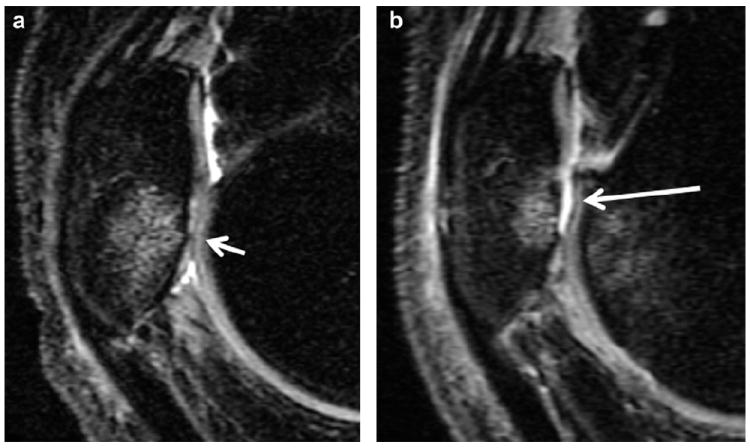
Sagittal, intermediate-weighted MR images demonstrate progression of a cartilage lesion at the PAT: at baseline (a) a small amount of partial-thickness cartilage loss at the inferior pole of the patella (short arrow) associated with bone marrow edema is seen and at the 12 month-follow-up (b) extensive full-thickness cartilage loss is shown at the same site (long arrow).
Twenty-one subjects developed one isolated new lesion each over the 2 years follow-up: 12 at the MF, four at the PAT and five at the T. Six subjects developed multiple lesions: in two subjects the lesions involved MF, MT and PAT compartments and in four both PAT and T compartments.
T2 relaxation
Statistically significant higher baseline T2 values were found in the progression compared to the non-progression cohort in all compartments (P < 0.05) except for the LT (P = 0.21; Fig. 2). Statistical significance was noted in the MFC (P = 0.0.03), MT (P = 0.02), LFC (P = 0.01), and PAT (P = 0.02). When examining the individual compartments of the subjects in the progression cohort, seven compartments (three LFC, one LT, one MFC, one MT and one PAT) in five different subjects had baseline T2 values ≥1 SD below the mean (in the bottom 16th percentile of all observed values). The logistic regression analysis demonstrated that elevated T2 values in the LFC [odds ratio (OR): 2.62, P = 0.02], LT (OR: 1.58, P = 0.15), MFC (OR: 2.12, P = 0.03), MT (OR: 2.12, P = 0.02) and PAT (OR: 2.20, P = 0.02) compartments were predictive of morphologic progression. Table V shows consistent results with higher ORs for individuals with progression for one SD increase in baseline T2 values. However, no statistically significant differences in T2 values were found when assessing the progression cohort subgroups with incident lesions, mild and severe progression of preexistent cartilage defects were compared (P > 0.05). Figure 3 shows an MR color map of a subject with non-progressive changes and low T2 values (a-b-c) as well as high T2 values in a subject with progressive disease (d-e-f).
Fig. 2.
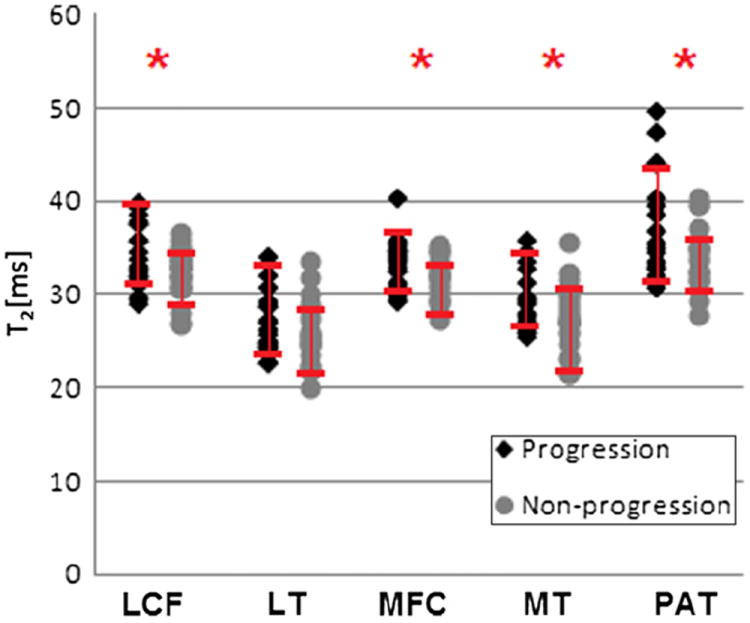
Dot plot showing T2 relaxation time values, stratified by progression vs non-progression. SDs are also depicted, asterisks (*) indicate statistical significance between the two groups (P < 0.05).
Table V.
ORs for one SD change in T2 and T1ρ
| MFC | MT | LFC | LT | PAT | |
|---|---|---|---|---|---|
| OR for one SD change in T2 | 2.12 | 2.12 | 2.616 | 1.58 | 2.19 |
| OR for one SD change in T1ρ | 2.71 | 1.79 | 2.15 | 1.97 | 2.28 |
Fig. 3.
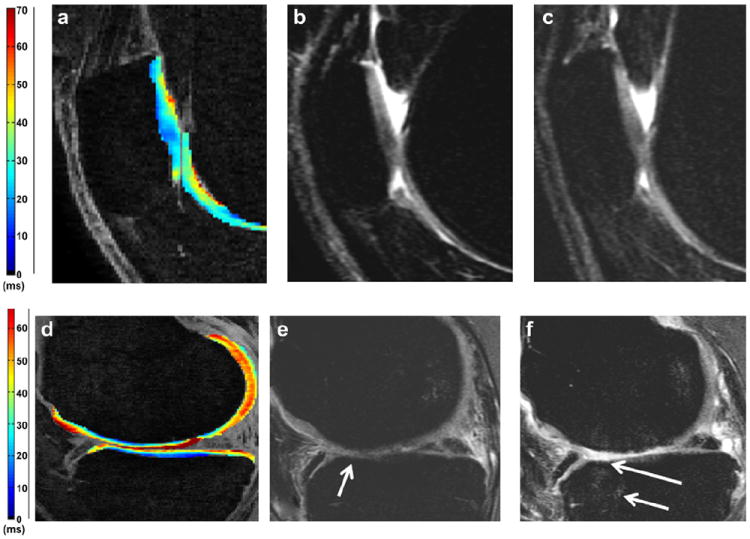
Sagittal T2 color maps (a, d) with baseline (b, e) and follow-up (c, f) sagittal intermediate-weighted MR images. Low T2 values (a) are associated with intact patella cartilage both at the baseline (b) and at the follow-up (c) (non-progressor). High T2 values (d) are associated with extensive progression of femoral and tibial cartilage degeneration: (e) partial femoral and tibial cartilage defects at baseline (arrow) progress in the follow-up (f) with full-thickness loss and partial-thickness cartilage loss at the tibia (long arrow). In addition new bone marrow edema pattern is shown at the femur and tibia (short arrow).
T1ρ relaxation
For T1ρ values, statistically significant higher values were also noted in the progression cohort compared to the non-progression cohort in all compartments except the MT (P = 0.12), Fig. 4. Statistical significance was noted in the MFC (P < 0.01), LFC (P = 0.03), LT (P = 0.01), and PAT (P = 0.01). When examining the individual compartments of the subjects of the progression cohort, 14 compartments (two LFC, four LT, two MFC, two MT and three PAT) in 12 different subjects had baseline T1ρ values ≥1 SD below the mean (in the bottom 16th percentile of all observed values). The logistic regression analysis demonstrated that baseline T1ρ values were associated with morphologic joint progression, in LFC (OR: 2.15, P = 0.01), LT (OR: 1.97, P = 0.03), MFC (OR: 2.71, P < 0.01), MT (OR: 1.79, P = 0.05) and PAT (OR: 2.28, P = 0.014).
Fig. 4.
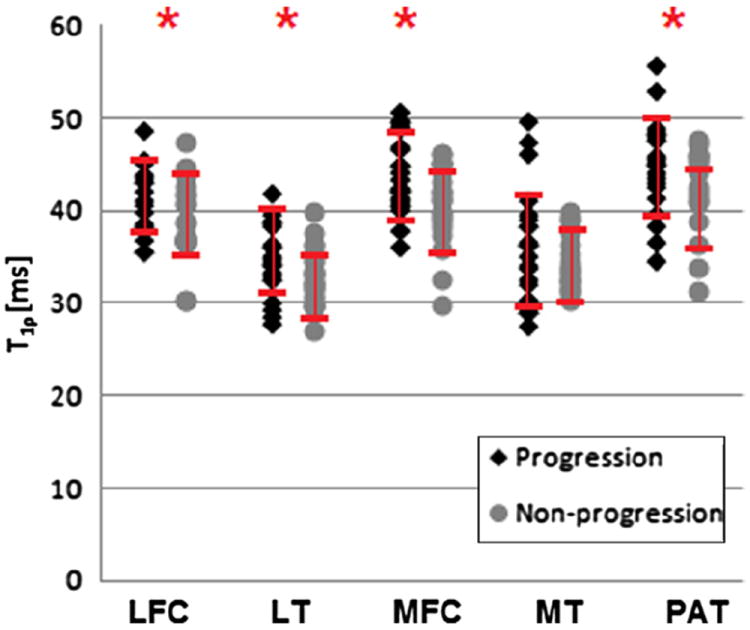
Dot plot showing T1ρ relaxation time values, stratified by progression vs non-progression. SDs are also depicted, asterisks (*) indicate statistical significance between the two groups (P < 0.05).
Again, there were no statistically significant differences in T1ρ values in the progression cohort between the subgroups with incident lesions, mild and severe progression of preexistent cartilage defects.
Figure 5 shows MR color maps with low T1ρ values in a subject with non-progressive changes (a-b-c) and high T1ρ values in a subject with progressive disease (d-e-f).
Fig. 5.
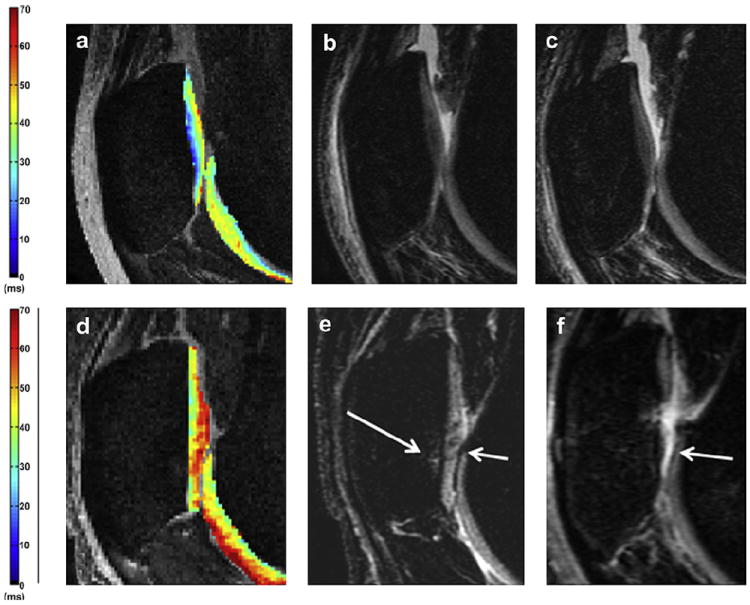
Sagittal T1ρ color maps (a,d) with baseline (b,e) and follow-up (c,f) sagittal morphological MR images. In (a) the T1ρ color map demonstrates low patellar T1ρ values at baseline (average value 30). Baseline (b) and follow-up (c) depict intact patellar cartilage with no degeneration (c). In (d) the T1ρ color map shows high patellar values at baseline (average value about 40). Baseline (e) and follow-up (f) demonstrate progression of a cartilage lesion at the patella: at baseline mild fissuring of the cartilage at the patella is shown (short arrow) associated with bone marrow edema pattern (long arrow) and at the follow-up (f) progression to extensive full-thickness cartilage loss is noted at this site (arrow).
T1ρ relaxation and T2 relaxation in different subcohorts
Based on radiographic evidence of OA (KL > 1) at baseline and MRI criteria of progression/non-progression, the population was divided into four subcohorts: 19 subjects in the NN subcohort, 15 subjects in the NP subcohort, nine in the OAN subcohort and 12 in the OAP subcohort.
Statistically significant lower baseline T2 values were found in the NN subcohort compared to the OAP subcohort in all compartments except the LT: MFC (P < 0.01), MT (P < 0.01), LFC (P = 0.02), LT (P = 0.12) and PAT (P < 0.01). For T1ρ, statistically significant lower values were also noted in the NN subcohort compared to the OAP subcohort in all compartments: MFC (P < 0.01), MT (P = 0.04), LFC (P < 0.01), LT (P < 0.01), PAT (P < 0.01). No other statistically significant differences were found throughout all compartments in the remaining subcohorts.
Discussion
The results of our study show that quantitative T2 and T1ρ relaxation time measurements predict the progression of cartilage degeneration in the knee, assessed with 3T MRI. Both T2 and T1ρ were able to separate progression and non-progression cohorts and the percentage differences as well as the ORs were also comparable. To the best of our knowledge this is the first study to demonstrate that both T2 and T1ρ relaxation time measurements maybe suitable to predict morphological progression of cartilage degenerative disease.
Clinical findings
Our finding that females showed more overall progression than males, though not significant, is similar to findings of other studies: Vavken et al. found a 0.63 ratio of gender difference in incidence of OA. Also McAlindon et al.26 demonstrated a higher prevalence of OA in women especially for the PAT-femur compartment where the prevalence in women older than 55 years was 8% and in men population was 2%. In our study clinical features were not significantly associated with progression and incidence of severe disease, which is also consistent with other studies: Link et al.27 did not find consistent correlations between pain and radiographic and MRI findings of OA (cartilaginous, ligamentous, and meniscal abnormalities) or between pain and radiographic grades that are used to assess OA. Kornaat et al. also suggested that focal or diffuse cartilaginous abnormalities were not associated with pain but associated with larger joint effusion and osteophytes28.
Morphological findings
The baseline WORMS values did not show significant differences between progression and non-progression cohorts. The limited sample of subjects may explain these results, which were borderline significant and demonstrated a statistical trend, with the progression cohort having higher scores, consistent with prior work which describes a positive association between severity and progression of lesions13,29. However, in this same sample, T1ρ and T2 were able to predict the morphological progression with statistical significance, thereby emphasizing the importance of these biochemical biomarkers.
A majority of our subjects showed more progression at the medial tibio-femoral compartment and at the PAT-femoral compartments than at the lateral femoral compartment. According to the literature, these two compartments are more frequently affected by OA of the knee26,30. Although these two compartments display similar pathological trends with regard to OA, their biomechanical, biochemical properties, along with risk factors, will starkly differ. Specific risk factors for femoro-patellar OA are patellar malalignment (lateral patellar tilt or lateral dislocation or PAT tracking) and muscle weakness. Risk factors for tibio-femoral include BMI, varus/valgus malalignment and gait disorders31. The common pathogenesis of those causes is an increased stress at the articulation, which leads progressively to the degeneration of the cartilage with associated joint space narrowing. Squatting and kneeling have no relation with tibio-femoral OA but are strongly associated with PAT-femoral OA32. The compartment that displayed limited progression in our study was the lateral tibio-femoral joint compartment. This compartment has been already demonstrated to be less frequently associated with OA in other studies30,32.
Quantitative findings
Previous studies have demonstrated that T2 and T1ρ relaxation times show biochemical changes at the knee cartilage in the absence of radiographic or MR evidence33,34, however, limited information is available whether they may also serve as predictors of progression of cartilage degenerative disease. Studies have shown that T2 is effective in detecting biochemical changes associated with OA2,35. T2 values are associated with water content and collagen within the cartilage matrix, and have been shown to increase in moderate degenerative joint disease35. T2 relaxation measurements have also been shown to increase with severity of OA36. These previous findings are all consistent with our findings that elevated T2 levels at baseline are associated with increased progression of OA.
T1ρ relaxation time has been indicated in quantification of biochemical changes within the cartilage at the knee, displaying sensitivity to the proteoglycan content in the extracellular matrix37-39. Elevated T1ρ relaxation times are associated with OA in the patellar and femoral cartilage37. This is consistent with our findings of elevated T1ρ times in the patellar and femoral cartilage within our progression cohort. We are not able to determine whether T2 or T1ρ values better predict cartilage damage and future studies will be necessary to clarify which technique is better suited for predicting progression.
The comparison of subcohorts obtained using MRI and radiographic criteria strengthened our results; in particular the comparison between the NN and OAP subcohorts found again statistically significant difference for all knee-compartments when T2 values were used as predictor of progression and in all knee-compartments except for MT when T1ρ values were used as predictor of progression of cartilage damage.
Both T1ρ and T2 values were demonstrated to be superior to the modified-WORMS in predicting cartilage lesion progression. In the literature the WORMS classification has often been used as a reference for detecting morphological cartilage lesions12,15, and most of the studies need a long term follow-up to demonstrate through WORMS that T1ρ and T2 values are reliable predictors of cartilage lesion progression11,13. Our results showed that the new and progression of cartilage lesions are earlier predicted by the T1ρ and T2 biomarkers and the modified-WORMS may confirm this data just with a longer follow-up.
The limitations of our study include a relatively small patient population (especially for the subcohort analysis) and a variety of degenerative disease ranging from normal knees to those with moderate OA. We were unable, due to the small number of patients, to exclude higher KL grades, or to focus on one specific grade with our analysis. Our follow-up time was also relatively short as OA related cartilage changes occur over a period of many years. Another possible limitation of the study was the availability of just one morphological sequence for the WORMS reading. The use of only one plane may underestimate cartilage lesions especially at patellar compartment. In this study, the definition of progression was based on morphological MR findings and not clinical findings – although MR findings maybe more sensitive and objective for measuring early signs of progression over shorter time periods. Finally the registration was able to mitigate most of the motion artifacts, which, however, persisted throughout all knee-compartments in three subjects only for the T2 sequence.
In conclusion our study has shown significant differences in baseline T2 and T1ρ relaxation measurements between a cohort of individuals with and without progression of degenerative cartilage MR lesions; progression could be predicted measuring T2 and T1ρ at the femoral condyles and femoro-patellar joints, where progression of disease was also most advanced. Based on these findings, T2 and T1ρ relaxation times may serve as predictors of cartilage degenerative disease progression, and in a clinical setting, may potentially have a role not only in identifying high-risk patients, but also in more effectively recommending preventative strategies to protect the cartilage in the joint to these patients. Future studies in larger cohorts, with more homogeneous patient characteristics, with clinical metrics of OA and longer follow-up times are clearly warranted.
Acknowledgments
We would like to thank Sharmila Majumdar, Ph.D. for in-depth discussions. This study was supported by NIH R01-AR46905.
Footnotes
Author contributions
Guarantors of integrity of entire study, TML, LN and AP; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, LN and AP; statistical analysis, AP and TML.
Conflict of interest
None.
References
- 1.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis Dec. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majumdar S, editor. Advances in MRI of the Knee for Osteoarthritis. 1. 2010. [Google Scholar]
- 3.Louer CR, Furman BD, Huebner JL, Kraus VB, Olson SA, Guilak F. Diet-induced obesity significantly increases the severity of post-traumatic arthritis in mice. Arthritis Rheum. 2012 Oct;64(10):3220–30. doi: 10.1002/art.34533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, et al. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum. 2011 Aug;63(8):2248–56. doi: 10.1002/art.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007 Sep;15(9):981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Richmond J, Hunter D, Irrgang J, Jones MH, Levy B, Marx R, et al. Treatment of osteoarthritis of the knee (nonarthroplasty) J Am Acad Orthop Sur. 2009 Sep;17(9):591–600. doi: 10.5435/00124635-200909000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traynor K. Osteoarthritis guidelines take balanced approach to therapy. Am J Health Syst Pharm. 2012 Jun 1;69(11):906–8. doi: 10.2146/news120038. [DOI] [PubMed] [Google Scholar]
- 8.Michael JWP, Schluter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010 Mar 5;107(9):152–69. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H. MRI-based knee cartilage T2 measurements and focal knee lesions correlate with BMI – 36 month follow-up data from the Osteoarthritis initiative. Arthritis Care Res (Hoboken) 2012 May 23; doi: 10.1002/acr.20672. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, et al. Baseline mean and heterogeneity of MR cartilage T(2) are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years – data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2012 Jul;20(7):727–35. doi: 10.1016/j.joca.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2012 Feb;64(2):248–55. doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls – data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13(5):R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects – data from the osteoarthritis initiative. Skeletal Radiol. 2012 Jun;41(6):633–41. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2 – initial experience with 1-year follow-up. Radiology. 2011 Feb;258(2):505–14. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, et al. Prediction of glycosaminoglycan content in human cartilage by age, T1 rho and T2 MRI. Osteoarthritis Cartilage. 2011 Feb;19(2):171–9. doi: 10.1016/j.joca.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jazrawi LM, Alaia MJ, Chang G, FitzGerald EF, Recht MP. Advances in magnetic resonance imaging of articular cartilage. J Am Acad Orthop Sur. 2011 Jul;19(7):420–9. doi: 10.5435/00124635-201107000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster Questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999 Sep;58(9):563–8. doi: 10.1136/ard.58.9.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe F. Determinants of WOMAC function, pain and stiffness scores: evidence for the role of low back pain, symptom counts, fatigue and depression in osteoarthritis, rheumatoid arthritis and fibromyalgia. Rheumatology. 1999 Apr;38(4):355–61. doi: 10.1093/rheumatology/38.4.355. [DOI] [PubMed] [Google Scholar]
- 19.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011 Jan-Feb;31(1):37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Han ET, Busse RF, Majumdar S. In vivo T(1rho) mapping in cartilage using 3D magnetization-prepared angle-modulated partitioned k-space spoiled gradient echo snapshots (3D MAPSS) Magn Reson Med. 2008 Feb;59(2):298–307. doi: 10.1002/mrm.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004 Mar;12(3):177–90. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3 T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010 Jun;18(6):776–86. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stehling C, Liebl H, Krug R, Lane NE, Nevitt MC, Lynch J, et al. Patellar cartilage: T2 values and morphologic abnormalities at 3.0-T MR imaging in relation to physical activity in asymptomatic subjects from the osteoarthritis initiative. Radiology. 2010 Feb;254(2):509–20. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, et al. Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthritis Cartilage. 2010 Nov;18(11):1408–16. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor JR, editor. An Introduction to Error Analysis. 2. Sausalito, California: University Science Books; 1997. [Google Scholar]
- 26.McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992 Jul;51(7):844–9. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003 Feb;226(2):373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 28.Kornaat PR, Bloem JL, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006 Jun;239(3):811–7. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 29.Kornaat PR, Bloem JL, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the osteoarthritis initiative. Radiology. 2011 Nov;261(2):507–15. doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaisson CE, Gale DR, Gale E, Kazis L, Skinner K, Felson DT. Detecting radiographic knee osteoarthritis: what combination of views is optimal? Rheumatology (Oxford) 2000 Nov;39(11):1218–21. doi: 10.1093/rheumatology/39.11.1218. [DOI] [PubMed] [Google Scholar]
- 31.Elahi S, Cahue S, Felson DT, Engelman L, Sharma L. The association between varus-valgus alignment and patellofemoral osteoarthritis. Arthritis Rheum. 2000 Aug;43(8):1874–80. doi: 10.1002/1529-0131(200008)43:8<1874::AID-ANR25>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Lin J, Li R, Kang X, Li H. Risk factors for radiographic tibiofemoral knee osteoarthritis: the wuchuan osteoarthritis study. Int J Rheumatol. 2010;2010:385826. doi: 10.1155/2010/385826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009 Sep;36(9):4059–67. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regatte RR, Akella SVS, Lonner JH, Kneeland JB, Reddy R. T-1p relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T-1p with T-2. J Magn Reson Imaging. 2006 Apr;23(4):547–53. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 35.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T-2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004 Jun;22(5):673–82. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 36.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004 Aug;232(2):592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Han ET, Ma CB, Link TM, Newitt DC, Majumdar S. In vivo 3T spiral imaging based multi-slice T(1rho) mapping of knee cartilage in osteoarthritis. Magn Reson Med. 2005 Oct;54(4):929–36. doi: 10.1002/mrm.20609. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, et al. In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage. 2007 Jul;15(7):789–97. doi: 10.1016/j.joca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regatte RR, Akella SV, Wheaton AJ, Lech G, Borthakur A, Kneeland JB, et al. 3D-T-1 rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004 Jul;11(7):741–9. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]


