Abstract
Selective attention in the presence of distraction is a key aspect of healthy cognition. The underlying neurobiological processes, have not, however, been functionally well characterized. In the present study, we used functional magnetic resonance imaging to determine how ecologically relevant distracting noise affects cortical activity in 27 healthy adults during two versions of the visual sustained attention to response task (SART) that differ in difficulty (and thus attentional load). A significant condition (noise or silence) by task (easy or difficult) interaction was observed in several areas, including dorsolateral prefrontal cortex (DLPFC), fusiform gyrus (FG), posterior cingulate (PCC), and pre-supplementary motor area (PreSMA). Post-hoc analyses of interaction effects revealed deactivation of DLPFC, PCC, and PreSMA during distracting noise under conditions of low attentional load, and activation of FG and PCC during distracting noise under conditions of high attentional load. These results suggest that distracting noise may help alert subjects to task goals and reduce demands on cortical resources during tasks of low difficulty and attentional load. Under conditions of higher load, however, additional cognitive resources may be required in the presence of noise.
Keywords: Attention, dorsolateral prefrontal cortex, fusiform gyrus, pre-supplementary motor area, posterior cingulate, distraction
1. Introduction
The ability to selectively attend to relevant stimuli in the environment while ignoring salient distractors is a key component of cognition. At least 5,000 drivers are killed each year in the U.S. due to “inattention” (www.census.gov), including accidents caused by environmental distraction. Attention deficit hyperactivity disorder, for which symptoms include being easily distracted (Wolraich et al. 2006), exerts a “significant negative impact of quality of daily life, including work, social life, and relationships” (Rösler et al. 2010). In schizophrenia, the inability “to choose which sources of input should be attended” is a debilitating cognitive symptom and has recently been suggested as an essential biomarker for treatment intervention (Luck et al. 2012).
To a large extent, the effect of irrelevant noise on task performance has been investigated in terms of its deleterious consequences. Early work by Broadbent found that noise interfered with vigilance (Broadbent 1951), response speed (Broadbent 1957), and mental arithmetic (Broadbent 1958). Similar findings have since been reported using many different tasks and subject populations (Smith 1989). However, irrelevant noise may also facilitate performance, particularly during monotonous, repetitive tasks (Smith 1989; Suter 1989). Indeed, as initially proposed by Yerkes and Dodson (1909) and expanded upon by Zentall and Zentall (1983), the effect of task-irrelevant stimuli may depend on task difficulty, as appropriate levels of stimulation may be required for optimum performance.
The neuronal processes that underlie the proposed interaction between task difficulty and distraction have not been well characterized. Insight into this process can be gained, however, by examining two variants of an attention task, the Sustained Attention to Response Task (SART), which may be comparable except for their respective difficulties. In the SART, a subject is shown a series of numbers and instructed to press a button when he sees any number except for “3”; if he sees a “3,” the subject is instructed to withhold from responding. The task has a much lower frequency of “3’s” than other numbers; thus the primary task objective is to inhibit the natural tendency to button press after each stimulus presentation (O’Connell et al. 2004). The Fixed version of the SART, in which targets are presented (one at a time) in numerical order and the stop target is therefore predictable, is easy and requires fewer trial-by-trial attentional resources. The Random version, in which numbers are presented in random order, requires the subject to more fully process each stimulus to perform the task accurately and is therefore more difficult, requiring higher attentional load. Behavioral evidence for increased processing is an increase in reaction time for the Random SART.
fMRI studies have characterized the functional neuroanatomy of the Fixed and Random SART. Both versions have shown involvement of a frontal-parietal attention network that includes the dorsolateral prefrontal cortex (DLPFC) and inferior parietal lobule (Fassbender et al. 2004). DLPFC activation in particular is associated with top-down control processes as demonstrated via neuroimaging in other attention tasks (Banich et al. 2000; Cohen et al. 1988; Hager et al. 1998; Sturm et al. 1999). The Random SART showed additional activity in the inferior frontal gyrus and basal ganglia, likely reflecting its additional demands on response inhibition. The Random SART also showed increased activity in visual cortex, reflecting its demands on sensory processing (Fassbender et al. 2004).
A later imaging study used randomly presented “alerting tones” during the Random SART, and showed deactivation of the frontal portion of the attention network despite no change in performance (O’Connell et al. 2011). The decreased activation in this area was interpreted to reflect decreased need for top-down attentional control due to the cueing, alerting effect of the tones. In addition, increased activity in the left DLPFC with tones was observed during a control task in which the subject was instructed to press after every stimulus, suggesting an “orienting” response to the tones (Corbetta and Shulman 2002; O’Connell et al. 2011).
Decreased activity in the DLPFC with tones during the Random SART suggests that exogenous stimulation may reduce demands on cortical attention networks. However, a number of questions remain. The tones in the O’Connell et al. (2011) study were task-relevant; it is unclear if task-irrelevant stimulation would have the same effect on Random SART-associated activity. In addition, the neurobiological effects of task-irrelevant auditory stimulation during tasks of comparatively low difficulty, such as the Fixed SART, are unknown. Finally, the alerting tones in the O’Connell et al. (2011) study were intermittent (every 8–12s) and of the same frequency (2 khz) and duration (30ms); it is unclear whether constant noise would have the same effect, or conversely increase the burden on attentional processing. Indeed, based on previous studies that examine the effect of cross-modal distraction on attention, one might predict that constant noise would increase response in areas crucial for processing the attended modality (Roland 1982; Langner et al. 2011).
In the present study, using functional magnetic resonance imaging (fMRI), we compared the neurophysiological effects of task-irrelevant “urban noise” stimulation on the Random and Fixed SART. The “urban noise” is a mixture of talk radio, music, and conversation one might find on a crowded city street, and is designed to mimic real-world sounds (Tregellas et al. 2009). We formulated two hypotheses: 1) Relative to Fixed SART, Random SART would additionally recruit areas important for attentional, inhibitory, and sensory processing, because the Random version is more difficult and requires more resources than the Fixed version; 2) a significant Task×Noise interaction would be observed in areas important for attentional and sensory processing (e.g. the DLPFC and visual cortex), suggesting that the effect of noise may differ depending on attentional load.
2. Methods
2.1. Subjects
Twenty-seven healthy subjects participated in this study. Mean age was 37.07 (SD = 12.68), 13 females, 14 males. Subjects provided written informed consent approved by the University of Colorado Institutional Review Board.
2.2. Task design
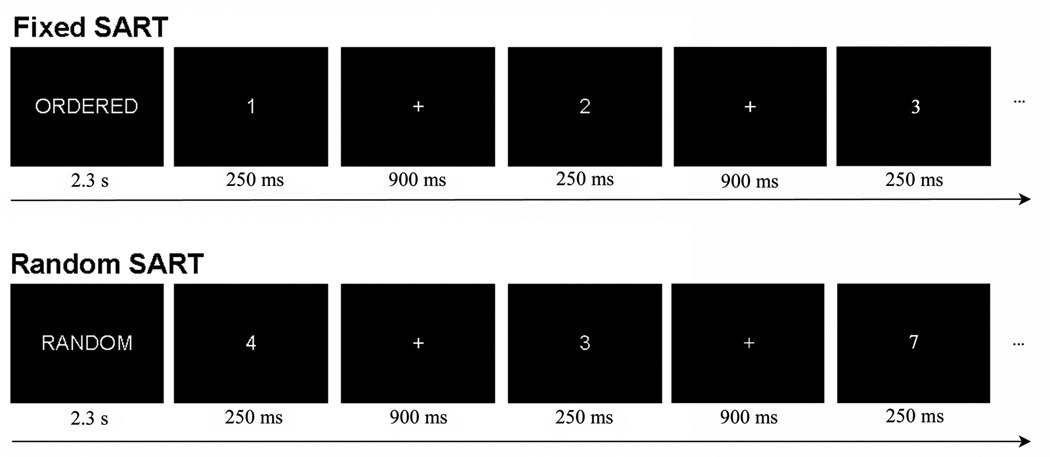
fMR images were obtained while subjects performed the Sustained Attention to Response Task (SART). Subjects were shown single-digit numbers presented one at a time, and instructed to press a button after every number except for the number “3,” in which case subjects were asked to withhold responding. The SART consisted of two conditions, Fixed SART and Random SART (Fig. 1). In the Fixed, or ‘Ordered’ condition, the numbers were presented in order, so the subject could predict when the no-go stimulus, or “3” will appear; in the Random condition, the numbers were presented pseudo-randomly. The subject was asked to respond as quickly and accurately as possible to help induce attentiveness.
Figure 1.
Schematic representation of the sustained attention to response task (SART). In the SART, the subject is required to button press after every number except for the number 3, in which case the subject withholds responding. Each number is presented for 250ms followed by a 900ms ITI. In the Fixed SART, the descriptive prompt “ORDERED” was displayed for 2.3s, followed by single-digit numbers presented in order (e.g. 1, 2, 3, 4…). In the Random SART, the prompt “RANDOM” was displayed for 2.3s, followed by single-digit numbers presented pseudo-randomly.
SART stimuli were presented as a block design, with ‘Ordered’ and ‘Random’ blocks pseudo-randomly interspersed throughout a session (Fig 1.). All stimuli were presented through MR-compatible goggles (Resonance Technology, Inc.). A 2.3s identifier cue (i.e. Ordered (Fixed) or Random) was presented before the first block, as well as each time the block switched from Ordered to Random (or vice-versa). The length of each block was 12.65s. Blocks that were preceded by an instruction had 9 trials; blocks that were not preceded by an instruction had 11 trials, thus making each block equal in duration. Each trial consisted of a 250ms stimulus (the single-digit number) followed by a 900ms intertrial interval; during the intertrial interval a fixation cross was presented to orient the subject. Number font was pseudo-randomized (40, 72, 94, 100, 120 type) as to increase the difference in feature detection processing requirements between Fixed and Random SART. Due to the predictability of Fixed SART, subjects may be able to correctly respond or withhold responding reflexively to the presence of any visual stimulus; however, the unpredictability of Random SART requires subjects to focus on specific stimulus features before making the appropriate response. Each session consisted of 56 blocks of trials and lasted for approximately 12m. Baseline data was collected from a 37.95s fixation period at the beginning and end of each session, and two 12.65s fixation sessions near the middle. Subjects were given a brief practice session outside of the scanner to introduce them to the task parameters.
To determine the effect of noise distraction on the functional neuroanatomy of the SART, we overlaid previously developed 80dB “urban white noise” distractors (Tregellas et al. 2009) during half of the blocks. Noise was presented in the magnet through MR-compatible headphones (Resonance Technology, Inc.). The “urban white noise” consisted of a mixture of audio clips, including segments of radio shows, classical music pieces, and background conversation (described fully in Tregellas et al. 2009). Volumes of all of these elements were mixed so that no one element was readily identifiable. The subjective experience of the sound mixture was that of standing in a busy crowd of people, in which multiple conversations were occurring, with a low level of indistinguishable background music and other sounds one might experience in a busy urban setting.
The primary performance measure on the SART was percent commission errors, defined as the percent of incorrect responses on no-go trials, i.e the percent of button presses following presentation of the number “3.” Pilot studies were first conducted outside the scanner to ensure that noise would not affect any performance measure. fMRI data could then be analyzed without the potentially contaminating effects of performance differences.
2.3. MR parameters
Functional scans were collected using a clustered volume approach as described previously (Edmister et al. 1999; Tregellas et al. 2009). Use of the clustered volume approach allowed stimuli to be presented while minimizing scanner noise. This technique has been shown to substantially improve signal detection in fMRI experiments using auditory stimuli (Edmister et al. 1999).
Studies were performed with a 3T GE Signa MR system using a standard quadrature head coil. Functional images were acquired with a gradient-echo T2* Blood Oxygenation Level Dependent (BOLD) contrast technique, with TR = 12650ms (as a clustered volume acquisition of 2000ms, plus an additional 10650ms silent interval), TE = 30ms, FOV = 220 mm2, 642 matrix, 38 slices, 3.5 mm thick, 0.5 mm gap, angled parallel to the planum sphenoidale. Additionally, one IR-EPI (TI = 505ms) volume was acquired to improve spatial normalization (see ‘Data Analysis’ below).
2.4. Data analysis
Data were analyzed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London). Data from each subject were realigned to the first volume, normalized to the Montreal Neurological Institute template, using a gray-matter-segmented inversion recovery echo planar image (IR-EPI) as an intermediate to improve coregistration between images (see Anbeek et al. (2005) for effectiveness of IR-EPI gray matter segmentation), and smoothed with an 8-mm FWHM Gaussian kernel. A 196-s high pass filter was applied to remove low-frequency fluctuation in the BOLD signal.
To account for both within-group and within-subject variance, a whole-brain random effects analysis was implemented. Parameter estimates were generated for each individual in a first-level analysis. First-level effects were modeled with a double-gamma function, without temporal derivatives, using the general linear model in SPM8. Motion regressors were included to account for motion-induced artifacts. Task-associated contrast images were generated for the four task conditions, FixedSilent, FixedNoise, RandomSilent, and RandomNoise. Fixation periods were used as an implicit baseline. The long TR required for a clustered volume acquisition (12.65s) resulted in 12 volumes acquired per condition, in addition to 8 volumes acquired for the fixation baseline. The resulting SPM contrast images were entered into a second-level, repeated measures ANOVA in SPM8. Results were considered significant at a cluster level threshold of p < 0.05 (FWE corrected for multiple comparisons) combined with a voxel level threshold of p < 0.001 (uncorrected). Whole-brain significant interaction effects were further explored with post-hoc paired t-tests using signal values extracted from an 8mm diameter sphere centered at the peak of activation. These values were obtained using the Marsbar toolbox in SPM8 (Brett et al. 2002).
In addition to the whole-brain analysis, an a priori hypothesis of response in DLPFC was evaluated using an anatomically defined region of interest (ROI) from WFU Pickatlas (Maldjian et al. 2003). The DLPFC ROI included consisted of Brodmann Areas 9 and 46 combined, excluding the superior frontal gyrus (as used by Tregellas et al. 2007). The mean response for all voxels in the anatomical ROI was extracted using the Marsbar toolbox.
3. Results
3.1. Effect of Noise on Fixed and Random SART Performance
As anticipated, no significant difference was observed between errors of commission on the Fixed SART in silence and noise (t =0.63, df = 26, p = 0.53) or the Random SART in silence and noise (t = 0.40, df = 26, p = 0.69). No significant difference was observed between omission errors in the Fixed SART in silence and noise (t = 0.12, df = 26, p = 0.90) or the Random SART in silence and noise (t = 0.18, df =26, p = 0.85). Likewise, no significant difference was observed between reaction times in the Fixed SART in silence and noise (t = 0.31, df = 26, p = 0.76) or the Random SART in silence and noise (t = 0.11, df = 26, p = 0.91) (Tables 1a–b).
Table 1.
| a. Behavioral Data, Fixed SART | |||
|---|---|---|---|
| Measure | Fixed Silent | Fixed Noise | p |
| % Errors of Commission | 4.42 ±1.01 | 3.69 ± 1.04 | 0.50 |
| % Errors of Omission | 4.13 ± 1.63 | 3.28 ± 1.37 | 0.31 |
| Reaction Time (ms) | 297 ± 7.87 | 289 ± 8.38 | 0.13 |
| b. Behavioral Data, Random SART | |||
|---|---|---|---|
| Measure | Random Silent | Random Noise | p |
| % Errors of Commission | 22.55 ± 3.71 | 21.52 ± 3.77 | 0.78 |
| % Errors of Omission | 1.03 ± 0.084 | 0.92 ± 0.52 | 0.74 |
| Reaction Time (ms) | 374 ± 15.36 | 373 ± 14.41 | 0.44 |
3.2. fMRI Results: Random > Fixed SART, Collapsed Across All Conditions
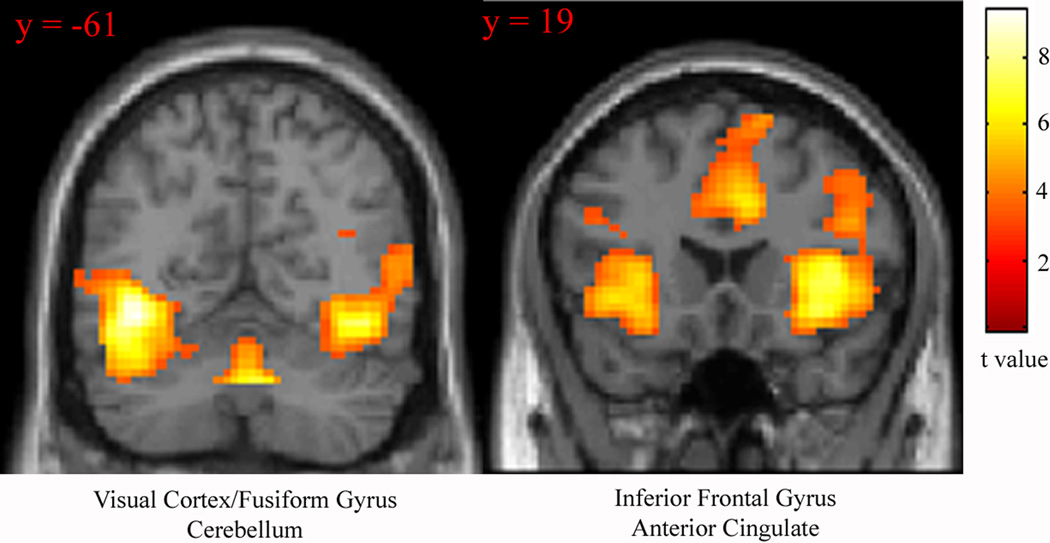
To test the hypothesis that the Random SART requires more visual processing than the Fixed SART, as well as to determine which additional resources were required for the Random version, the contrast Random > Fixed SART (collapsed across noise and silence conditions) was examined. Significant activation was observed in primary and accessory visual cortex, cingulate gyrus, inferior frontal gyrus, insula, cerebellum, and thalamus (Figure 2, Table 2).
Figure 2.
Regions recruited for the contrast Random > Fixed SART, collapsed across silence and noise conditions. Statistical parametric maps thresholded at p < 0.001 for visualization. Images displayed in neurologic convention (R on R).
Table 2.
MNI coordinates and statistics for the contrast Random > Fixed SART, collapsed across all noise and silence conditions. Regions grouped by contiguous clusters. T values drawn from local maxima.
| Brain Areas | Hemi | Brodmann Area | t value | x,y,z (mm) | Cluster (voxel size) |
|---|---|---|---|---|---|
| fusiform gyrus | L | 19 | 9.16 | −39, −64, −8 | 2395 |
| middle occipital gyrus | L | 18 | 9.07 | −39, −91, 1 | |
| middle occipital gyrus | L | 18 | 8.47 | −30, −97, −2 | |
| middle temporal gyrus | R | 37 | 7.83 | 45, −64, 11 | 1594 |
| superior temporal gyrus | R | 22 | 7.69 | 39, −58, 11 | |
| lingual gyrus | R | 17 | 7.28 | 21, −91, 4 | |
| inferior frontal gyrus | R | 45 | 7.37 | 42, 23, 4 | 2119 |
| inferior fronal gyrus | R | 47 | 7.13 | 33,26,−2 | |
| inferior frontal gyrus | R | 47 | 7.03 | 36, 20, −8 | |
| inferior frontal gyrus | L | 45 | 6.70 | −30, 26, 1 | 469 |
| basal ganglia | L | 4.61 | −18,2, −11 | ||
| anterior cingulate | R | 32 | 5.80 | 9, 23, 31 | 716 |
| thalamus | R | 5.65 | 3, −31, 1 | 371 | |
| thalamus | L | 5.59 | 3, −13, 10 |
3.3. fMRI Results: Fixed > Random SART, Collapsed Across All Conditions
Whole brain analysis of the contrast Fixed > Random SART revealed significant activation of the precuneus (peak (x,y,z) 9, −70, 52, t = 5.93, k = 243 voxels).
3.4. fMRI Results: Interaction contrast
To test the hypothesis that task-irrelevant noise stimuli is associated with contrasting effects on Random and Fixed SART, the interaction contrast (noise × task) was examined. Whole-brain analysis revealed significant interaction effects in middle frontal gyrus, medial frontal gyrus/pre-supplementary motor area (PreSMA), cerebellum, fusiform gyrus, midbrain and posterior cingulate (Figures 3A–C, Table 3).
Figure 3.
Brain regions showing significant response for the interaction contrast ((Random SART during noise > Random SART during silence > (Fixed SART during noise > Fixed SART during silence)). Statistical parametric maps thresholded at p < 0.001 for visualization. Images displayed in neurologic convention (R on R).
Table 3.
MNI coordinates and statistics for brain regions showing greater responses for the whole brain interaction contrast ((Random SART during noise > Random SART during silence > (Fixed SART during noise > Fixed SART during silence)). Regions grouped by contiguous clusters. T values drawn from local maxima.
| Brain Areas | Hemi | Brodmann Area | t value | x,y,z (mm) | Cluster (voxel size) |
|---|---|---|---|---|---|
| cerebellum | R | 5.22 | 42, −64, −20 | 132 | |
| fusiform gyrus | R | 19 | 4.76 | 33, −73, −14 | |
| midbrain | L | 5.00 | −3, −19, −8 | 113 | |
| midbrain | R | 4.82 | 9, −13, −2 | ||
| basal ganglia | R | 4.35 | 21, −4,1 | ||
| posterior cingulate | R | 29 | 4.97 | 6, −46, 16 | 119 |
| posterior cingulate | L | 29 | 3.91 | −6. −49, 13 | |
| pre-SMA | L | 6 | 4.37 | −9, 5, 61 | 246 |
| pre-SMA | R | 6 | 4.04 | 6, −13, 64 | |
| superior frontal gyrus | L | 8 | 3.78 | −6, 17, 52 |
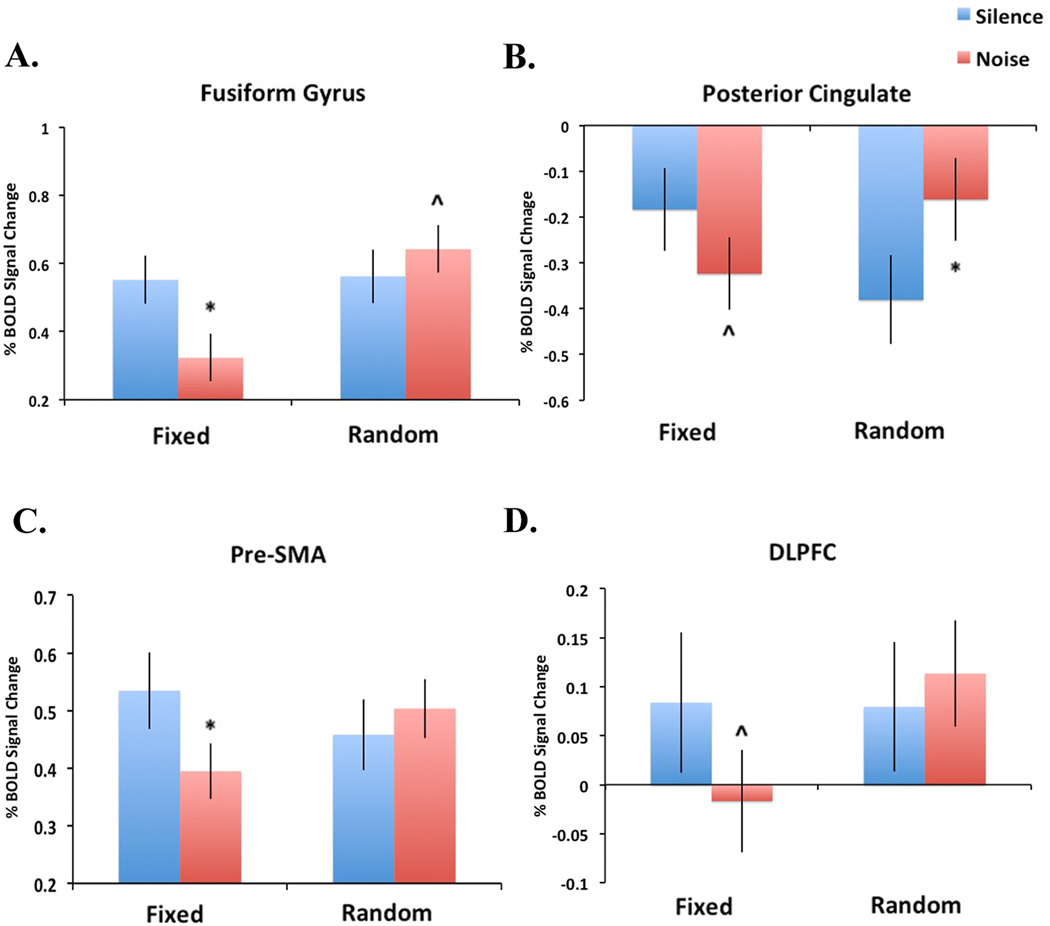
To further explore these results, post-hoc analyses of significant interaction effects were performed (Figure 4). These tests revealed significantly decreased response in the fusiform gyrus during the FixedNoise condition relative to FixedSilent (t = 3.77, p = 0.001) and a nearly significant increase in response in the fusiform gyrus during the RandomNoise condition relative to RandomSilent (t = 1.86, p = 0.07). In the posterior cingulate, a nearly significant decrease in response was observed during the FixedNoise condition compared to FixedSilent (t = 1.94, p = 0.06) and significantly increased response was observed during the RandomNoise condition relative to RandomSilent (t = 2.98, p < 0.01). In the pre-SMA, significantly decreased response was observed in the FixedSilent condition relative to FixedNoise (t = 2.86, p < 0.01) and no difference was observed between the RandomNoise and RandomSilent conditions (t = 1.17, p = 0.25).
Figure 4.
Direction of interaction effects. In figures 4A–C, the Y-axis represents the percent BOLD signal change (relative to baseline) extracted from an 8mm sphere centered at the peak of significant clusters reported in Figure 3. In figure 4D, the Y-axis represents the mean voxelwise percent BOLD signal change (relative to baseline) for the DLPFC anatomical ROI. Error bars represent the standard error. *p<0.05; ^p<0.1.
3.5 fMRI Results: DLPFC ROI
To test the hypothesis that task-irrelevant noise stimuli is associated with contrasting effects on Random and Fixed SART in the DLPFC, the mean voxelwise percent signal change was examined for all four task conditions from an anatomically-defined ROI of the left DLPFC. Using this analysis, a significant interaction effect was observed in the left DLPFC (t = 3.37, df = 26, p = 0.002; peak coordinate (x,y,z) −27, 38, 46; Figure 3D). Post-hoc analysis of the direction of this effect revealed a nearly significant decrease in response in the DLPFC during the FixedNoise relative to FixedSilent condition (t = 2.00, p = 0.056) but no difference in response between the RandomNoise and RandomSilent conditions (t = 0.7, p = 0.49).
4. Discussion
4.1. General discussion
The primary goal of the present study was to compare the effects of task-irrelevant, “real-world” auditory stimulation on versions of an attention task that differed in their difficulty/attentional load. The principal finding was a significant task (easy or difficult) by condition (noise or silent) interaction in a network of posterior and anterior brain regions. Post-hoc tests to describe the interaction showed decreased response during noise for the easy Fixed SART in the fusiform gyrus, posterior cingulate, preSMA, and DLPFC. Increased response in the fusiform gyrus and posterior cingulate during noise for the more difficult Random SART was also observed; noise did not affect response in the DLPFC or preSMA for the more difficult task. These findings suggest that task-irrelevant stimuli may have dramatically different effects on attention-related processing, depending on the difficulty of the task and the consequent burden on the attentional processes being utilized. In the following sections, results are discussed with regard to specific regions and their functions.
4.2. Response differences between Random and Fixed SART
The contrast Random > Fixed SART revealed robust response in a network of posterior and anterior regions, including areas involved in primary and accessory visual processing (occipital gyrus, fusiform gyrus), motor inhibition and target detection (inferior frontal gyrus) (Aron 2010; Hampshire et al. 2009), and conflict monitoring/error detection (anterior cingulate) (Botvinick et al. 2004; Kerns et al. 2004; Totah et al. 2009). Recruitment of these areas is not surprising considering the nature of the two tasks: relative to the Fixed SART, the Random SART requires more precise processing of task-related external stimuli (i.e. numbers), results in more commission errors, and requires a higher level of response-generated motor inhibition (O’Connell et al. 2008). Robust activity in occipital areas in particular suggests that the Random SART, relative to the Fixed SART, requires more visual processing as previously described.
The contrast Fixed > Random SART revealed a significant activation of the precuneus. The precuneus is associated with episodic memory and introspection (Cavanna and Trimble 2006), and is active during retrieval of imagined pictures (Lundstrom et al. 2003). Increased activity may represent anticipatory mental imagery of the predictable stimulus targets during the Fixed SART, or intrusion of task-unrelated mental representations.
4.3. Neurobiological effect of noise under low load conditions (Fixed SART)
In the present study, the addition of noise during the Fixed SART deactivated the left DLPFC. Along with the dorsal anterior cingulate (and other regions), a number of fMRI and PET studies have implicated this region in top-down attentional control (Banich et al. 2000; Botvinick et al. 2004; Carter et al. 1999; Cohen et al. 1988; Hager et al. 1998; Sturm et al. 1999; Weissman et al. 2006). The finding that noise decreased recruitment of this area during the Fixed SART without impairing performance suggests that noise may reduce the need for DLPFC-mediated top-down control of attention during the task. For comparison, previous work by O’Connell et al. (2011) showed deactivation of the right DLFPC during alerting tones during the Random SART; an ROI placed in the right DLPFC in the present study did not show a significant task×noise interaction (data not shown). Vanderhasselt and De Raedt (2009) have proposed that the left DLPFC may be involved in maintaining a task set, whereas the right DLPFC may be important for response conflict-driven changes in top-down control. Accordingly to this interpretation, deactivation of left DLPFC suggests a reduced dependence on cortical resources to maintain the Fixed SART task set (i.e. stop on “3”) during noise. Modulation of the right DLPFC during the Random SART is consistent with an increase in response conflict in this version of the task due to the unpredictability of the stimuli.
Noise was also associated with deactivation of the posterior cingulate during the Fixed SART. Along with the precuneus, the PCC is a component of the default mode network (Buckner et al 2008). Activation of the PCC is positively correlated with lapses in attention (Weissman et al. 2006) and the number of “task-independent” thoughts (Buckner et al. 2008). Deactivation of this region may reflect increased ability of the subject to stay on task during noise.
Deactivation was also observed during noise in the preSMA in the Fixed SART. PreSMA activation is associated with motor readiness (Cunnington et al. 2005), preparation for motor response inhibition (Aron 2011; Swann et al. 2011), and may be inversely correlated with response threshold (i.e. the amount of diagnostic information required before making an action) (Forstmann et al. 2008; Mansfield et al. 2011). The preSMA is subject to modulation by the DLPFC (Rosenberg-Katz et al. 2012) through which it may influence all of the above processes. In regards to the present findings, the functional implications of deactivation of this region during noise are unclear. It may simply be a downstream effect of reduced activity in the DLPFC. Alternatively, it may reflect increased response threshold induced by the noise; this possibility seems unlikely, however, considering that noise did not significantly change reaction time. Finally, it may simply reflect a reduced need for voluntary motor planning, as deactivation occurred during noise despite no change in any performance measure. Additional studies are needed to determine the behavioral significance of preSMA deactivation during tasks that require low attentional load.
The fusiform gyrus also showed reduced activation during noise. The fusiform gyrus, an accessory visual processing area, is frequently recruited during attention tasks, particularly when subjects are instructed to pay attention to a visual modality while ignoring task-irrelevant stimuli from a different modality (Langner et al. 2011). In this framework, the observed result is somewhat surprising. However, the Fixed SART may already require limited processing of external stimuli (i.e. each number) due to its predicable nature; thus, additional visual processing resources may not be required to perform the task during auditory stimulation. Indeed, the finding that activity in fusiform gyrus was decreased during noise may reflect subjects’ (voluntarily or involuntarily) processing the auditory stimulation while “tuning out” the visual stimuli during the Fixed SART. The latter finding is consistent with the view of Lavie (2005) who proposed that under conditions of low load, “any capacity not taken up in perception of task-relevant stimuli would ‘spill over’ to the perception of task-irrelevant distractors.”
4.4. Neurobiological effect of noise under high load conditions (Random SART)
Surprisingly, noise did not affect response in the Random SART. Our findings did not support the hypothesis that the DLPFC would be recruited during the Random SART in noise relative to silence. A small increase in fusiform gyrus activity was observed during noise on the Random SART, consistent with the reported effect of cross-modal distraction on regions essential for processing the attended modality (Langner et al. 2011). Increased PCC activity was also observed during noise, which may reflect increased resource allocation towards cued, anticipatory processing of visual stimuli (Small et al. 2003). Future studies are needed to determine if degraded stimuli (e.g. faded or disjointed numbers) further increase the difficulty/attentional load for the Random SART and thus enhance the effect of the noise distractor.
4.5. Alternative interpretation #1: Effect of noise is arousal-dependent
The presumption employed in the present study is that the Random SART is more difficult and requires a higher processing load than the Fixed SART, and the differences in the functional effects of noise interpreted as such. However, a reasonable alternative view is that the Random SART is simply more arousing than the Fixed SART, and that the effect of noise is a function of arousal rather than (or in addition to) task difficulty, per se.
The Random SART has been proposed to be more arousing than the Fixed SART (Robertson and Garavan 2004), due to the difference in stimulus predictability between the two tasks. Indeed, it has been proposed that the primary challenge of the Fixed SART is that the subject may have difficulty staying alert (Roberson and Garavan 2004). A relationship between arousal and performance has been shown in many studies, starting with work by Yerkes and Dodson (1908), who first demonstrated that performance has an inverted-U shaped dependence on arousal. In this framework, the urban noise may arouse subjects, pushing them closer to an “optimal” performance level during the Fixed SART, and thereby reducing the need for DLPFC-mediated attentional control. In interpreting such results, it may be useful to draw an analogy to the arousing effect of turning on the radio during a long drive on a deserted highway late at night. Future studies may combine electroencephalography (to measure arousal) with fMRI to determine the relationships between exogenous noise, arousal, SART performance, and SART-associated brain activity.
4.6. Alternative interpretation #2: Endogenous attention, exogenous attention and the SART
According to a recent hypothesis proposed by Chun et al. (2010), the construct of attention may be divided into two subtypes: endogenous, or internal attention, and exogenous, or external attention. Endogenous attention refers to “the selection, modulation, and maintenance of internally generated information, such as task rules (and) responses.” Exogenous attention is defined as “selection and modulation of sensory information, as it initially comes into the mind, generally in a modality-specific representation…(in other words) perceptual attention.” Based on these definitions, the Fixed SART may be more dependent upon internal attention mechanisms. Its predictability requires little concentration on the details of each stimulus, given that the next number is already known before it is presented. Rather, its primary challenge lies in staying engaged on the task despite its monotonous nature (Johnson et al. 2007). In contrast, the Random SART may be more dependent upon exogenous attention. It requires more precise concentration on each external stimulus to complete the task accurately.
In this context, noise may be playing an entirely different role on each version of the SART. In the boring, predictable Fixed SART, noise may represent an alerting stimulus, helping to reorient the subject towards task goals. The subject may thus become less dependent on internal attention processes (perhaps represented by the DLPFC), explaining the decrease in activity in this region (among others). The noise may not interfere with exogenous attention networks as the Fixed SART is only minimally dependent on these processes. However, in the Random SART, noise may represent a competing external stimulus, increasing the burden on cortical areas (e.g. the fusiform gyrus) and/or recruiting additional filtering mechanisms.
4.7. Limitations
The clustered, or “sparse” acquisition technique was employed in order to minimize the confounding effect of scanner noise (Edmister et al. 1999; Talavage and Hall 2012). However, use of the technique reduced the number of volumes acquired during scanning and may have increased the likelihood of Type II (false negative) error. It is possible that Type II error may explain the lack of a significant interaction effect in the whole-brain analysis (after correcting for multiple comparisons). In support of this interpretation, a significant interaction effect was observed in the DLPFC using an alternative approach (comparison of mean response under each condition using an anatomical ROI).
Additionally, although we have examined top-down control (or lack thereof) by focusing on activity in the DLPFC, it is possible that top-down control is represented by activity in other areas that the present study is underpowered to detect. Future studies using functional connectivity may be useful to map the cortical substrate upstream of sensory cortex recruitment during distracting conditions.
4.8. Conclusions
In conclusion, the present study revealed a significant interaction between attention task difficulty/load and distracting noise on cortical activity in a network of brain areas. Post-hoc tests revealed decreased response during noise in a network of brain regions under conditions of low difficulty/load, as well as an increased response in visual processing areas during noise under conditions of higher difficulty/load. Future studies may examine the neurobiological effects of ecologically relevant noise distraction in clinical populations.
Highlights.
We examined the effects of distracting urban noise on the neurobiology of attention
Noise decreased activity in anterior and posterior areas under low attentional load
Noise increased activity in posterior areas under high load
Possible roles for arousal and type of attention (external/internal) are discussed
Acknowledgements
The authors thank Debra Singel for help with fMRI data acquisition. The research was supported by the VA Biomedical Laboratory and Clinical Science Research and Development Service, the National Association for Research in Schizophrenia and Affective Disorders (NARSAD) and the Blowitz-Ridgeway Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Contributor Information
Jason Smucny, Email: Jason.Smucny@ucdenver.edu.
Donald C. Rojas, Email: Don.Rojas@ucdenver.edu.
Lindsay C. Eichman, Email: Lindsay.Eichman@ucdenver.edu.
Jason R. Tregellas, Email: Jason.Tregellas@ucdenver.edu.
References
- Anbeek P, Vincken KL, van Bochove GS, van Osch MJ, van der Grond J. Probablistic segmentation of brain tissue in MR imaging. NeuroImage. 2005;27(4):795–804. doi: 10.1016/j.neuroimage.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Aron AR. From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biol Psychiatry. 2011;69(12):e55–e68. doi: 10.1016/j.biopsych.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, et al. fMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci. 2000;12(6):988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox (abstract) Available on CD-ROM in NeuroImage. 2002;16(2) [Google Scholar]
- Broadbent DE. Noise, paced performance and vigilance tasks. Med Res Council, App Psych Res Unit Rep. 1951;165(51):8. doi: 10.1111/j.2044-8295.1953.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Effects of noises of high and low frequency on behaviour. Ergonomics. 1957;1:21–29. [Google Scholar]
- Broadbent DE. Effect of noise on an "intellectual" task. J Ac Soc Am. 1958;30:824–827. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioral correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chun MM, Golomb JD, Turk-Browne NB. A taxonomy of internal and external attention. Ann Rev Psychol. 2011;62:73–101. doi: 10.1146/annurev.psych.093008.100427. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Semple WE, Gross M, Holcomb HH, Dowling MS, Nordahl TE. Functional localization of sustained attention: Comparison to sensory stimulation in the absence of instruction. Neuropsych, Neuropsychol, & Beh Neurol. 1998;1:3–20. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Moser E. Premovement activity of the presupplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Hum Mov Sie. 2005;24(5–6):644–656. doi: 10.1016/j.humov.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum Brain Mapp. 1999;7(2):89–97. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javit DC, Roberston IH, et al. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Cog Brain Res. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, et al. Striatum and preSMA facilitate decision-making under time pressure. Proc Natl Acad Sci USA. 2008;105(45):17538–17542. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon J, Eysenck MW. Distraction and cognitive performance. Eur J Cog Psych. 1989;1(2):161–179. [Google Scholar]
- Hager F, Volz H-P, Gaser C, Mentzel H-J, Kaiser W, Sauer H. Challenging the anterior attentional system with a continuous performance task: a functional magnetic resonance imaging approach. Eur Arch Psychiatry Clin Neurosci. 1998;248:161–170. doi: 10.1007/s004060050034. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Thompson R, Duncan J, Owen AM. Selective tuning of the right inferior frontal gyrus during target detection. Cog Affect Behav Neurosci. 2009;9(1):103–112. doi: 10.3758/CABN.9.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Robertson KH, Kelly SP, Silk TJ, Barry E, Daibhis A, et al. Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropscyhologia. 2007;45(10):2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Boers F, Sturm W, Willmes K, Eickhoff SB. Modality-specific perceptual expectations selectively modulate baseline activity in auditory, somatosensory, and visual cortices. Cereb Cortex. 2011;21(12):2850–2862. doi: 10.1093/cercor/bhr083. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused? Selective attention under load. Trends Cogn Sci. 2005;9(2):75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Ford JM, Sarter M, Lustig C. CNTRICS final biomarker selection: Control of attention. Schizophr Bull. 2012;38(1):53–61. doi: 10.1093/schbul/sbr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchetectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mansfield EL, Karayanidis F, Jamadar S, Heathcote A, Forstmann BU. Adjustments of response threshold during task switching: a model-based functional magnetic resonance imaging study. J Neurosci. 2011;31(41):14688–14692. doi: 10.1523/JNEUROSCI.2390-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell C, Manly T, Robertson IJ, Hevenor SJ, Levine B. An fMRI of sustained attention with endogenous and exogenous engagement. Brain Cogn. 2004;54(2):133–135. doi: 10.1016/s0278-2626(03)00268-9. [DOI] [PubMed] [Google Scholar]
- O’Connell C, Roberston IH, Levine B. The prosthetics of vigilant attention: random cuing cuts processing demands. Neuropsychology. 2011;25(4):535–543. doi: 10.1037/a0022767. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Garavan H. Vigilant Attention. In: Gazzaniga Michael S., editor. The Cognitive Neurosciences. third ed. Cambridge, MA: MIT Press; 2004. pp. 563–578. [Google Scholar]
- Roland PE. Cortical regulation of selective attention in man. A regional cerebral blood flow study. J Neurophysiol. 1982;48:1059–1078. doi: 10.1152/jn.1982.48.5.1059. [DOI] [PubMed] [Google Scholar]
- Rosenberg-Katz K, Jamshy S, Singer N, Podlipsky I, Kipervasser S, Andelman F, et al. Enhanced functional synchronization of medial and lateral PFC underlies internally-guided action planning. Front Hum Neurosci. 2012;6:79. doi: 10.3389/fnhum.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösler M, Casas M, Konofal E, Buitelaar J. Attention deficit hyperactivity disorder in adults. World J Biol Psychiatry. 2010;11(5):684–698. doi: 10.3109/15622975.2010.483249. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam M-M. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. NeuroImage. 2003;18(3):633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Smith A. A review of the effects of noise on human performance. Scand J Psychol. 1989;30(3):185–206. doi: 10.1111/j.1467-9450.1989.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, et al. Functional anatomy of intrinsic alertness: Evidence for a fronto-parietalthalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Suter AH. Tech. Mem. 3-89. U.S. Army Human Engineering Lab.; Aberdeen Proving Ground, MD: 1989. The effects of noise on performance. [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: electrophysiological responses and functional and structural connectivity. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavage TM, Hall DA. How challenges in auditory fMRI led to general advancements for the field. Neuroimage. 2012;62(2):641–647. doi: 10.1016/j.neuroimage.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NKB, Kim YB, Homayoung H, Boghaddam B. Anterior cingulate neurons represent errors and prepatory attention within the same behavioral sequence. J Neurosci. 2009;29(20):6418–6426. doi: 10.1523/JNEUROSCI.1142-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R. Increased hemodynamic response in the hippocampus, thalamus, and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92(1–3):262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Ellis J, Shatti S, Du YP, Rojas DC. Increased hippocampal , thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. Am J Psychiatry. 2009;166(3):354–360. doi: 10.1176/appi.ajp.2008.08030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt M-A, De Raedt R. Dorsolateral prefrontal cortex and Stroop performance: taclking the lateralization. Psychonomic Bulletin and Rev. 2009;16(3):609–612. doi: 10.3758/PBR.16.3.609. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberst KC, Visscher KM, Woldorff MG. The neural basis of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wolraich ML. Attention-deficit hyperactivity disorder. Semin Pediatr Neurol. 2006;13(4):279–285. doi: 10.1016/j.spen.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Yerkes RB, Dodson JH. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol and Psych. 1908;18:459–482. [Google Scholar]
- Zentall SS, Zentall TR. Optimal stimulation: A model of disordered activity and performance in normal and deviant children. Psych Bull. 1983;94(3):446–471. [PubMed] [Google Scholar]
Web References
- [Date last accessed: May 22, 2012]; http://www.census.gov/compendia/statab/2012/tables/12s1109.pdf.