Abstract
Purpose
Hydroxylation of the antidepressant and smoking deterrent drug bupropion is a clinically important bioactivation and elimination pathway. Bupropion hydroxylation is catalyzed selectively by cytochrome P4502B6 (CYP2B6). CYP2B6-catalyzed bupropion hydroxylation has been used as an in vitro and in vivo phenotypic probe for CYP2B6 activity and CYP2B6 drug interactions. Bupropion is chiral, used clinically as a racemate, and disposition is stereoselective. Nevertheless, it is unknown whether CYP2B6-catalyzed bupropion hydroxylation is stereoselective.
Methods
Hydroxylation of racemic bupropion by recombinant CYP2B6 and human liver microsomes was evaluated using a stereoselective assay.
Results
At therapeutic concentrations, hydroxylation of (S)-bupropion was 3-fold and 1.5-greater than (R)-bupropion, respectively, by recombinant CYP2B6 and human liver microsomes. In vitro intrinsic clearances were likewise different for bupropion enantiomers.
Conclusions
Stereoselective bupropion hydroxylation may have implications for the therapeutic efficacy of bupropion as an antidepressant or smoking cessation therapy, and for the use of bupropion as an in vivo phenotypic probe for CYP2B6 activity.
Keywords: bupropion, cytochrome P4502B6, stereochemistry, CYP2B6
Introduction
The antidepressant and smoking cessation drug bupropion (Fig 1) is extensively metabolized, primarily via t-butylhydroxylation to hydroxybupropion, and non-P450-dependent reduction to erythro- and threohydrobupropion (1). Hydroxybupropion is an active metabolite which contributes to the antidepressant effect of the parent drug (2, 3). Hydroxybupropion has a longer elimination half-life than the parent drug, peak cerebrospinal fluid and plasma concentrations exceed those of bupropion by 4- to 7-fold, and the plasma metabolite/parent area under the curve ratio is approximately ten or greater after a single dose (4, 5).
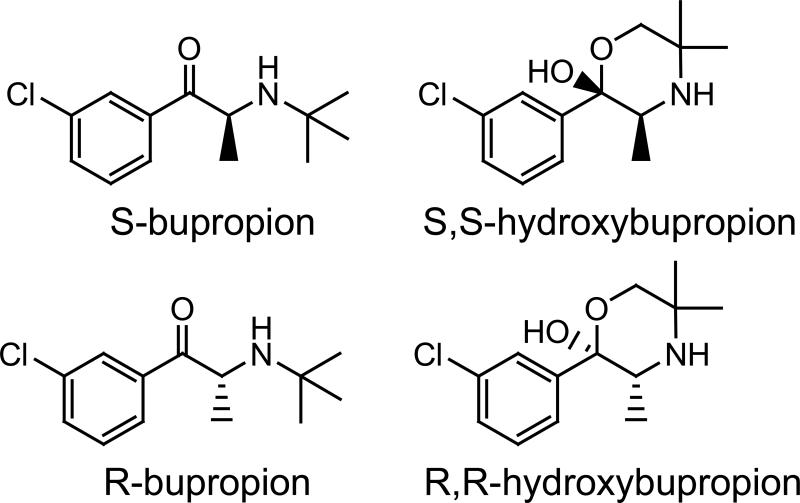
Figure 1.
Structures
Bupropion is used clinically as a racemate. Hydroxylation of the enantiomers results in the creation of a second chiral center, hence there is the potential for four diastereomeric hydroxylated metabolites (6). Nonetheless, only (S,S)- and (R-R)-hydroxybupropion have been found in plasma in humans, presumably due to steric hinderance precluding formation of (R,S) and (S,R)- hydroxybupropion. The hydroxybupropion enantiomers differ significantly in their pharmacologic effects. For example, (S,S)-hydroxybupropion was orders of magnitude more potent than (R,R)-hydroxybupropion in a mouse depression model and in antagonism of acute nicotine effects in mice, and as an inhibitor of monoamine uptake and nicotinic acetylcholine receptor antagonism. The affinity of (S,S)-hydroxybupropion at the dopamine transporter comparable to that of bupropion (2, 3).
Clinical bupropion disposition is stereoselective. Plasma concentrations of (R,R)-hydroxybupropion are approximately 20-fold greater than (S,S)-hydroxybupropion (6-8). In contrast, plasma concentrations of (R)-bupropion are only about 2-fold greater than those of (S)-bupropion (8).
Several investigations have established that racemic bupropion hydroxylation is catalyzed exclusively by recombinant and human liver microsomal CYP2B6 (9-11). CYP2B6 genetic polymorphisms have been reported to affect the anti-smoking efficacy of bupropion (12). Hence CYP2B6-catalyzed bupropion hydroxylation is a clinically important bioactivation and elimination pathway.
CYP2B6 catalyzes the inactivation of a diverse array of therapeutic drugs such as methadone, bupropion, meperidine, propofol, efavirenz, ketamine, and tamoxifen, and the bioactivation of cyclophosphamide. It also metabolizes drugs of abuse such as nicotine, ecstasy, and phencyclidine (12-17). CYP2B6 metabolizes as much as 8% of drugs (18). CYP2B6 exhibits interindividual variability in expression and activity (19), and is susceptible to induction and inhibition (14, 20-23). Bupropion hydroxylation, catalyzed exclusively by CYP2B6, has been used as an in vitro probe to determine CYP2B6 constitutive activity, the influence of genetic polymorphisms, and drug interactions (23-27). Clinically, bupropion clearance or hydroxylation have been used as in vivo probes for CYP2B6 activity and drug interactions, and the phenotypic effects of CYP2B6 polymorphisms (4, 22, 27, 28).
The mechanism of stereoselective bupropion disposition in humans is unknown. It is unknown if stereoselectivity in plasma (R,R)- and (S,S)-hydroxybupropion concentrations arises from differences in diastereomer formation. The purpose of this investigation was to test the hypothesis that bupropion hydroxylation by CYP2B6 is stereoselective.
Materials and Methods
Chemicals and Reagents
The internal standards bupropion-d9 and hydroxybupropion-d6 were purchased from Toronto Research Chemicals (Ontario, Canada). Hydroxybupropion and CYP2B6 with coexpressed NADPH-P450 oxidoreductaase and cytochrome b5 were purchased from BD Biosciences (San Jose, CA). Human liver microsomes were obtained as described previously (29). Bupropion and all other reagents were ACS grade or reagent grade and purchased from Sigma Chemical Co. (St. Louis, MO).
Enzyme incubations
Incubation mixtures consisted of 0.5 mg/ml protein (HLM) or 2 pmol/ml CYP2B6, racemic bupropion or hydroxybupropion, and an NADPH regenerating system (3 mM MgCl2, 1 mM EDTA, 1 mM NADP, 5 mM glucose-6-phosphate, 1 U/ml glucose-6-phosphate dehydrogenase, preincubated at 37°C for 10 min) in 200 μl 50 mM potassium phosphate buffer (pH 7.4), performed in 96 well plates. Reactions (37°C) were initiated by adding the NADPH-generating system and terminated by adding 200 μl of ice-cold acetonitrile internal standard mixture (2.5 ng/ml each hydroxybupropion-d6 enantiomer). Mixtures were then vortexed and centrifuged. The supernatant was transferred to a clean plate and concentrated under nitrogen for 20 min at 37°C. The concentrated solution was transferred to an autosampler plate and 50 μl was injected for LC/MS/MS analysis of hydroxybupropion. To determine linearity, CYP2B6 incubations were evaluated at 2 and 80 μM racemic bupropion and samples removed at 5, 10, 15, and 20 min for analysis. To evaluate potential further metabolism, hydroxybupropion was incubated for 0, 10, and 20 min with CYP2B6 or human liver microsomes and then quantified. Substrate saturation curves with CYP2B6 and HLM were evaluated using 2, 5, 10, 25, 50, 75, 100, 150, 250, 500, 750, 1000 μM racemic bupropion and 20 min incubations.
Clinical protocol
Plasma and urine concentrations of bupropion and hydroxybupropion isomers were determined in a subject who received 150 mg oral immediate release racemic bupropion. The protocol was approved by the Washington University Institutional Review Board and performed after obtaining written consent from the subject. Venous blood samples and urine were obtained for 48 hr. Plasma and urine was stored at -20°C prior to analysis.
Plasma concentrations of bupropion enantiomers and (R,R)- and (S,S)-hydroxybupropion were determined using a validated LC-MS/MS assay (8).
Analytical methods
LC/MS/MS analysis of bupropion and hydroxybupropion in incubations was performed on an Applied Biosystems 4000 QTRAP triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Foster City, CA) and a Shimadzu integrated LC system (Shimadzu, Columbia, MD) with a chiral α1-acid glycoprotein column (100×2 mm, 5 μm) and chiral guard cartridge (ChromTech, Apple Valley, MN), as described previously for plasma (8). Mobile phase A was 20 mM formate buffer, pH 5.7 and mobile phase B was methanol. Mobile phase was delivered at a flow rate of 0.22 ml/min with the following program: 10% B for 0.5 min, linear gradient to 30% B between 0.5 and 1.0 min, held at 30% until 5 min, linear gradient to 50% until 8 min, held at 50% B until 15 min, then re-equilibrated to initial conditions between 15 and 20 min. Under these conditions, retention times for R- and S-bupropion were 8.4 and 10.1 min, and those for (S,S)- and (R,R)-hydroxybupropion were 9.3 and 13.9 min, respectively, from extracted plasma. In incubation samples, retention times for R- and S-bupropion were 4.9 and 6.0 min, and those for (S,S)- and (R,R)-hydroxybupropion were 5.3 and 8.8 min, respectively.
The final sample solvent was different for plasma and enzyme incubations samples, resulting in different retention times for the two assays. To verify that sample solvent did not affect elution order, a plasma sample with an excess of R-bupropion and (R,R)-hydroxybupropion (the first bupropion peak and the second hydroxybupropion peak) was extracted in duplicate by the described solid phase extraction method. To one sample was added 2M ammonium formate as described in the plasma assay. The other sample was dried and reconstituted in 200 μL water and 200 μL acetonitrile. The sample was then dried again for 20 min as described in the incubation assay. Both samples had the same elution order: the first bupropion peak and the second hydroxybupropion peak had the highest area, indicating that the analyte elution order was the same regardless of the sample solvent.
Data Analysis
Results are expressed as the mean ± standard deviation, unless otherwise noted. Velocity vs. substrate concentration data were analyzed by non-weighted non-linear regression analysis (SigmaPlot 9.01, Systat, Point Richmond, CA) using a simple Michaelis-Menten model. Results are expressed as the parameter estimate ± standard error of the estimate.
Results
Identification of enantiomer elution
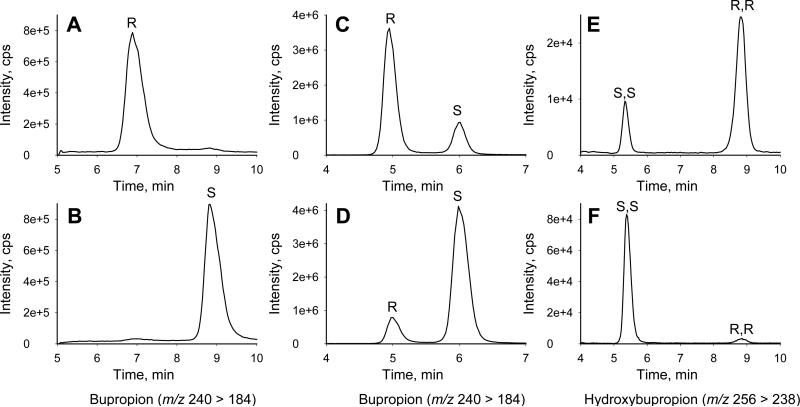
The chromatographic elution order of R- and S-bupropion enantiomers has not been reported. Chiral HPLC assays did not identify R or S-bupropion (7, 30). Furthermore, although two of the four possible hydroxybupropion diastereomers (S,S- and R,R-) have been identified in plasma (6, 7), the bupropion enantiomers from which these metabolites arise have not been identified. Initial experiments identified the HPLC elution order of the bupropion enantiomers and the enantiomeric origin of hydroxybupropion metabolites. Hydroxybupropion peaks at 9.3 and 13.5 min were identified as (S,S)- and (R,R)-, respectively, based on the analysis of patient plasma samples (vide infra), where (R,R)-hydroxybupropion has been previously identified as the most abundant diastereomer (6, 7). Small quantities of R- and S-bupropion enantiomers (separated by semi-preparative HPLC and >95% enantiomerically pure; Fig 2 A and B), were incubated with CYP2B6. Incubation of the bupropion enantiomer eluting at 7 min resulted primarily in R,R-hydroxybupropion (Fig 2, A, C and E), while incubation of the enantiomer at 9 min resulted primarily in S,S-hydroxybupropion (Fig 2, B, and F). Based on these results, the first and second bupropion peaks were assigned as R- and S- bupropion, respectively. Although partial racemization of bupropion occurred during the incubation (Fig 2, C and D), this did not preclude enantiomeric identification.
Figure 2.
Chiral LC-MS/MS analysis of bupropion and hydroxybupropion. (A, B) Injection of individual bupropion enantiomers following semi-preparative separation of racemic bupropion. (C, D) Bupropion enantiomers after 20 min incubation with CYP2B6 and NADPH. Partial racemization is apparent. (E, F) Hydroxybupropion formation following 20 min incubation of individual bupropion enantiomers with CYP2B6. Retention times in incubation samples (C-F) were shifted earlier compared with authentic samples (A, B) due to the presence of the acetonitrile used for protein precipitation.
Bupropion enantiomers metabolism by CYP2B6
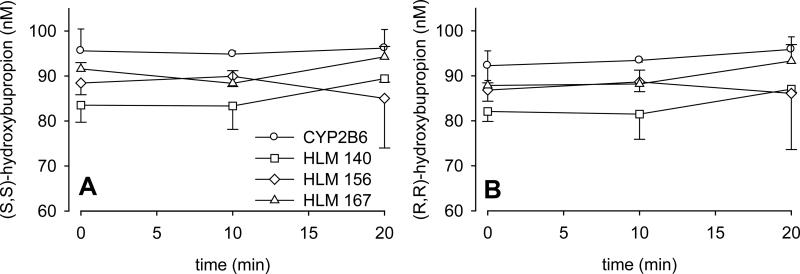
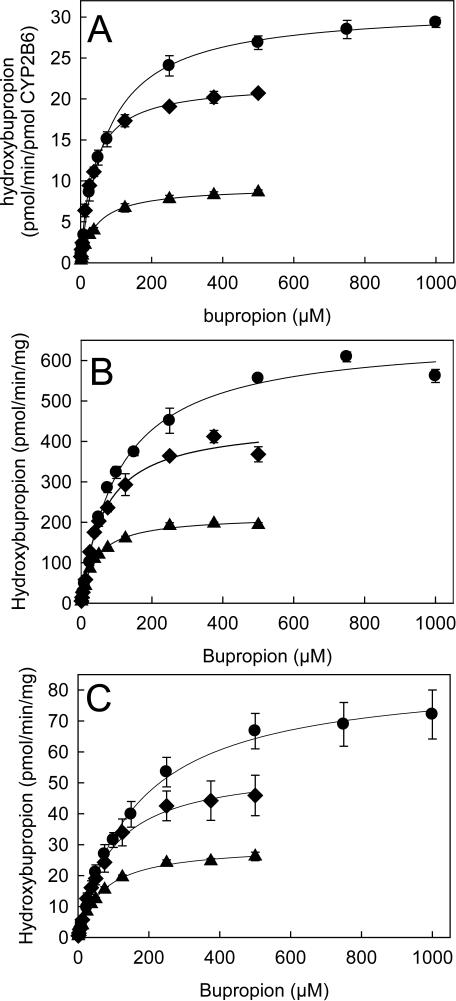
Racemic bupropion was incubated with CYP2B6 at both therapeutic concentrations and at the reported Km for racemic bupropion metabolism (2 and 80 μM, respectively) (31). Hydroxylation of both enantiomers was linear with time for at least 20 min (not shown). The rate of (S,S)- hydroxybupropion formation was approximately 3-fold greater than that of (R,R)-hydroxybupropion. To evaluate the possibility of further hydroxybupropion enantiomers metabolism at differential rates, as an explanation for this difference, racemic hydroxybupropion was incubated with CYP2B6 (and also with microsomes three different human livers) (Fig 3). There was no decrease in hydroxybupropion concentration, eliminating further metabolism of this metabolite by CYP2B6 under these conditions. Concentration-dependent metabolism of racemic bupropion by recombinant CYP2B6 is shown in Fig 4A. Eadie-Hofstee plots were linear (not shown), therefore data were analyzed using a single enzyme model. Kinetic parameters are listed in Table I. The Vmax and intrinsic clearance for S-bupropion hydroxylation were 2- to 3-fold greater than those of R-bupropion.
Figure 3.
Stability of hydroxybupropion enantiomers in metabolic incubations. Racemic hydroxybupropion was incubated with CYP2B6 and human liver microsomes for 20 min, and analyzed by LC-MS/MS. Panels A and B show (S,S)- and (R,R)-hydroxybupropion concentrations, respectively. Each data point is the mean ± SD (n=3).
Figure 4.
Substrate saturation curve for racemic bupropion hydroxylation. Hydroxybupropion (◆ S,S-hydroxybupropion, ▲ R,R-hydroxybupropion, ● total hydroxybupropion) was quantified using a chiral assay. Each data point is the mean ± SD (n=3). Lines are rates predicted from parameters determined by nonlinear regression analysis of hydroxybupropion formation. (A) metabolism by recombinant CYP2B6, (B and C) metabolism by HLM 158 and 167, respectively.
Table I.
Kinetic parameters for bupropion hydroxylation by recombinant CYP2B6.a
| Metabolite | Vmax (pmol/min/pmol CYP2B6) | Km (μM) | Vmax/Km (μl/min/pmol CYP2B6) |
|---|---|---|---|
| (R,R)-hydroxybupropion | 9.3 ± 0.2 | 46 ± 3 | 0.21 |
| (S,S)-hydroxybupropion | 22.0 ± 0.2 | 34 ± 1 | 0.65 |
| Total hydroxybupropion | 31.2 ± 0.4 | 74 ± 3 | 0.42 |
Racemic bupropion was incubated with recombinant CYP2B6. Results are the parameter estimate ± the standard error of the estimate
Bupropion enantiomers metabolism by human liver microsomes
Rates of bupropion hydroxylation in 3 different human liver microsomes varied substantially (Table II). Nonetheless, all showed the same enantioselectivity, with the rate of (S,S)-hydroxybupropion formation 1.5-fold greater than that of (R,R)-hydroxybupropion. Concentration-dependent metabolism of racemic bupropion by human liver microsomes is shown in Fig 4 B and C. Kinetic parameters are listed in Table III. The Vmax and intrinsic clearance for S-bupropion hydroxylation were 2-fold greater than those of R-bupropion.
Table II.
Bupropion hydroxylation by human liver microsomes.a
| 2 μM bupropion |
80 μM bupropion |
|||||
|---|---|---|---|---|---|---|
| Metabolite | HLM140 | HLM158 | HLM167 | HLM140 | HLM158 | HLM167 |
| (R,R)-hydroxybupropion | 0.054 ± 0.007 | 3.6 ± 0.2 | 0.26 ± 0.07 | 2.22 ± 0.06 | 76 ± 3 | 6.6 ± 0.1 |
| (S,S)-hydroxybupropion | 0.073 ± 0.005 | 4.7 ± 0.4 | 0.32 ± 0.12 | 3.29 ± 0.08 | 117 ± 2 | 10.4 ± 0.1 |
Racemic bupropion was incubated with human liver microsomes (HLM). Results (pmol/min/mg protein) are the mean ± SD of 3 determinations
Table III.
Kinetic parameters for bupropion hydroxylation by recombinant human liver microsomes.a
| Metabolite | Vmax (pmol/min/mg) | Km (μM) | Vmax/Km (μl/min/mg) |
|---|---|---|---|
| HLM 158 | |||
| (R,R)-hydroxybupropion | 216 ± 2 | 40 ± 2 | 5.3 |
| (S,S)-hydroxybupropion | 448 ± 10 | 63 ± 4 | 7.1 |
| Total hydroxybupropion | 662 ± 11 | 109 ± 6 | 6.1 |
| HLM-167 | |||
| (R,R)-hydroxybupropion | 30 ± 1 | 68 ± 3 | 0.4 |
| (S,S)-hydroxybupropion | 56 ± 2 | 90 ± 10 | 0.6 |
| Total hydroxybupropion | 85 ± 1 | 162 ± 14 | 0.5 |
Racemic bupropion was incubated with uman liver microsomes (HLM). Results are the parameter estimate ± the standard error of the estimate
Stereoselective human disposition of bupropion and hydroxybupropion
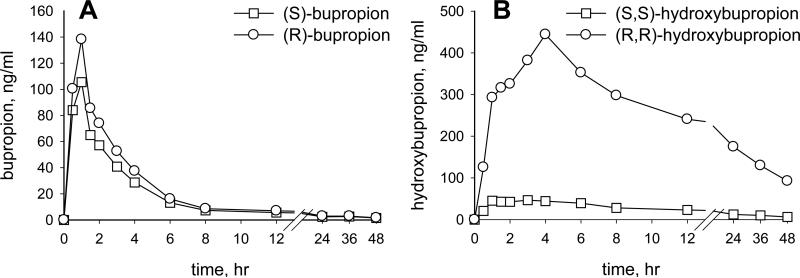
Figure 5 presents plasma bupropion and hydroxybupropion concentrations from a subject who received 150 mg oral immediate release racemic bupropion. There was an enantiomeric excess of both (R)-bupropion (64% of total bupropion) and (R,R)-hydroxybupropion (94% of total hydroxybupropion). In urine, (R)-bupropion and (R,R)-hydroxybupropion were 76-78% of the total (not shown). Results are consistent with a previous report (8).
Figure 5.
Plasma bupropion (A) and hydroxybupropion (B) concentrations from a research subject who received 150 mg oral immediate release bupropion. Plasma was analyzed by LC-MS/MS.
Discussion
This investigation demonstrates that bupropion hydroxylation catalyzed by CYP2B6 and human liver microsomes is stereoselective. CYP2B6 hydroxylates both (S)- and (R)-bupropion. From racemic bupropion, (S)-bupropion was metabolized at more than three times the rate of (R)-, both at therapeutic concentrations and based on the in vitro intrinsic clearance. In human liver microsomes, with racemic bupropion, (S,S)-hydroxybupropion concentrations were generally 1.5-fold those of (R,R)-hydroxybupropion. Apparent stereoselectivity in hydroxybupropion formation was not explained by further stereoselective metabolism of hydroxybupropion enantiomers.
Metabolism of both bupropion enantiomers by CYP2B6 creates the potential for a enantiomeric metabolic interaction, as has been described previously for other CYP2B6 substrates (17). A preliminary experiment suggested that the rate of enantiopure R-bupropion hydroxylation was approximately twice that of equimolar R-bupropion in the racemate (not shown). Since bupropion is only administered as a racemate, this potential interaction is of unknown importance.
The mechanism of stereoselective bupropion and hydroxybupropion disposition clinically is unknown. (R,R)-hydroxybupropion plasma concentrations are approximately 20-fold greater than those of (S,S)-hydroxybupropion after immediate-release oral racemic bupropion (6-8). Possible explanations include differences in bupropion enantiomer concentrations, stereoselective bupropion hydroxylation, or stereoselective hydroxybupropion metabolism and/or elimination. Nonetheless, (R)-bupropion plasma concentrations are only about 2-fold greater than those of (S)-bupropion. Stereoselective metabolism of hydroxybupropion enantiomers is unlikely, since no hydroxybupropion metabolism was evidenced in vitro in this investigation, and no further oxidative or reductive metabolite of hydroxybupropion was identified in vivo (32). Somewhat (approximately 2-fold) faster plasma elimination of (R,R)- vs (S,S)-hydroxybupropion has been observed clinically (7, 8), but this is insufficient to explain the substantial difference between hydroxybupropion diastereomer plasma concentrations. The present results show that greater (R,R)- hydroxybupropion plasma concentrations cannot be explained by greater R-bupropion metabolism. Indeed, mildly stereoselective (S)-bupropion hydroxylation and (S,S)-hydroxybupropion formation in human liver microsomes stand in contrast to 20-fold greater (R,R)- than (S,S)-hydroxybupropion plasma concentrations. Clinical studies are needed to better understand stereoselective bupropion metabolism and disposition.
Stereoselectivity in bupropion metabolism has not been previously addressed, perhaps because racemization was thought to obviate potential implications of bupropion chirality. Bupropion enantiomers rapidly (42%, 62%, and >94% in 2, 4, and 24 hr, respectively) racemize in phosphate buffer (pH 7.4) (33). Hydroxybupropion also racemizes under similar conditions, albeit much more slowly (0.1% and 2% in 2 and 24 hr, respectively) (33). Despite the potential for racemization, there was clear enantiomeric excess in plasma and urine of (R)-bupropion, and more notably (R,R)-hydroxybupropion, even after administration of the racemate. As yet unidentified factors may retard racemization in vivo compared with in vitro rates.
Stereoselective bupropion hydroxylation may have implications for the therapeutic efficacy of bupropion as an antidepressant or smoking cessation therapy. It was suggested that (S,S)-hydroxybupropion may be a better candidate for smoking cessation than bupropion (3), yet dismissed by others because plasma concentrations are minute compared with those of (R,R)-hydroxybupropion (2). Nevertheless, plasma hydroxybupropion enantiomer concentrations may not be the sole determinant of clinical efficacy. CYP2B6 protein is expressed in various regions of the human brain, including the cerebrum (34, 35). If human brain CYP2B6, like human hepatic CYP2B6, metabolizes (S)-bupropion faster than (R)-bupropion, then there may be implications for therapeutic efficacy.
Stereoselective bupropion hydroxylation may also have implications for the use of bupropion as an in vivo CYP2B6 phenotypic probe. Various metrics of racemic bupropion metabolism have been used as probes for CYP2B6, including plasma bupropion clearance and area under the curve of concentration vs time (AUC), plasma hydroxybupropion maximum concentration and AUC, and plasma hydroxybupropion/bupropion AUC ratios (4, 22, 27, 28). These metrics have variable sensitivities and specificities. Racemic hydroxybupropion AUC and maximum concentration variably reflect CYP2B6 induction and inhibition (4, 22, 27), and data are less robust for genetic polymorphisms (28). The plasma AUC ratio of hydroxybupropion to bupropion is confounded because hydroxybupropion is elimination-rate rather than formation-rate limited (metabolite half-life exceeds that of the parent) (1). Bupropion indices are less sensitive, because hydroxylation is only a minor metabolic pathway for bupropion (less than 4% of the dose recovered in urine) (1, 36). Thus CYP2B6 induction or inhibition may not affect bupropion disposition (4, 27), and measurement of parent concentrations alone for CYP2B6 phenotyping has been cautioned against (27). In contrast, clinical disposition of bupropion and hydroxybupropion enantiomers differs from that of the racemate, and responds more selectively to CYP2B6 modulation, suggesting that stereoselective bupropion hydroxylation may afford an improved in vivo phenotypic probe for CYP2B6 activity (37).
Conclusion
Recombinant and human liver microsomal CYP2B6-catalyzed hydroxylation of racemic bupropion is stereoselective. Stereoselective bupropion hydroxylation may have implications for the therapeutic efficacy of bupropion as an antidepressant or smoking cessation therapy, and for the use of bupropion as an in vivo phenotypic probe for CYP2B6 activity.
Acknowledgements
Supported by NIH R01DA14211 and K24DA00417
Abbreviations
- CYP2B6
cytochrome P4502B6
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
References
- 1.Lai AA, Schroeder DH. Clinical pharmacokinetics of bupropion: a review. J Clin Psychiatry. 1983;44:82–84. [PubMed] [Google Scholar]
- 2.Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003;474:85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- 3.Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol. Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- 4.Palovaara S, Pelkonen O, Uusitalo J, Lundgren S, Laine K. Inhibition of cytochrome P450 2B6 activity by hormone replacement therapy and oral contraceptive as measured by bupropion hydroxylation. Clin. Pharmacol. Ther. 2003;74:326–333. doi: 10.1016/S0009-9236(03)00202-9. [DOI] [PubMed] [Google Scholar]
- 5.Jefferson JW, Pradko JF, Muir KT. Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations. Clin Ther. 2005;27:1685–1695. doi: 10.1016/j.clinthera.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Suckow RF, Zhang MF, Cooper TB. Enantiomeric determination of the phenylmorpholinol metabolite of bupropion in human plasma using coupled achiral-chiral liquid chromatography. Biomed Chromatogr. 1997;11:174–179. doi: 10.1002/(SICI)1099-0801(199705)11:3<174::AID-BMC681>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Xu H, Loboz KK, Gross AS, McLachlan AJ. Stereoselective analysis of hydroxybupropion and application to drug interaction studies. Chirality. 2007;19:163–170. doi: 10.1002/chir.20356. [DOI] [PubMed] [Google Scholar]
- 8.Coles R, Kharasch ED. Stereoselective analysis of bupropion and hydroxybupropion in human plasma and urine by LC/MS/MS. J Chromatogr B. 2007;857:67–75. doi: 10.1016/j.jchromb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab. Dispos. 2000;28:1176–1183. [PubMed] [Google Scholar]
- 10.Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos. 2000;28:1222–1230. [PubMed] [Google Scholar]
- 11.Faucette SR, Hawke RL, Shord SS, Lecluyse EL, Lindley CM. Evaluation of the contribution of cytochrome P450 3A4 to human liver microsomal bupropion hydroxylation. Drug Metab. Dispos. 2001;29:1123–1129. [PubMed] [Google Scholar]
- 12.Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, Tyndale RF. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biol Psychiatry. 2007;62:635–641. doi: 10.1016/j.biopsych.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Ekins S, Wrighton SA. The role of CYP2B6 in human xenobiotic metabolism. Drug Metab. Rev. 1999;31:719–754. doi: 10.1081/dmr-100101942. [DOI] [PubMed] [Google Scholar]
- 14.Turpeinen M, Raunio H, Pelkonen O. The functional role of CYP2B6 in human drug metabolism: substrates and inhibitors in vitro, in vivo and in silico. Curr Drug Metab. 2006;7:705–714. doi: 10.2174/138920006778520633. [DOI] [PubMed] [Google Scholar]
- 15.Sridar C, Kent UM, Notley LM, Gillam EM, Hollenberg PF. Effect of tamoxifen on the enzymatic activity of human cytochrome CYP2B6. J Pharmacol Exp Ther. 2002;301:945–952. doi: 10.1124/jpet.301.3.945. [DOI] [PubMed] [Google Scholar]
- 16.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome CYP3A and CYP2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–269. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321:389–399. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 18.Nolan D, Phillips E, Mallal S. Efavirenz and CYP2B6 polymorphism: implications for drug toxicity and resistance. Clin Infect Dis. 2006;42:408–410. doi: 10.1086/499369. [DOI] [PubMed] [Google Scholar]
- 19.Zanger UM, Klein K, Saussele T, Blievernicht J, M HH, Schwab M. Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics. 2007;8:743–759. doi: 10.2217/14622416.8.7.743. [DOI] [PubMed] [Google Scholar]
- 20.Hesse LM, Sakai Y, Vishnuvardhan D, Li AP, Von Moltke LL, Greenblatt DJ. Effect of bupropion on CYP2B6 and CYP3A4 catalytic activity, immunoreactive protein and mRNA levels in primary human hepatocytes: comparison with rifampicin. J Pharm Pharmacol. 2003;55:1229–1239. doi: 10.1211/0022357021657. [DOI] [PubMed] [Google Scholar]
- 21.Faucette SR, Wang H, Hamilton GA, Jolley SL, Gilbert D, Lindley C, Yan B, Negishi M, LeCluyse EL. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab Dispos. 2004;32:348–358. doi: 10.1124/dmd.32.3.348. [DOI] [PubMed] [Google Scholar]
- 22.Loboz KK, Gross AS, Williams KM, Liauw WS, Day RO, Blievernicht JK, Zanger UM, McLachlan AJ. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: Effect of induction by rifampin and ethnicity. Clin Pharmacol Ther. 2006;80:75–84. doi: 10.1016/j.clpt.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Walsky RL, Astuccio AV, Obach RS. Evaluation of 227 drugs for in vitro inhibition of cytochrome P450 2B6. J Clin Pharmacol. 2006;46:1426–1438. doi: 10.1177/0091270006293753. [DOI] [PubMed] [Google Scholar]
- 24.Turpeinen M, Nieminen R, Juntunen T, Taavitsainen P, Raunio H, Pelkonen O. Selective inhibition of CYP2B6-catalyzed bupropion hydroxylation in human liver microsomes in vitro. Drug Metab. Dispos. 2004;32:626–631. doi: 10.1124/dmd.32.6.626. [DOI] [PubMed] [Google Scholar]
- 25.Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics. 2004;14:225–238. doi: 10.1097/00008571-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Richter T, Murdter TE, Heinkele G, Pleiss J, Tatzel S, Schwab M, Eichelbaum M, Zanger UM. Potent mechanism-based inhibition of human CYP2B6 by clopidogrel and ticlopidine. J. Pharmacol. Exp. Ther. 2004;308:189–197. doi: 10.1124/jpet.103.056127. [DOI] [PubMed] [Google Scholar]
- 27.Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2005;77:553–559. doi: 10.1016/j.clpt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Kirchheiner J, Klein C, Meineke I, Sasse J, Zanger UM, Murdter TE, Roots I, Brockmöller J. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003;13:619–626. doi: 10.1097/00008571-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Klees TM, Sheffels P, Thummel KE, Kharasch ED. Pharmacogenetic determinants of human liver microsomal alfentanil metabolism and the role of cytochrome P450 3A5. Anesthesiology. 2005;102:550–556. doi: 10.1097/00000542-200503000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Munro JS, Walker TA. Bupropion hydrochloride: the development of a chiral separation using an ovomucoid column. J Chromatogr A. 2001;913:275–282. doi: 10.1016/s0021-9673(01)00639-2. [DOI] [PubMed] [Google Scholar]
- 31.Walsky RL, Obach RS. Verification of the selectivity of (+)N-3-benzylnirvanol as a CYP2C19 inhibitor. Drug Metab Dispos. 2003;31:343. doi: 10.1124/dmd.31.3.343. [DOI] [PubMed] [Google Scholar]
- 32.Petsalo A, Turpeinen M, Tolonen A. Identification of bupropion urinary metabolites by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:2547–2554. doi: 10.1002/rcm.3117. [DOI] [PubMed] [Google Scholar]
- 33.Fang QK, Han Z, Grover P, Kessler D, Senanayake* CH, Wald SA. Rapid access to enantiopure bupropion and its major metabolite by stereospecific nucleophilic substitution on an α-ketotriflate. Tetrahedron: Asymmetry. 2000;11:3659–3663. [Google Scholar]
- 34.Gervot L, Rochat B, Gautier JC, Bohnenstengel F, Kroemer H, de Berardinis V, Martin H, Beaune P, de Waziers I. Human CYP2B6: Expression, inducibility and catalytic activities. Pharmacogenetics. 1999;9:295–306. [PubMed] [Google Scholar]
- 35.Miksys S, Lerman C, Shields PG, Mash DC, Tyndale RF. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology. 2003;45:122–132. doi: 10.1016/s0028-3908(03)00136-9. [DOI] [PubMed] [Google Scholar]
- 36.DeVane CL, Laizure SC, Stewart JT, Kolts BE, Ryerson EG, Miller RL, Lai AA. Disposition of bupropion in healthy volunteers and subjects with alcoholic liver disease. J. Clin. Psychopharmacol. 1990;10:328–332. [PubMed] [Google Scholar]
- 37.Kharasch ED, Mitchell D, Coles R. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for CYP2B6 activity (abstract). Clin Pharmacol Ther. 2008 doi: 10.1177/0091270008314254. in press. [DOI] [PubMed] [Google Scholar]