Abstract
Numerous experimental Leishmania vaccines have been developed to prevent the visceral and cutaneous forms of Leishmaniasis, which occur after exposure to the bite of an infected sand fly, yet only one is under evaluation in humans. KSAC and L110f, recombinant Leishmania poly-proteins delivered in a stable emulsion (SE) with the TLR 4 agonists monophosphoryl lipid A (MPL) or glucopyranosyl lipid A (GLA) have shown protection in animal models. KSAC+GLA-SE protected against cutaneous disease following sand fly transmission of L. major in susceptible BALB/c mice. Similar poly-protein adjuvant combinations are the vaccine candidates most likely to see clinical evaluation. We assessed immunity generated by KSAC or L110f vaccination with GLA-SE following challenge with L. major by needle or infected sand fly bite in resistant C57BL/6 mice. Poly-protein vaccinated mice had a 60-fold increase in CD4+IFN-γ+ T cell numbers versus control animals at 2 weeks post needle inoculation of L. major and this correlated with a 100-fold reduction in parasite load. Immunity did not, however, reach levels observed in mice with a healed primary infection. Following challenge by infected sand fly bite, poly-protein vaccinated animals had comparable parasite loads, greater numbers of neutrophils at the challenge site, and reduced CD4+ IFN-γ+:IL-17+ ratios versus non-vaccinated controls. In contrast, healed animals had significantly reduced parasite loads and higher CD4+ IFN-γ+:IL-17+ ratios. These observations demonstrate that vaccine-induced protection against needle challenge does not necessarily translate to protection following challenge by infected sand fly bite.
Introduction
Leishmania are protozoan parasites that are transmitted to the skin of a mammalian host following the bite of an infected Phlebotomine sand fly. The clinical outcomes of infection with different Leishmania spp. is highly diverse, ranging from self-limiting, cutaneous lesions following infection with L. major, to fatal systemic involvement of the liver, spleen, and bone marrow following infection with L. donovani (1). A defining feature of cutaneous disease caused by L. major is that dermal lesions eventually heal, but are thought to continue to harbor viable parasites for the lifetime of the individual. This chronic state is associated with the maintenance of a powerful cell-mediated immune response such that individuals with a healed primary infection are protected against re-infection. Consequently, intentional infection with L. major parasites at discrete locations on the body has been successfully employed as a live ‘vaccine’ (2-5). However, issues surrounding the inoculation of viable parasites in immuno-compromised individuals and adverse reactions at the site of leishmanization in some individuals have precluded its use as an intervention strategy (5, 6).
Rodents are natural reservoirs of L. major and the study of cutaneous disease in resistant laboratory mice where lesions heal and parasites establish stable chronic infection is thought to reflect the human experience with this parasite (7, 8). In these murine models, cell-mediated immunity is primarily associated with Th1 CD4+ and CD8+ T cells, most often defined by their ability to produce IFN-γ in response to parasite antigens (8, 9). Therefore, the cell-mediated immune response in humans or mice with a healed primary infection represents the ‘gold-standard’ of protective immunity. Reproducing this protective immune response with protein, DNA-based, or live-attenuated vaccines has been an active area of study because it is assumed this would be the first step towards the development of a safe and effective Leishmania vaccine (10-19). However, few experimental vaccines have progressed beyond animal models into human clinical trials due to a lack of consensus regarding their likelihood of success and/or the costs associated with producing a vaccine acceptable for human use (18-20). Of the so-called ‘first-generation’ whole cell-killed promastigote vaccines that have been used in people, while shown to be safe, none have succeeded in providing the level of protection to natural infection that would justify their further use and development (21, 22).
Recently, several defined poly-proteins containing multiple antigenic epitopes from Leishmania delivered in a stable water-in-oil emulsion (SE) with monophosphoryl lipid A (MPL) or glucopyranosyl lipid A (GLA), TLR 4 agonists suitable for use in people, have provided protection in susceptible BALB/c and resistant C57BL/6 mice following needle challenge with Leishmania spp. (23-27). One of these poly-proteins, KSAC, containing kinetoplastid membrane protein 11 (KMP11), sterol 24-c-methyltransferase (SMT), A2, and cysteine proteinase B (CPB), was shown to protect BALB/c mice against early lesion development following sand fly transmission of L. major, and this was associated with an approximately 50-fold reduction in parasite load at 5 weeks post infected sand fly challenge (28). These observations are promising since Phase I clinical trials have also shown that at least some of these antigen-adjuvant combinations are safe for use in people, and recombinant Leishmania poly-proteins lend themselves well to meeting good manufacturing practice (GMP) and regulatory approval (18, 29-31).
In the resistant C57BL/6 model, we and others have reported that vaccination with autoclaved L. major (ALM) delivered with the TLR9 agonist CpG oligodeoxynucleotides, a Th1 adjuvant, results in significant protection following needle challenge (11, 12, 32). However, this protection is significantly weaker than that observed in mice with a healed primary infection, and fails to protect following challenge by exposure to the bites of L. major infected sand flies (32). The failure of the ALM+CpG vaccine was not due to a difference in the virulence of inoculated parasites, but rather was associated with the unique immunomodulatory conditions at the site of sand fly inoculation, and in particular the prolonged recruitment of neutrophils (32). In the studies reported here the objective was to assess protective immunity generated by poly-protein+GLA-SE vaccination in the context of ALM+CpG vaccinated and healed mice following needle and infected sand fly challenge in a resistant mouse model of cutaneous Leishmaniasis.
Materials and Methods
Mice
Female C57BL/6 mice were obtained from Jackson Laboratories. All mice were maintained in the National Institute of Allergy and Infectious Diseases animal care facility under specific pathogen-free conditions.
Leishmania cell lines and preparation for needle inoculation
Leishmania major Friedlin strain was obtained from the Jordan Valley NIH/FV1 (MHOM/IL/80/Friedlin). Leishmania major RYN was obtained from a lesion biopsy of a laboratory worker accidentally exposed to Lutzomia longipalpis sand flies that were experimentally infected with a strain of L. major (WR2885) originating in Iraq, as described previously (33). All parasites were grown in-vitro as described previously (33). Infective-stage metacyclic promastigotes were isolated from stationary cultures (4–6 day-old) by negative selection of non-infective forms using peanut agglutinin (PNA, Vector Laboratories Inc)(34).
Healed mice and ALM+CpG, KSAC+GLA-SE, and L110f+GLA-SE-vaccination
Briefly, healed mice were generated by infection with 104 L. major (FV1 or RYN) metacyclic promastigotes subcutaneously (s.c.) in the footpad, and used at 23 weeks post-primary infection when footpad lesions had completely resolved. Autoclaved Leishmania antigen (ALM) plus CpG oligodeoxynucleotides (ODN) vaccinated mice were injected s.c. in the footpad with 50 μg of ALM plus 50 μg of CpG ODN sequence 1826 (Coley Pharmaceutical Group), provided by Dr. P. Darrah (VRC/NIH), three times, at 2-week intervals. Additional groups of mice were vaccinated s.c. in the base of the tail with 10μg of antigen (KSAC or L110f) plus 20μg of GLA-SE in 100μl, three times, at 2-week intervals (antigens and adjuvant were kindly provided by Dr. S. Reed, IDRI). In some experiments mice were injected with KSAC plus 5 μg of GLA-SE, and where indicated, Age-Matched Control (AMC) mice were injected with Saline plus GLA-SE alone. In some experiments AMC and KSAC+GLA-SE vaccinated mice were boosted three days prior to sand fly challenge with 10μg of KSAC antigen plus 20μg of GLA-SE.
Sand Fly Infection and Exposure of Mice to Infected Sand flies
Colonized female Phlebotomus duboscqi sand flies were infected with L. major RYN as described previously (33). After 13-14 days, parasite infections within the sand fly midgut were assessed to determine suitability for transmission according to our previously published protocol (33). On the day of challenge, mouse ears were exposed to the bites of four infected flies for 2-3 hours in the dark at 23°C and 50% humidity. The average number of flies with or without a blood meal was used to assess feeding intensity between treatment groups, and no significant differences were found (data not shown).
Determination of Lesion Size and Parasite Load
Ear lesion diameters or ear lesion area were measured (in mm) weekly as described in the text. Ears with more than one lesion are reported as total lesion diameter or total lesion area per ear. Two to 4 weeks following challenge by needle inoculation or 6 weeks following challenge by exposure to infected sand fly bites, mice were euthanized and a single cell suspension of individual ears was prepared as described previously (32). A portion of each ear was re-suspended in parasite growth medium and serially diluted in a 96-well flat-bottom microtiter plate, overlaying 100ul onto 50μl of NNN medium containing 20% defibrinated rabbit blood. The number of viable parasites in each ear was determined from the highest dilution at which promastigotes could be grown out after 7 to 10 days of incubation at 26°C.
Phenotypic analysis of ear-derived cell populations
Ear-derived cells were incubated with an anti-Fc-γ III/II (CD16/32) receptor Ab (2.4G2, BD Biosciences) in RPMI without phenol red (Gibco) containing 1.0% FCS for 10 minutes followed by incubation for 20 minutes with a combination of six of the following antibodies: PE-Cy7 or APC anti-CD11b (M1/70 BD Biosciences); Per-CP Cy5.5 anti-Ly6C (HK1.4, eBioscience); FITC or PE anti-Ly6G (1A8, BD Biosciences); APC or PE anti-CD11c (HL3, BD Biosciences); Per-CP Cy5.5 anti-CD11c (N418, BioLegend); APC anti-F4/80 (BM8, eBioscience), FITC anti-I-Ab (AF6-120.1, BD Biosciences); Alexafluor-700 or Alexafluor-750 anti-mouse MHC II (M5/114.15.2, eBioscience). Isotype controls employed were rat IgG1 (R3-34) and rat IgG2b (A95-1 or eBR2a). Data were collected using FacsDIVA software on a FacsCANTO flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar). Gating of ear-derived cells was carried out as described previously (35, 36). Cells were analyzed following pooling of ears from mice of the same group as indicated in the text.
Re-stimulation of ear-derived T cells for cytokine analysis by flow cytometry or Multi-plex ELISA
T cells were re-stimulated with parasite antigens as described previously (25, 32). Briefly, whole ear single-cell suspensions were incubated at 37°C in 5% CO2 for 12-14 hours in flat-bottom 48-well plates with 2.5×105 bone marrow-derived dendritic cells (BMDCs) or 1×106 T cell-depleted (Miltenyi Biotech), irradiated, naïve spleen cells (APCs), with or without 50 μg/ml freeze-thaw Leishmania antigen (L.m.-Ag), 40 μg/ml KSAC or 40 μg/ml L110f. During the last 4 hours of culture, 1μg/ml of Brefeldin A (Golgiplug; BD Biosciences) was added. Following culture, washed cells were labeled with Live/Dead fixable VIOLET or AQUA (Invitrogen) to exclude dead cells and anti-Fc III/II (CD16/32) receptor Ab (2.4G2), followed by PE-Cy7 anti-mouse CD4 (RM4-5) for 20 minutes. Cells were then fixed with BD Cytofix/Cytoperm (BD Biosciences) and stained with a combination of the following anti-mouse antibodies: V500, APC-Cy7, or PerCP-Cy5.5 anti-CD3 (145-2C11), FITC anti-IFN-γ (XMG1.2), PE- or Alexafluor-700 anti-TNF-α (MP6-XT22), APC anti-IL-2 (JES6-5H4), PerCP-Cy5.5 anti-IL17A (eBio17B7) or PE anti-IL-10 (JES5-16E3). Intracellular staining was carried out for 45 minutes on ice. Isotype controls employed were rat IgG1 (R3-34) and rat IgG2b (A95-1 or eBR2a). All Abs were from eBiosciences or BD Biosciences. Data were collected using FacsDIVA software on a FacsCANTO flow cytometer (BD Biosciences), and analyzed using FlowJo software (TreeStar). Forward-scatter and side-scatter width was employed to exclude cell doublets from analysis. For measurement of chemokines and cytokines in culture supernatants, pooled, single-cell suspensions of ear tissue, prepared as described above, were incubated at 37°C in 5% CO2 for 12 hours in 48-well round bottom plates with 2.5×105/ml BMDC with or without L.m.-Ag, KSAC, or L110f in a total volume of 1 ml. Following incubation, the concentration of chemokines and cytokines in the culture supernatant was determined by multiplex analysis (Affymetrix).
Statistics
Lesion diameters and parasite loads were compared using the Mann-Whitney test. All p-values are two-sided. Statistical calculations were done in Graphpad PRISM 5.0c (www.graphpad.com). Level of significance is reported as follows; *, 0.05>p>0.005; **. 0.005>p0.0005; ***, p<0.0005.
Ethics Statement
All animal experiments were performed under an Animal Study Protocol approved by the NIAID Animal Care and Use Committee using guidelines established by the Animal Welfare Act and the PHS Policy on Humane Care and Use of Laboratory Animals.
RESULTS
Vaccination with poly-protein+GLA-SE or autoclaved L. major (ALM)+CpG mediates protection against needle challenge with Leishmania major
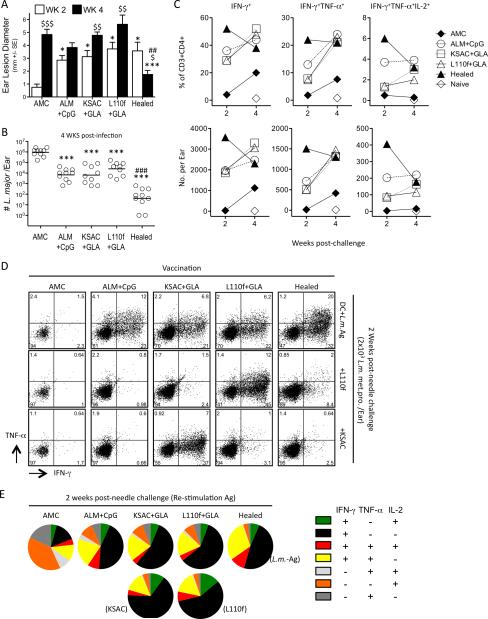
C57BL/6 mice were vaccinated with ALM+CpG, KSAC+GLA-SE or L110f+GLA-SE three times at 2-week intervals, as described previously (25, 32). Healed mice were used 23 weeks after primary infection with 104 live L. major metacyclic promastigotes in the footpad. Seventeen weeks following the last vaccine dose, mice were challenged by needle inoculation of the ear dermis with 2×103 purified L. major metacyclic promastigotes. Analysis of ear lesion diameters at 2 weeks post-challenge revealed that healed and vaccinated mice had significantly larger lesions compared to age-matched control (AMC) mice (Figure 1A), likely due to the recruitment of cells primed by pre-exposure to Leishmania antigens. By 4 weeks post-challenge the mean lesion diameter of AMC and vaccinated animals was not significantly different. In contrast, at 4 wks p.i., healed mice had significantly reduced lesions in comparison to 2 wks, and to the AMC and each of the vaccinated groups. Assessment of parasite numbers per ear at 4 weeks p.i. revealed that each of the vaccinated groups significantly reduced the parasite load per ear versus AMC, although not to same degree as healed animals (Figure 1B). Analysis of cytokine production by ear-derived CD3+CD4+ T cells in response to whole-parasite antigen at 2 and 4 weeks post needle inoculation revealed that vaccinated and healed mice mounted robust immune responses compared to AMC (Fig 1C). However, only healed mice contained approximately 2-fold greater frequencies and total numbers of IFN-γ+ cells at the earlier, 2 week time-point (Figure 1C & D), confirming our previous observation that the strongest correlate for protection is a robust immune response early after challenge (32). In addition, while the response in healed mice to whole parasite antigen decreased over time, reflecting the greatly reduced antigen load, responses in each of the other groups increased (Figure 1C), suggesting that the higher parasite loads in these ears continued to drive T cell expansion. All of the vaccinated groups of mice harbored cells with the capacity to produce multiple cytokines (Figure 1, C-E), although healed mice showed higher frequencies of IFN-γ+TNF-α+ double and IFN-γ+TNF-α+IL-2+ triple producers among CD4+CD3+ (Figure 1, D) and cytokine positive (Figure 1E) T cells at 2 weeks p.i.. As expected, the largest population of cytokine-producing cells in control animals produced IL-2 alone, reflecting the early phase of the primary immune response (Figure 1E). Elicitation of IFN-γ production by ear cells from ALM+CpG-vaccinated or healed mice was more efficient with killed, whole-parasite antigen (L.m.-Ag) versus the L110f or KSAC poly-proteins, reflecting the diversity of the Leishmania antigen-specific response (Figure 1D). Each of the poly-proteins also elicited some cytokine producing cells in healed mice, confirming that T cells specific for these antigens are part of this highly protective secondary immune response. In contrast, responses in mice vaccinated with either KSAC or L110f were elicited most efficiently by re-stimulation with the corresponding poly-protein. This result suggests that poly-protein vaccination activates large numbers of antigen-specific cells by two weeks post-challenge, but that some of these cells are responding to peptides that are not adequately processed or presented when whole parasite antigen was used for re-stimulation. In vitro CD4+ T-cell re-stimulation with whole parasite antigen rather than poly-protein appears to best reflect the corresponding reduction in parasite numbers in vivo.
Figure 1. Vaccination with poly-protein+GLA-SE or autoclaved L. major (ALM)+CpG mediates protection against needle challenge with Leishmania major.
Ears of Age Matched Control (AMC), Healed, and ALM+CpG, KSAC+GLA-SE(20μg), or L110f+GLA-SE(20μg) vaccinated mice were challenged by needle inoculation with 2×103 L.m. metacyclic promastigotes and subsequently analyzed at the indicated time-points. (A) Mean lesion diameter (+/-1 SE). (B) Parasite loads in individual ears at 4 weeks following challenge. (C-E) Intracellular staining for cytokine-producing CD4+CD3+ T cells after in-vitro re-stimulation of pooled ear-derived cells from the same groups of mice employed in 1, A and B. (C) Kinetic of analysis of IFN-γ+, IFN-γ+TNF-α+ or IFN-γ+TNF-α+IL-2+ cells following re-stimulation with BMDCs plus whole killed parasite (L.m.-Ag). (D) Responses following re-stimulation with BMDCs plus L.m.-Ag, KSAC, or L110f antigen at 2 weeks post needle challenge. (E) Graphical representation of the cytokine profile of CD3+CD4+ T cells making at least 1 cytokine in response to Ag re-stimulation at 2 weeks post needle challenge. Asterisk(*) symbols represent significant differences versus the AMC group at the same time point; currency($) symbols represent significant differences between the 2 and 4 week time points within the same group; hash(#) symbols represent significant differences between the healed group and each of the vaccinated groups at 4 weeks. This experiment was done once.
These observations demonstrate that defined poly-protein vaccines containing only a fraction of the potentially available Leishmania antigens and employing an adjuvant suitable for use in humans result in an approximately 60-fold increase in the number of IFN-γ+ T cells per ear versus control animals at 2 weeks post-challenge. In addition, poly-protein+GLA-SE vaccinated mice reduced parasite loads to the same degree as mice vaccinated with ALM+CpG, a non-living vaccine that confers consistently strong protection against needle challenge in animal models (11, 12, 32).
Lesion size in vaccinated mice correlates with neutrophil numbers and an early IL-17 response
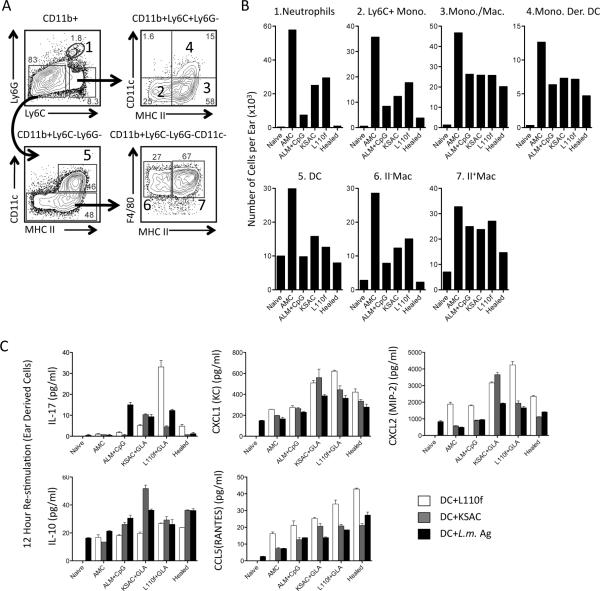
Because innate cells, in addition to adaptive immunity, influence inflammation, pathology, and parasite clearance (32, 35-37) we investigated the composition of these cells at the site of infection 4 weeks following needle challenge (Figure 2, A and B). Healed mice had the lowest number of each CD11b+ subset investigated, correlating with the reduction in lesion size, parasite load, and numbers of antigen-responsive T cells in these animals versus vaccinated mice at 4 weeks post-challenge. In contrast, ears of KSAC and L110f vaccinated animals had larger numbers of certain innate cell types, specifically neutrophils, Ly6C+ monocytes, DCs, and F4/80+MHCII- macrophages versus ALM+CpG-vaccinated mice, despite comparable control of parasite numbers (Figure 2B). Because neutrophils can interfere with primary and secondary CD4+ T cell activation and represented the largest fold-increase between poly-protein and either ALM+CpG or healed animals, we wished to investigate the factors that may be influencing their recruitment. Recently, it has been shown that inoculation of L. major with the CpG oligonucleotide adjuvant generates IL-17-producing CD4+ T cells and results in IL-17-dependent neutrophil recruitment to the skin (38). IL-17-mediated recruitment of neutrophils has also been implicated in the pathogenesis of L. major infection in BALB/c mice (39). Analysis of culture supernatants from ear-derived cells 14 days following challenge revealed that each of the vaccinated groups of mice had elevated levels of IL-17 in response to L.m.-Ag versus control or healed animals (Figure 1C). This was most dramatic when cells from L110f-vaccinated animals were stimulated with the L110f protein. IL-17 mediates neutrophil recruitment via induction of neutrophilic chemokines such as CXCL1 and CXCL2 from innate and non-immune cells (40). We found elevated levels of CXCL1 and CXCL2 in poly-protein+GLA vaccinated animals, and this was most clear when cells were re-stimulated with the protein employed for vaccination. These observations suggest that an early IL-17 response may enhance innate cell recruitment in vaccinated animals via induction of CXCL1 and CXCL2. IL-17 production in ALM+CpG vaccinated mice did not correlate with increased levels of CXCL1 and CXCL2, and this was reflected in lower neutrophil numbers at 4 weeks. Because regulatory cytokines such as IL-10 or T cell recruitment can also influence the outcome of infection, we investigated the levels of IL-10 and the Th1 recruiting chemokine CCL5(RANTES), Figure 2C. We found similar levels of IL-10 among vaccinated and healed animals, apart from an increase in KSAC vaccinated mice re-stimulated with the KSAC poly-protein. In addition, CCL5 levels were comparable among the vaccinated mice with slightly higher levels observed in healed animals. Therefore, regulation by IL-10 or lack of Th1 recruiting chemokines does not appear to explain the difference in protection comparing vaccinated and healed animals.
Figure 2. Analysis of total myeloid populations and cytokine/chemokine production at the site of infection.
(A) Representative gating strategy where subpopulations of CD11b+ myeloid cells are defined by the following markers: Ly6CintLy6G+ neutrophils (region 1); Ly6ChiLy6G-CD11c-MHCII- inflammatory monocytes (Ly6C+ Mono., region 2) Ly6ChiLy6G-CD11c-MHCII+ monocytes/macrophages (Mono./Mac., region 3); Ly6ChiLy6G-CD11c+MHCII+ monocyte-derived dendritic cells (Mono. Der. DC, region 4); Ly6C-Ly6-GCD11c+MHCII+ dendritic cells (DC; region 5); Ly6C-Ly6G-CD11c-MHCII- macrophages (II- Mac., region 6); Ly6C-Ly6G-CD11c-MHCII+ macrophages (II+ Mac., region 7). (B) Relative cell number of the indicated cell types per ear following analysis of pooled ear samples from the mice depicted in Figure 1 at 4 weeks post-challenge. (C) Detection of IL-17, CXCL1(KC), CXCL2(MIP-2), IL-10, and CCL5(RANTES), by multiplex analysis following in-vitro re-stimulation of ear-derived cells for 12 hours with the indicated antigen at 2-weeks post-infection. Error bars represent the standard deviation between replicates of a pooled sample. This experiment was done once.
Poly-protein+GLA-SE vaccination fails to protect against infected sand fly challenge in resistant C57BL/6 mice
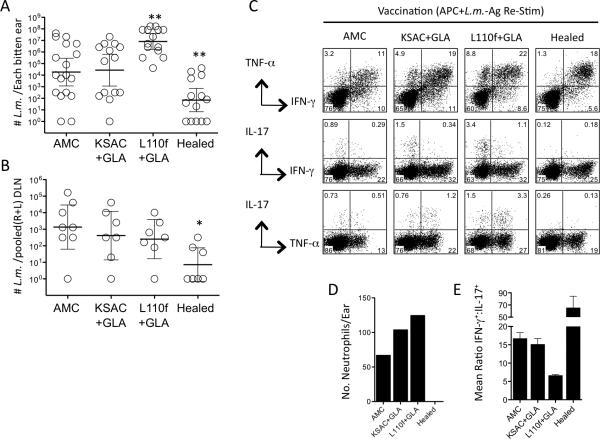
We previously reported that ALM+CpG vaccination protects against needle, but not infected sand fly challenge (32). We wished to determine whether poly-protein+GLA-SE vaccination, which also protects against needle challenge, (Figure 1B), would protect against infected sand fly challenge. Analysis of parasite loads in the ears and draining lymph nodes of poly-protein vaccinated or healed animals six weeks following infected sand fly challenge revealed that only animals with a healed primary infection reduced parasite numbers versus control animals, and this pattern was apparent in both the ear and ear-draining lymph node, Figure 3, A and B. Surprisingly, vaccination with L110f resulted in significantly increased parasite numbers in the ear versus control or KSAC vaccinated animals, despite higher or equivalent frequencies of IFNγ+CD4+ T cells, respectively (Figure 3A and C). The failure of KSAC vaccinated mice to reduce parasite loads versus control animals also occurred despite an increase in the frequency of IFNγ+ cells versus control mice. In contrast, healed mice contained lower total frequencies of IFNγ cells compared to vaccinated mice, likely reflecting the strong reduction in parasite numbers at 6 weeks post-challenge in these mice. Ears of KSAC- and L110f-vaccinated animals contained more neutrophils then control animals (Figure 3D) and, at least in the case of KSAC-vaccinated mice, this was not attributable to increased parasite loads as assessed at 6 weeks. Co-staining for IL-17 and IFNγ or TNF production by CD4+ T cells revealed that IL-17+ cells made up a greater proportion of the secondary immune responses in poly-protein vaccinated versus healed mice (Figure 3C). The majority of IL-17+ cells in poly-protein+GLA vaccinated mice co-produced TNF, while the opposite was true in AMC and healed mice. At the 6-week time point examined, the ratio of CD4+IFN-γ+:CD4+IL-17+ T cells correlated with vaccine success or failure against sand fly challenge, with healed mice having the highest ratio, and the L110f mice having a 2.5-fold lower ratio compared to AMC or KSAC vaccinated animals. The IFN-γ:IL-17 ratio also correlated with the number of neutrophils per ear. These observations demonstrate that challenge by exposure to L. major-infected sand flies alters the requirements for protective immunity as compared to challenge by needle inoculation.
Figure 3. Poly-protein+GLA-SE vaccination fails to protect against infected sand fly challenge in resistant C57BL/6 mice.
Ears of Age Matched Control (AMC), Healed, KSAC+GLA-SE, or L110f+GLA-SE vaccinated mice were exposed to the bites of infected sand flies and analyzed 6 weeks later. (A) Parasite loads in individual ears. (B) Parasite loads in pooled (right plus left) ear DLNs. (C) Intracellular staining for cytokine-producing CD4+CD3+ T cells after in-vitro re-stimulation of pooled ear-derived cells with APCs plus L.m.-Ag. (D) Number of CD11b+Ly-6CintLy6G+ neutrophils per ear. (E) Graphical representation of the mean ratio (n=2) of CD4+IFN-γ+:CD4+IL-17+ T cells in the ear. This experiment was done once.
Lower adjuvant dose, or boosting shortly before challenge, does not enhance the efficacy of KSAC+GLA-SE vaccination against infected sand fly challenge
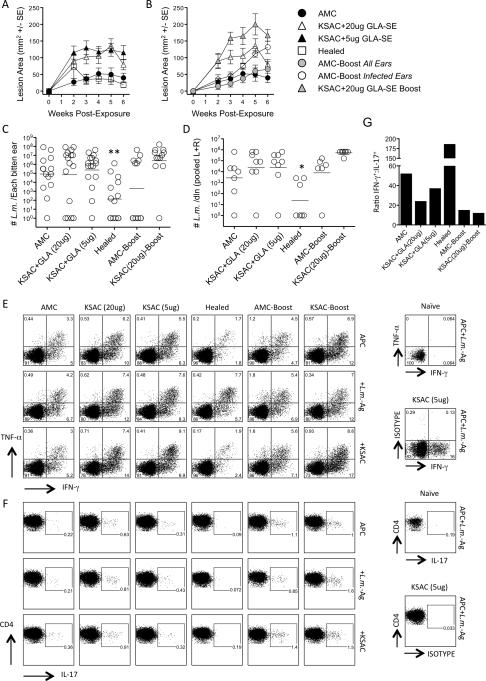
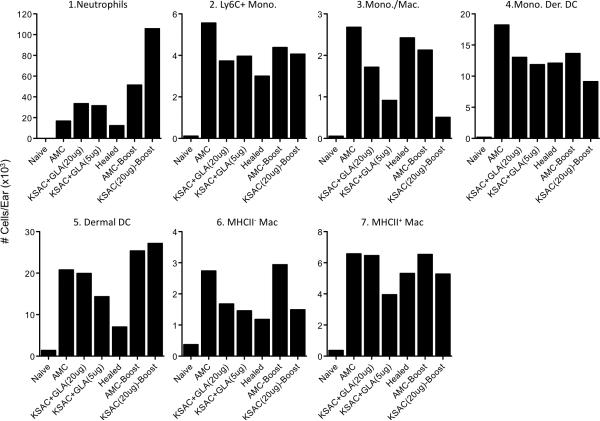
In an attempt to improve vaccine efficacy, we focused our attention on the KSAC poly-protein vaccine since L110f resulted in an enhancement of parasite load. To address the possibility that over-administration of the GLA-SE adjuvant was responsible for inducing IL-17-dependent neutrophil recruitment, we included a group of animals vaccinated with a lower dose (5μg) of GLA-SE. And to address the possibility that the vaccination protocol failed to maintain a sufficient number of Leishmania-specific cells available for recall at the time of challenge, we boosted vaccinated and control mice with KSAC+GLA-SE just prior to challenge. Lowering the adjuvant dose did not lead to a reduction in lesion size or parasite load following infected sand fly challenge as compared to mice given the 20μg dose (Figure 4, A, C and D). In fact, KSAC-vaccinated mice had increased lesion sizes compared to control mice, and this was not attributable to differences in parasite numbers as assessed at 6 weeks post-challenge. In contrast, healed mice, which also had an early increase in lesion size versus control animals at 2 weeks post-challenge, had smaller lesion sizes compared to vaccinated animals starting at 3 weeks (p<0.01) and significantly fewer parasites per ear compared to all other groups at 6 weeks. The relatively flat lesion kinetic in AMC mice shown in Figure 4A is the result of the wide variation in parasite dose delivered by sand fly bite such that ears receiving low doses of parasites are beginning to present with lesions while ears receiving a high dose are resolving their lesions (41). We also assessed IFN-γ, TNF-α, and IL-17 production by dermal-derived CD4+ T cells following re-stimulation with APCs alone, APC+L.m.-Ag, or APC+KSAC protein (Figure 4E and F). Vaccination with 5μg vs. 20μg GLA in emulsion plus KSAC resulted in lower frequencies of IFN-γ+ cells, but this was also accompanied by a decrease in the frequency of IL-17+ cells, such that the ratio of IFN-γ+ to IL-17+ CD4 T cells increased from 23 to 37:1 following L.m.-Ag restimulation (Figure 4G). However, this increased ratio was still lower than the 186:1 observed in mice with a healed primary lesion or the 52:1 ratio observed in control mice. Once again, ears of KSAC vaccinated animals contained approximately twice the number of neutrophils at the site of challenge versus control or healed mice, (Figure 5). KSAC vaccination with 5 vs 20μg of GLA-SE did not alter the number of neutrophils at the site of infection, but did trend towards fewer monocyte/macrophages, DC's and MHCII+ macrophages.
Figure 4. Lower adjuvant dose, or boosting shortly before challenge, does not enhance the efficacy of KSAC+GLA-SE vaccination against infected sand fly challenge.
Ears of AMC, Healed, and KSAC+GLA-SE(20μg)- or KSAC+GLA-SE(5μg)-vaccinated mice were exposed to the bites of infected sand flies and analyzed 6 weeks later. A separate group of AMC or KSAC+GLA-SE(20μg) vaccinated mice were boosted with KSAC+GLA-SE(20μg) 3 days before exposure to infected sand flies. (A and B) Mean lesion area (+/-1 SE) following exposure to infected sand fly bites. Data in A and B are from the same experiment, but are separated for clarity. (C) Parasite loads in individual ears. (D) Parasite loads in pooled (right plus left) ear DLNs. (E) Intracellular staining for IFN-γ+ and/or TNF-α+ CD4+CD3+ T cells after in-vitro re-stimulation of pooled ear-derived cells with APCs plus L.m.-Ag or KSAC. (F) Frequency of IL-17+ cells among the same cells in E. (G) Graphical representation of the ratio of CD4+IFN-γ+:CD4+IL-17+ T cells in the ear following APC+L.m.-Ag re-stimulation. Asterisk(*) symbols represent significant differences versus the AMC group. Error bars in (A and B) represent the standard error of the mean lesion area per ear. This experiment was done once.
Figure 5. Analysis of CD11b+ myeloid populations at the site of infected sand fly challenge reveals enhanced neutrophil numbers in vaccinated animals.
Relative cell number of the indicated cell types per ear as defined in Figure 2 following analysis of pooled ear samples from the mice depicted in Figure 4.
Boosting KSAC+GLA-SE(20μg) vaccinated mice 3 days prior to challenge with a fourth dose of KSAC+GLA-SE did not improve the outcome of infected sand fly challenge (Figure 4, B, C and D) Rather, boosting led to a significant exacerbation of lesion size starting at 3 weeks post challenge (p=0.029) as compared to KSAC-vaccinated animals that were not boosted.(Figure 4B). In the age-match control group that was injected with KSAC+GLA-SE three days prior to infected sand fly challenge (AMC-Boost) half of the ears did not present with lesions and did not contain parasites. The most likely explanation for this is that transmission did not occur following exposure to infected sand flies. Analysis of ear lesion size on those AMC-Boost ears that were infected at the time of analysis (open circles in 4B) revealed these ears had significantly larger lesions at 6 weeks post-transmission versus non-boosted age matched controls (p=0.014), see Figure 4B. Boosting AMC or KSAC vaccinated mice led to slight increases in the frequency of IFN-γ+ cells (Figure 4E). However, this was accompanied by a 2-4 fold increase in the frequency of IL-17 producing cells, such that the ratio of IFN-γ+:IL-17+ cells decreased from 52 to 15 in AMC versus AMC-Boost mice and from 24 to 12 in KSAC versus KSAC-Boost mice (Figure 4G). This correlated with a 3-fold increase in neutrophil numbers per ear (Figure 5). Therefore, lowering the adjuvant dose did increase the CD4+IFN-γ+:CD4+IL-17+ ratio, but this increase did not reach the levels seen in control or healed animals, and did not reduce neutrophil numbers or lesion size. In addition, while boosting slightly increased the frequencies of IFN-γ-producing T cells, the most dramatic effect was an enhancement of the IL-17 response and increased neutrophil numbers and lesion size, strongly suggesting this is an inappropriate vaccination protocol. Neither alteration to the vaccine protocol led to improved control of parasite numbers.
DISCUSSION
The observations reported here add to our previous finding that vaccine-induced protection against needle challenge in resistant C57BL/6 mice does not necessarily translate to protection following challenge by exposure to the bites of L. major-infected sand flies. In addition, immune responses elicited by L.m.-Ag re-stimulation of cells from the site of challenge in protein plus adjuvantvaccinated mice again failed to match the early qualitative and quantitative intensity of the response in mice with a healed primary infection. We have argued that a rapid and robust response is critical to deal with the immune modulatory conditions present at the dermal site of infected sand fly bite, which includes the massive and prolonged recruitment of neutrophils (32, 42). Leishmania major parasites are not killed by neutrophils and both CD4+ T cell priming by dendritic cells and IFN-γ production in vaccinated mice are down modulated in the presence of neutrophils (32, 35, 36). More critically, the efficacy of the whole cell killed ALM+CpG vaccine against infected sand fly challenge was enhanced in neutrophil depleted mice (32). Furthermore, the ALM+CpG vaccine still conferred protection against needle challenge with parasites derived from infected sand flies, arguing that the failure of the non-living vaccines to protect against sand fly challenge is not due to greater virulence of sand fly transmitted parasites (32). In contrast to infected sand fly challenge, needle challenge results in a transient recruitment of smaller numbers of neutrophils making needle challenge less likely to require such robust secondary immunity in order to observe a reduction in parasite load (32). Therefore, the most likely explanation for vaccine failure against infected sand fly challenge is the inability of the vaccine to replicate the early kinetics of the secondary immunity observed in animals with a healed primary infection. So far as we are aware, there does not exist a Leishmania vaccine that replicates the protective immune response observed in healed mice.
Following infected sand fly challenge, L110f-vaccinated mice had increased parasite loads versus AMC- and KSAC-vaccinated animals despite comparable levels of IFN-γ+ and multi-cytokine-producing cells versus the other vaccinated groups. This increased parasite load correlated with the highest frequencies of IL-17+CD4+ T cells and the largest numbers of neutrophils per ear. Previous studies have shown KSAC to provide superior protection against Leishmania challenge compared to a vaccine containing L110f (23, 28). In the BALB/c model, Gomes et al. also found that L110f vaccination significantly reduced parasite loads following needle but not infected sand fly challenge, similar to what we have shown here employing L110f and KSAC vaccination and published previously with the ALM+CpG vaccine (32). Sand fly transmission of L. mexicana was also shown to abrogate a delay in lesion progression mediated by soluble Leishmania antigen+rIL-12 vaccination following needle challenge of BALB/c mice (43). Lastly, protection conferred by L111f+MPL-SE in mice against experimental needle challenge with L. infantum did not translate to protection in dogs following natural exposure (26, 44) although it is immunotherapeutic in mild to moderate cases of canine VL (45, 46). Therefore, abrogation of protective immunity following challenge by sand fly bite appears to be a general observation and this may be largely due to immunomodulation by neutrophils (32, 36).
Our observations employing the KSAC+GLA-SE vaccine should be viewed in the context of those by Gomes et. al. (28) in which KSAC vaccination protected otherwise susceptible BALB/c mice against early lesion development and reduced parasite loads approximately 50-fold following sand fly transmission of the FV1 strain of L. major. Protection against lesion development also occurred when infected sand fly challenge was delayed to 12 weeks post-vaccination and employed the L. major WR2885 clinical isolate, a challenge protocol similar to the one employed here. There is a not a clear explanation for the differences between the protection conferred by the same vaccine in these two different mouse strains. Importantly, this discrepancy is not due to an inability of KSAC vaccination to induce Th1 immunity in C57Bl/6 mice, since vaccination protected against needle challenge and antigen-specific IFN-γ+ cells were readily detected at the site of challenge. In addition, direct comparison of pre-challenge levels of KSAC-specific IFN-γ in C57Bl/6 versus BALB/c mice vaccinated with KSAC revealed a trend towards higher levels in C57BL/6 animals (Goto et. al.). However, protection in a resistant mouse requires that the vaccine improve upon what is ultimately a very effective primary response. In comparison, vaccination of BALB/c mice may sufficiently ablate a disease-promoting Th2 response to prevent or slow parasite replication without necessarily inducing the level of Th1 immunity at the site of challenge observed in resistant animals. For example, vaccination with the live, attenuated lpg2-/- L. major parasite protected BALB/c, but not C57BL/6 mice and was associated with a greatly diminished Th2 response rather than a strongly up-regulated Th1 response (14, 47). In the studies by Goto et. al. (23), the KSAC vaccinated BALB/c mice that were protected from needle challenge had significantly reduced levels of splenic IL-4 versus control animals, but equivalent levels of IFN-γ. By contrast, the KSAC+GLA-SE vaccinated BALB/c mice that were protected against sand fly challenge did show significantly increased IFN-γ secretion by splenic cells both pre- and post-challenge; whether or not their Th2 response was abrogated was not reported (28). The influence of KSAC vaccination on the strength and Th1/Th2 nature of subsequent immune responses to L. major transmitted by sand fly bite in these two mouse strains is likely to be in addition to any influence of neutrophils on the expression of this immunity, since sand fly bite is expected to induce neutrophil recruitment in each case.
Recent observations suggest that a sufficient resting period between boosting doses employing the GLA-SE adjuvant may be required for optimal generation of IFN-γ-producing T cells (S. Bertholet, personal communication). Therefore, the 2-week intervals used in our prime-boost protocol versus the 3-week intervals used by Gomes et al. as well as others (23) may have been too short and led to less-efficient priming of Th1 immunity than what might otherwise be possible in the C57BL/6 background, though this still would not explain the protection conferred against needle challenge.
The poly-protein+GLA-SE vaccines represent the only defined candidates currently in clinical trial for use as therapeutic or prophylactic vaccines against Leishmaniasis in humans. In the C57BL/6 mouse model employed here, vaccination with KSAC or L110f did not provide protection against infected sand fly challenge. The likely explanation for this finding is that vaccination did not sufficiently improve upon the already protective Th1 response mounted in this resistant mouse strain. Lack of protection also seemed to correlate with IL-17 production, increased numbers of neutrophils per ear, and increased lesion size. Vaccine associated IL-17 production was not limited to sand fly transmitted infections since IL-17 was also detected following needle challenge. The impact of enhanced neutrophil numbers may be especially pronounced in the context of sand fly transmission where, compared to needle challenge, a stronger and more prolonged period of neutrophil recruitment has been shown to down-modulate secondary immune responses (32). Our observations once again emphasize that mice with a healed primary infection mount the most appropriate secondary response upon challenge, as evidenced by the robust control of parasite numbers following exposure to the bites of infected sand flies. Importantly, and in contrast to protein plus adjuvant vaccination, this secondary response is almost completely devoid of IL-17 producing CD4+ cells, suggesting that IL-17 does not have a role in the protective secondary response to sand fly transmitted L. major infection.
Despite the observations reported here, the current need is for continued evaluation in people, along with a better understanding of the immune response in individuals with a healed primary infection (48). What our data do suggest is that excessive boosting with immune-stimulatory adjuvants may alter an otherwise protective response. An outstanding question is whether boosting, which is intended to expand Leishmania-specific cells, requires the use of an adjuvant once a Th1 response has been primed. Recent findings by Okwar et al. suggest that heat-killed L.m.-antigen alone is sufficient to induce a protective response comparable to that observed in healed mice provided the antigen is administered five times (49). It will be important to test this boosting regimen against infected sand fly challenge.
The observations reported here should provide critical information for the further development of a Leishmania vaccine, including the factors determining protection following needle versus infected sand fly challenge and the appropriate use of powerful immuno-stimulatory adjuvants.
Acknowledgements
The authors wish to thank Kim Beacht for technical assistance.
Footnotes
This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases
References
- 1.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nature reviews. Microbiology. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 2.Greenblatt CL. The present and future of vaccination for cutaneous leishmaniasis. Prog Clin Biol Res. 1980;47:259–285. [PubMed] [Google Scholar]
- 3.Kellina OI. Problem and current lines in investigations on the epidemiology of leishmaniasis and its control in the U.S.S.R. Bull Soc Pathol Exot Filiales. 1981;74:306–318. [PubMed] [Google Scholar]
- 4.Nadim A, Javadian E, Tahvildar-Bidruni G, Ghorbani M. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales. 1983;76:377–383. [PubMed] [Google Scholar]
- 5.Handman E. Leishmaniasis: current status of vaccine development. Clin Microbiol Rev. 2001;14:229–243. doi: 10.1128/CMR.14.2.229-243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Momeni AZ, Aminjavaheri M. Successful treatment of non-healing cases of cutaneous leishmaniasis, using a combination of meglumine antimoniate plus allopurinol. European journal of dermatology : EJD. 2003;13:40–43. [PubMed] [Google Scholar]
- 7.Aebischer T, Moody SF, Handman E. Persistence of virulent Leishmania major in murine cutaneous leishmaniasis: a possible hazard for the host. Infection and immunity. 1993;61:220–226. doi: 10.1128/iai.61.1.220-226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nature reviews. Immunology. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 9.Muller I, Kropf P, Louis JA, Milon G. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infection and immunity. 1994;62:2575–2581. doi: 10.1128/iai.62.6.2575-2581.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 11.Rhee EG, Mendez S, Shah JA, Wu CY, Kirman JR, Turon TN, Davey DF, Davis H, Klinman DM, Coler RN, Sacks DL, Seder RA. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. The Journal of experimental medicine. 2002;195:1565–1573. doi: 10.1084/jem.20020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 13.Palatnik-de-Sousa CB, Barbosa Ade F, Oliveira SM, Nico D, Bernardo RR, Santos WR, Rodrigues MM, Soares I, Borja-Cabrera GP. FML vaccine against canine visceral leishmaniasis: from second-generation to synthetic vaccine. Expert Rev Vaccines. 2008;7:833–851. doi: 10.1586/14760584.7.6.833. [DOI] [PubMed] [Google Scholar]
- 14.Uzonna JE, Spath GF, Beverley SM, Scott P. Vaccination with phosphoglycan-deficient Leishmania major protects highly susceptible mice from virulent challenge without inducing a strong Th1 response. J Immunol. 2004;172:3793–3797. doi: 10.4049/jimmunol.172.6.3793. [DOI] [PubMed] [Google Scholar]
- 15.Okwor I, Uzonna J. Persistent parasites and immunologic memory in cutaneous leishmaniasis: implications for vaccine designs and vaccination strategies. Immunologic research. 2008;41:123–136. doi: 10.1007/s12026-008-8016-2. [DOI] [PubMed] [Google Scholar]
- 16.Mendez S, Gurunathan S, Kamhawi S, Belkaid Y, Moga MA, Skeiky YA, Campos-Neto A, Reed S, Seder RA, Sacks D. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J Immunol. 2001;166:5122–5128. doi: 10.4049/jimmunol.166.8.5122. [DOI] [PubMed] [Google Scholar]
- 17.Selvapandiyan A, Dey R, Nylen S, Duncan R, Sacks D, Nakhasi HL. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J Immunol. 2009;183:1813–1820. doi: 10.4049/jimmunol.0900276. [DOI] [PubMed] [Google Scholar]
- 18.Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2012;30:134–141. doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaye PM, Aebischer T. Visceral leishmaniasis: immunology and prospects for a vaccine. Clinical microbiology and infection. 2011;17:1462–1470. doi: 10.1111/j.1469-0691.2011.03610.x. [DOI] [PubMed] [Google Scholar]
- 20.Costa CH, Peters NC, Maruyama SR, de Brito EC, Jr., Santos IK. Vaccines for the leishmaniases: proposals for a research agenda. PLoS neglected tropical diseases. 2011;5:e943. doi: 10.1371/journal.pntd.0000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noazin S, Khamesipour A, Moulton LH, Tanner M, Nasseri K, Modabber F, Sharifi I, Khalil EA, Bernal ID, Antunes CM, Smith PG. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis: a meta-analysis. Vaccine. 2009;27:4747–4753. doi: 10.1016/j.vaccine.2009.05.084. [DOI] [PubMed] [Google Scholar]
- 22.Noazin S, Modabber F, Khamesipour A, Smith PG, Moulton LH, Nasseri K, Sharifi I, Khalil EA, Bernal ID, Antunes CM, Kieny MP, Tanner M. First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine. 2008;26:6759–6767. doi: 10.1016/j.vaccine.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 23.Goto Y, Bhatia A, Raman VS, Liang H, Mohamath R, Picone AF, Vidal SE, Vedvick TS, Howard RF, Reed SG. KSAC, the first defined polyprotein vaccine candidate for visceral leishmaniasis. Clinical and vaccine immunology : CVI. 2011;18:1118–1124. doi: 10.1128/CVI.05024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skeiky YA, Coler RN, Brannon M, Stromberg E, Greeson K, Crane RT, Webb JR, Campos-Neto A, Reed SG. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine. 2002;20:3292–3303. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 25.Bertholet S, Goto Y, Carter L, Bhatia A, Howard RF, Carter D, Coler RN, Vedvick TS, Reed SG. Optimized subunit vaccine protects against experimental leishmaniasis. Vaccine. 2009;27:7036–7045. doi: 10.1016/j.vaccine.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infection and immunity. 2007;75:4648–4654. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coler RN, Skeiky YA, Bernards K, Greeson K, Carter D, Cornellison CD, Modabber F, Campos-Neto A, Reed SG. Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infection and immunity. 2002;70:4215–4225. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes R, Teixeira C, Oliveira F, Lawyer PG, Elnaiem DE, Meneses C, Goto Y, Bhatia A, Howard RF, Reed SG, Valenzuela JG, Kamhawi S. KSAC, a defined Leishmania antigen, plus adjuvant protects against the virulence of L. major transmitted by its natural vector Phlebotomus duboscqi. PLoS neglected tropical diseases. 2012;6:e1610. doi: 10.1371/journal.pntd.0001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakravarty J, Kumar S, Trivedi S, Rai VK, Singh A, Ashman JA, Laughlin EM, Coler RN, Kahn SJ, Beckmann AM, Cowgill KD, Reed SG, Sundar S, Piazza FM. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine. 2011;29:3531–3537. doi: 10.1016/j.vaccine.2011.02.096. [DOI] [PubMed] [Google Scholar]
- 30.Llanos-Cuentas A, Calderon W, Cruz M, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Bertholet S, Laughlin EM, Kahn SJ, Beckmann AM, Cowgill KD, Reed SG, Piazza FM. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with sodium stibogluconate for the treatment of mucosal leishmaniasis. Vaccine. 2010;28:7427–7435. doi: 10.1016/j.vaccine.2010.08.092. [DOI] [PubMed] [Google Scholar]
- 31.Nascimento E, Fernandes DF, Vieira EP, Campos-Neto A, Ashman JA, Alves FP, Coler RN, Bogatzki LY, Kahn SJ, Beckmann AM, Pine SO, Cowgill KD, Reed SG, Piazza FM. A clinical trial to evaluate the safety and immunogenicity of the LEISHF1+MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine. 2010;28:6581–6587. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 32.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, Sacks DL. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS pathogens. 2009;5:e1000484. doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamper LW, Patrick RL, Fay MP, Lawyer PG, Elnaiem DE, Secundino N, Debrabant A, Sacks DL, Peters NC. Infection parameters in the sand fly vector that predict transmission of Leishmania major. PLoS neglected tropical diseases. 2011;5:e1288. doi: 10.1371/journal.pntd.0001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sacks DL, Hieny S, Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. J Immunol. 1985;135:564–569. [PubMed] [Google Scholar]
- 35.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribeiro-Gomes FL, Peters NC, Debrabant A, Sacks DL. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS pathogens. 2012;8:e1002536. doi: 10.1371/journal.ppat.1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Trez C, Magez S, Akira S, Ryffel B, Carlier Y, Muraille E. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS pathogens. 2009;5:e1000494. doi: 10.1371/journal.ppat.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu W, Huang L, Mendez S. A live Leishmania major vaccine containing CpG motifs induces the de novo generation of Th17 cells in C57BL/6 mice. European journal of immunology. 2010;40:2517–2527. doi: 10.1002/eji.201040484. [DOI] [PubMed] [Google Scholar]
- 39.Lopez Kostka S, Dinges S, Griewank K, Iwakura Y, Udey MC, von Stebut E. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol. 2009;182:3039–3046. doi: 10.4049/jimmunol.0713598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. The Journal of allergy and clinical immunology. 2009;123:986–994. doi: 10.1016/j.jaci.2009.03.033. quiz 995-986. [DOI] [PubMed] [Google Scholar]
- 41.Kimblin N, Peters N, Debrabant A, Secundino N, Egen J, Lawyer P, Fay MP, Kamhawi S, Sacks D. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc Natl Acad Sci U S A. 2008;105:10125–10130. doi: 10.1073/pnas.0802331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters NC, Sacks DL. The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cellular microbiology. 2009;11:1290–1296. doi: 10.1111/j.1462-5822.2009.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogers ME, Sizova OV, Ferguson MA, Nikolaev AV, Bates PA. Synthetic glycovaccine protects against the bite of leishmania-infected sand flies. The Journal of infectious diseases. 2006;194:512–518. doi: 10.1086/505584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gradoni L, Foglia Manzillo V, Pagano A, Piantedosi D, De Luna R, Gramiccia M, Scalone A, Di Muccio T, Oliva G. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine. 2005;23:5245–5251. doi: 10.1016/j.vaccine.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Trigo J, Abbehusen M, Netto EM, Nakatani M, Pedral-Sampaio G, de Jesus RS, Goto Y, Guderian J, Howard RF, Reed SG. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine. 2010;28:3333–3340. doi: 10.1016/j.vaccine.2010.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miret J, Nascimento E, Sampaio W, Franca JC, Fujiwara RT, Vale A, Dias ES, Vieira E, da Costa RT, Mayrink W, Campos Neto A, Reed S. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine. 2008;26:1585–1594. doi: 10.1016/j.vaccine.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kebaier C, Uzonna JE, Beverley SM, Scott P. Immunization with persistent attenuated Delta lpg2 Leishmania major parasites requires adjuvant to provide protective immunity in C57BL/6 mice. Infection and immunity. 2006;74:777–780. doi: 10.1128/IAI.74.1.777-780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nylen S, Khamesipour A, Mohammadi A, Jafari-Shakib R, Eidsmo L, Noazin S, Modabber F, Akuffo H. Surrogate markers of immunity to Leishmania major in leishmanin skin test negative individuals from an endemic area re-visited. Vaccine. 2006;24:6944–6954. doi: 10.1016/j.vaccine.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 49.Okwor I, Kuriakose S, Uzonna J. Repeated inoculation of killed Leishmania major induces durable immune response that protects mice against virulent challenge. Vaccine. 2010;28:5451–5457. doi: 10.1016/j.vaccine.2010.05.077. [DOI] [PubMed] [Google Scholar]