Summary
Podosomes are cytoskeletal-based structures involved in extracellular matrix (ECM) remodeling and cellular motility. A new study now implicates podosomes in pore formation during myoblast fusion.
Cell-cell fusion is a highly regulated event that is critical for many physiological and pathological events, including fertilization, muscle development, and immune response. During skeletal muscle development, fusion of muscle cells generates multinucleate and functional muscle fibers and aberrant fusion has been implicated in dystrophic muscle diseases [1, 2]. Recently, a number of groups have studied myoblast fusion during body wall muscle formation in Drosophila melanogaster as a genetically tractable in vivo model system to study cell-cell fusion [1]. In a new study published in the Journal of Cell Biology, Sens et al. [3] investigated the role of actin assembly in formation of the fusion pore during Drosophila myoblast fusion. Interestingly, they find that an invasive actin-rich podosome-like structure is used by fusion competent myoblasts (FCM) to adhere to and fuse with muscle founder cells.
Previously, it was known that actin filaments accumulate transiently at the site of myoblast fusion [4–6], dependent on signaling from heterotypic adhesion molecules and downstream regulators of branched actin assembly, including Rac, SCAR and WASP [7]. Furthermore, both the SCAR and WASP complex activators of the branched actin nucleating Arp2/3 complex were known to be essential for myoblast fusion [5, 6, 8–10]. However, the nature of the fusion structure and the roles of individual actin regulators were poorly understood. To determine whether the prominent actin accumulations at pre-fusion sites were unique to a muscle cell subtype, Sens et al. [3] expressed GFP-actin under the control of FCM or founder cell promoters and costained for all actin filaments in embryos with fluorescent phalloidin. Interestingly, the large actin foci were exclusively found in FCM cells and were associated with a deformation in the founder cell membrane. Transmission electron microscopy studies showed finger-like FCM cell protrusions apparently invading into the founder cells at the site of cell-cell fusion.
Invasive actin-rich finger-like protrusions have been well characterized in cells that invade or remodel tissue and are termed invadopodia in cancer cells and podosomes in normal cells (or collectively, invadosomes) [11, 12]. However, a role in cell-cell fusion has not been previously described, and their main function is thought to be degradation of extracellular matrix (ECM) (Fig 1), in part due to active trafficking of ECM-degrading proteinases to sites of protrusion formation. The myoblast structures seemed to be a potential variation of podosomes, as they were morphologically similar by electron microscopy and even had adhesion ring structures, albeit cell-cell rather than cell-ECM adhesions. If they really were podosomes, their data suggested that podosomes are more versatile than previously appreciated and also that they are formed in vivo during developmental “invasions”.
Figure 1. Comparison of myoblast podosome (A) with ECM-degrading podosome (B).
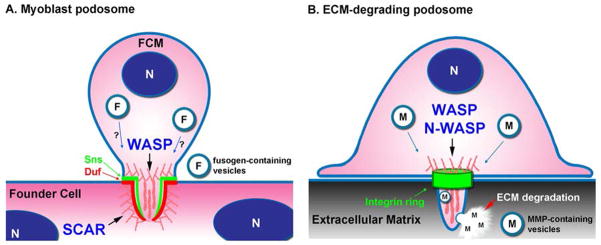
(A) Actin assembly is required on both the fusion competent myoblast (FCM) and founder cell membranes for initiation of fusion pore formation. Although WASP and SCAR have redundant functions in the formation of actin foci in FCM podosome structures, WASP is essential for invasion of FCM podosomes into founder cells and SCAR induces formation of a thin actin layer at the prefusion site in founder cells. Similar to traditional podosomes (B), myoblast podosomes have an adhesion ring; however the molecular components are Ig-superfamily cell-cell adhesion receptors found in the FCM (Sns ring) and founder cells (Duf ring). Fusogen-containing vesicles may be trafficked to podosomes to promote cell fusion. (B) WASP/N-WASP promotes formation of branched actin-rich ECM-degrading podosomes. Rings containing integrins and focal adhesion proteins are formed around ECM-degrading podosomes. Matrix-metalloproteinases (MMPs) are embedded in vesicles, transported to podosomes, and secreted to induce ECM degradation. N: nucleus.
To determine whether the FCM actin-rich protrusions resembled podosomes molecularly, Sens et al. [3] manipulated the SCAR and WASP regulators of branched actin assembly and determined the effects on F-actin foci formation and myoblast fusion. Loss of both protein complexes in scar, sltr double mutants led to loss of the FCM actin focus, verifying that it is a branched actin-based structure. However, the actin focus was present in single scar or sltr mutants, suggesting compensation of one branched actin regulator for the other. More interesting, however, was the finding that despite continued formation of the F-actin foci on the FCM side of the pre-fusion site, loss of WASP but not SCAR complexes led to defective formation of invasive protrusions and lack of FCM invasion into founder cells. By contrast, loss of SCAR affected actin assembly at the founder cell membrane side of the pre-fusion site. Since WASP, but not SCAR, homologues are known to be essential for podosome formation and function in mammalian cells, these data support the concept that the invasive myoblast protrusions are similar or identical to podosomes.
Sens et al. suggest that actin assembly is required on both the FCM and founder cell membranes for initiation of fusion pore formation. In FCM cells, WASP activity promotes formation of actin-rich invasive podosomes that protrude into founder cells and allow extensive membrane contact at the prefusion site. In founder cells, SCAR promotes formation of a thin actin layer at the prefusion site and is necessary for cell fusion. These two actin structures may allow close enough apposition and/or curvature of the membranes for fusion to initiate. They may also serve as docking sites for vesicles containing fusogenic factors, such as lipid rafts (Fig 1) [13, 14]. Notably, Golgi-derived vesicles with electron-dense rims have been described trafficking toward muscle cell contact sites in Drosophila embryos [5]. Moreover, invadopodia in cancer cells have been shown to be dependent on the lipid raft protein caveolin [15] and both podosomes and invadopodia are sites of active vesicle trafficking [11, 12]. Since both WASP and SCAR are essential for myoblast fusion, the authors were not able to dissect further the individual roles of these actin regulators in later events. However, they did analyze the structure of the fusion site in wild type embryos by a high pressure freezing/freeze substitution electron microscopy preparation and found a single macro fusion pore filled with ribosomes and other organelles but not actin. Thus, after fusion pore initiation, rapid disassembly of actin and pore expansion is likely to occur.
Overall, the study by Sens et al. [3] provides an elegant example of the versatility of actin-based structures for cellular invasion processes in vivo. Although a previous study had shown that leukocytes use podosomes as adhesion and invasion structures during transcytosis of endothelial cells [16], podosomes had not been previously identified as mediators of direct fusion of two cells. Indeed, the dogma in the field has been that podosomes are structures that mediate ECM adhesion and degradation. A final novel contribution of this paper is the identification of podosomes in an in vivo setting, which has been limited [17, 18], potentially due to the small size and transient nature [11, 19]. Future studies should shed further light upon the adaptability of these invasive structures.
References
- 1.Chen EH, Olson EN. Towards a molecular pathway for myoblast fusion in Drosophila. Trends Cell Biol. 2004;14:452–460. doi: 10.1016/j.tcb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Volonte D, Peoples AJ, Galbiati F. Modulation of myoblast fusion by caveolin-3 in dystrophic skeletal muscle cells: implications for Duchenne muscular dystrophy and limb-girdle muscular dystrophy-1C. Mol Biol Cell. 2003;14:4075–4088. doi: 10.1091/mbc.E03-03-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sens KL, Zhang S, Jin P, Duan R, Zhang G, Luo F, Parachini L, Chen EH. An invasive podosome-like structure promotes fusion pore formation during myoblast fusion. J Cell Biol. 2010;191:1013–1027. doi: 10.1083/jcb.201006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesper DA, Stute C, Buttgereit D, Kreiskother N, Vishnu S, Fischbach KF, Renkawitz-Pohl R. Myoblast fusion in Drosophila melanogaster is mediated through a fusion-restricted myogenic-adhesive structure (FuRMAS) Dev Dyn. 2007;236:404–415. doi: 10.1002/dvdy.21035. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134:4357–4367. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochlin K, Yu S, Roy S, Baylies MK. Myoblast fusion: when it takes more to make one. Dev Biol. 2010;341:66–83. doi: 10.1016/j.ydbio.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger S, Schafer G, Kesper DA, Holz A, Eriksson T, Palmer RH, Beck L, Klambt C, Renkawitz-Pohl R, Onel SF. WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. J Cell Sci. 2008;121:1303–1313. doi: 10.1242/jcs.022269. [DOI] [PubMed] [Google Scholar]
- 9.Massarwa R, Carmon S, Shilo BZ, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell. 2007;12:557–569. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Schafer G, Weber S, Holz A, Bogdan S, Schumacher S, Muller A, Renkawitz-Pohl R, Onel SF. The Wiskott-Aldrich syndrome protein (WASP) is essential for myoblast fusion in Drosophila. Dev Biol. 2007;304:664–674. doi: 10.1016/j.ydbio.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 13.Martens S, McMahon HT. Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9:543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 14.Mukai A, Kurisaki T, Sato SB, Kobayashi T, Kondoh G, Hashimoto N. Dynamic clustering and dispersion of lipid rafts contribute to fusion competence of myogenic cells. Exp Cell Res. 2009;315:3052–3063. doi: 10.1016/j.yexcr.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, Fukami K. Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res. 2009;69:8594–8602. doi: 10.1158/0008-5472.CAN-09-2305. [DOI] [PubMed] [Google Scholar]
- 16.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity. 2007;26:784–797. doi: 10.1016/j.immuni.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol. 2010;189:13–22. doi: 10.1083/jcb.200912096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rottiers P, Saltel F, Daubon T, Chaigne-Delalande B, Tridon V, Billottet C, Reuzeau E, Genot E. TGFbeta-induced endothelial podosomes mediate basement membrane collagen degradation in arterial vessels. J Cell Sci. 2009;122:4311–4318. doi: 10.1242/jcs.057448. [DOI] [PubMed] [Google Scholar]
- 19.Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]


