Abstract
Cancer of the oral cavity is a serious disease, affecting about 30,000 individuals in US annually. There are several animal models of oral cancer, but each has certain disadvantages. As a new model, we investigated whether topical application of the tobacco smoke carcinogen, dibenzo[a,l]pyrene (DB[a,l]P) is mutagenic and carcinogenic in the oral cavity of the B6C3F1 lacI and B6C3F1 mouse, respectively. B6C3F1 lacI mice received DB[a,l]P (0, 3, 6, 12 nmol) 3× per week. B6C3F1 mice received the same doses and also 24 nmol. At 38 weeks mutagenesis was measured in oral tissues in lacI mice. For the high dose group, the mutant fraction (MF) in upper mucosa and tongue increased about twofold relative to that in vehicle-alone. The increases were statistically significant. The mutational profile in the DB[a,l]P-induced mutants was compared with that induced by benzo[a]pyrene (BaP) in oral tissue. BaP is mutagenic in many tissues when administered by gavage. The mutational profile for DB[a,l]P was more similar to that reported for p53 mutations in head and neck cancers than was that of BaP. At 47 weeks, oral squamous cell carcinomas (OSCC) were found in 31% of the high-dose B6C3F1 group. Elevations of p53 and COX-2 protein were observed in tumor and dysplastic tissue. As DB[a,l]P induces mutations and tumors in the oral cavity, and has a mutational profile in oral tissue similar to that found in p53 in human OSCC, the treatment protocol described here may represent a new and relevant model for cancer of the oral cavity.
Keywords: DB[a,l]P; oral cancer; mutagenesis; mutational profile; tobacco smoking
The annual incidence of cancer of the oral cavity in the US is about 30,000 cases and the 5-year survival rate only about 60%.1 Treatment is often disfiguring and in certain countries the incidence rate is much higher.2 A large majority of the cases in the US result from tobacco and ethanol exposure.2 Experimental animal models would be useful in identifying chemopreventive agents and following disease progression. However, there are few models and these have certain disadvantages. For instance, 4-nitroquinoline-N-oxide is an oral carcinogen in the rat and mouse.3–6 However, it is not a tobacco smoke carcinogen, and, in contrast to many tobacco smoke procarcinogens,7 is not oxidatively activated to its ultimate genotoxic form.8 Another model uses 7,12-dimethylbenzanthracene in the hamster cheek pouch.9 7,12-Dimethylbenzanthracene is oxidatively activated, but is not found in tobacco smoke or the environment. Also, the hamster cheek pouch is essentially designed for storage and transport of food, and this model is a questionable surrogate for the human oral cavity. Polycyclic aromatic hydrocarbons are thought to be important tobacco smoke carcinogens resulting from pyrolysis of tobacco.10 The tobacco smoke polycyclic aromatic hydrocarbon, benzo[a]pyrene (BaP), induces tongue tumors in a long-term feeding bioassay, but forestomach is the main target and BaP is quite toxic at the high doses necessary to induce tongue tumors.11 In one report, tobacco-specific nitrosamines induced tumors in the rat oral cavity, but the incidence was low and required a surgical application of the carcinogens.12 Hence it seems reasonable that additional models could play an important role in investigations of carcinogenesis in the oral cavity.
Here we have investigated whether the powerful carcinogen, dibenzo[a,l]pyrene (DB[a,l]P), is mutagenic and carcinogenic in the oral cavity of the lacI mouse upon topical application. As mutagenesis represents an important step in carcinogenesis, mutagenesis can represent an early biomarker for cancer and precancer. DB[a,l]P is an extremely powerful carcinogen in several animal models, and a component of tobacco smoke13–17 and thus seems an appropriate model compound for an oral carcinogen. As DB[a,l]P is reported to be a much more powerful mutagen and carcinogen than BaP in mouse lung,13,18 we reasoned it would likely be a more potent carcinogen than BaP in the oral cavity and if DB[a,l]P is also mutagenic, mutagenesis could serve as an early surrogate for carcinogenesis. Furthermore, the topical administration of DB[a,l]P by administration into the oral cavity is likely to result in more selective targeting to the oral cavity. In addition to potency differences, it was also reported that there were differences in the mutational specificities of BaP and DB[a,l]P in the lung.18 Here the mutational profiles of the two carcinogens in the oral cavity were compared with their mutational profiles previously reported in lung, where DB[a,l]P is a much more potent carcinogen. We also compared the mutational profiles of BaP and DB[a,l]P with that of P53 in human oral cavity tumors.
Methods
Chemicals
Protease K and RNase A were purchased from Sigma Chemical Co. The syntheses of DB[a,l]P was carried out following our recently reported Suzuki cross-coupling reaction approach.19 Briefly, 5,6-dihydrobenz[d,e]anthracene, prepared from benzanthrone,20 was brominated using Br2/AcOH to afford 7-bromobenzanthracene derivative which was subjected to palladium-catalyzed Suzuki coupling reaction with 2-formylphenylboronic acid. Refluxing of the resulting aldehyde derivative with aniline followed by t-BuOK assisted cyclization yielded the DB[a,l]P.
Animals
Female B6C3F1 (Jackson Laboratories, Bar Harbor, ME) and Big Blue™ B6C3F1(Stratagene, La Jolla, CA), 6 weeks of age, were used in carcinogenesis and mutagenesis study, respectively. Mice were quarantined for 1 week; then they were transferred to the bioassay laboratory. All mice were kept on a 12-h light:12-h dark cycle, maintained at 50% relative humidity and (21 ± 2)°C and were fed a semi-purified, modified AIN-93M diet (5% corn oil) and water ad libitum. The bioassay was carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and was approved by Institutional Animal Care and Use Committee at Penn State Medical School.
Animal treatment
In the carcinogenesis bioassay, four groups of female B6C3F1 mice (20/group) at the age of 8 weeks old received DB[a,l]P in 30 µl DMSO (3, 6, 12, and 24 nmol) or DMSO alone administered into the oral cavity three times a week for 38 weeks. Mice were weighed weekly until termination at 47 weeks after the first carcinogen administration. During the progress of the bioassay, mice were culled from the group and sacrificed if we observed a sudden weight loss of more than 20% or a tumor size exceeding 2 cm in diameter. At termination, mice were sacrificed by carbon dioxide asphyxiation and soft tissues of the oral cavity including tongue, pharynx and cheek were collected and fixed in 10% neutral buffered formalin. Tissues were processed in an automated Tissue-Tek VIP processor and paraffin-embedded with a Tissue-Tek TEC embedding station. Sections were cut at 6 µm for routine hematoxylin and eosin (H&E) staining. All tissues were examined by an ACVP diplomate pathologist blinded to treatment and were graded for the presence of hyperplasia, dysplasia, carcinoma in situ (CIS) or invasive squamous cell carcinoma (SCC) according to established criteria.21
In the mutagenesis study, Big Blue™ B6C3F1 (lacI) mice (6/group) received DB[a,l]P in DMSO (0, 3, 6, and 12 nmol), three times a week and mice were sacrificed 38 weeks after the first carcinogen administration. The tongues were excised and stored at −80°C until isolation of DNA. Oral tissue from BaP-treated mice used for the comparison of mutational spectra was taken from mice previously treated with BaP.22
DNA isolation
Tongues were cut lengthwise in half and one half was taken for DNA isolation and the other half frozen and stored. The pallet and pharynx were pooled and homogenized together to give a mixture designated “upper mucosa.” Each tissue was gently homogenized by hand in a microcentrifuge tube using a Teflon pestle in three volumes of 10 mM Tris-HCl (pH, 8.0), 10 mM EDTA, 150 mM NaCl per gram tissue weight (w/v). SDS and protease K were added to this homogenate to obtain final concentrations of 10 and 1 mg/ml, respectively. The mixture was incubated overnight at 37°C or 2–3 hr at 50°C, and then for 30 min at 37°C with 0.1 mg/ml RNase A. After incubation one-third volume of 6M ammonium acetate (pH 7.4) was added, the mixture was gently mixed and then centrifuged at 14,000 rpm in an Eppendorf microfuge. The supernatant was carefully removed, leaving a small volume behind, to avoid transfer of any of the precipitate. To the supernatant an equal volume of isopropyl alcohol was added at room temperature to precipitate DNA. The supernatant was removed, the DNA was washed once with 70% ethanol, suspended in 10 mM Tris-HCl (pH, 8.0), 1 mM EDTA and left overnight at room temperature to dissolve.
Mutagenesis assay
Phage packaging was carried out using a homemade packaging extract prepared from bacterial strains supplied by Dr. Peter Glazer (Yale University School of Medicine, New Haven, CT); and the positive selection cII mutation assay was performed as previously described.23 At least three packaging reactions were carried out for each DNA sample. The mean mutant fractions of each group were compared with those from the control (vehicle-alone) group using a one-tailed t-test.
Amplification and sequencing
Mutants were cored from petri dishes and the agar plug was mixed with 100 µl phage buffer. Ten microliters of the buffer were then spread on a selective plate to confirm mutant phenotype and purify mutant phages. Fifty mutant plaques from DB[a,l]P and BaP treated oral tissue and controls were randomly selected from at least four selective plates per compound. The purified mutant plaques were then subjected to amplification and sequencing of the cII gene by PCR. Sequencing was performed by Roderick Haesevoets, University of Victoria, BC, Canada.
Amplification
the primer sequences were: forward: 5′-AAAAAGGGCATCAAATTAAACC-3′, reverse: 5′-CCGAAGTTGAGTATTTTTGCTGT-3′.
The reaction mixture (100 µl reaction) consisted of: H2O, 59.1 µl; 100 mM dNTP mix,1.0 µl; 10× buffer,10 µl; cII forward primer (10 µM) 2.0 µl; cII reverse primer (10.0 µM) 2.0 µl; 50 mM MgCl2,3.5 µl; Taq, 2.4 µl; sample, 20 µl; 10 × buffer: 100 mM Tris HCl pH 9.0, 500 mM KCl, 1.0% TritonX100. The PCR conditions were: 94.0°, 4.0 min; 30 cycles: 95.0°, 30 sec; 55.0°; 30 sec; 72.0°, 2.5 min; 4.0°, hold.
Purification
PCR Product Pre-Sequencing Kit (USB); as per kit instructions.
Sequencing
Primer sequences, cII forward: 5′-ACCACACCTATGGTGTATG-3′, cII reverse, 5′-GTCATAATGACTCCTGTTGA-3′ (only used to confirm a mutation if the sequence from cII forward primer was not clear). The reaction mixture was prepared from a CEQ Dye Terminator Cycle Sequencing (DTCS) Quick Start Kit (Beckman-Coulter), PCR conditions: 96.0°, 5.0 min, 30 cycles: 96.0°, 20 sec; 52.0°, 25 sec; 60.0°, 4.0 min; 4.0°, hold.
Electrophoresis/base calling/trace generation
Sequencing was done on a CEQ8000 Capillary Electrophoresis DNA Sequencer (Beckman-Coulter) and the analysis software was: SeqMan II v6.1 (DNASTAR).
Immunohistochemical analysis
Bouin’s fixed, paraffin-embedded tissue blocks were sectioned at 5 microns. Slides were prepared as described previously.24 After washing, the slides were incubated with 1:50 dilution of the rabbit polyclonal primary antibody, p53 (CM5, Novocastra) or COX-2 (Cayman) for 30 min at room temperature, incubated with Dako Envision+™ anti-rabbit labeled polymer conjugated with horseradish peroxidase (HRP) for 20 min at room temperature, developed using Dako DAB+™, and counter-stained with Meyer’s modified hematoxylin.
Statistics
Significant pairwise differences in tumor incidence between treated groups and controls were determined using Fisher’s Exact test followed by adjusted post-hoc testing. The overall differences in body weights during the bioassay were tested using one-way analysis of variance (ANOVA) followed by Dunnett’s procedure to determine whether the effects of treatment during and after carcinogen administration differed from carcinogen alone. The differences in survival were analyzed by the Log-Rank test.
For mutagenesis the mean mutant fractions of each group were compared with those from the control (vehicle-alone) group using a one-way ANOVA. A post hoc comparison of each nonzero dosage against the control was performed using Dunnett’s test. The distributions of AT containing mutations by group were compared using Fisher’s Exact test which showed that the distributions are statistically significantly different (p = 0.002).
Results
Mutagenesis
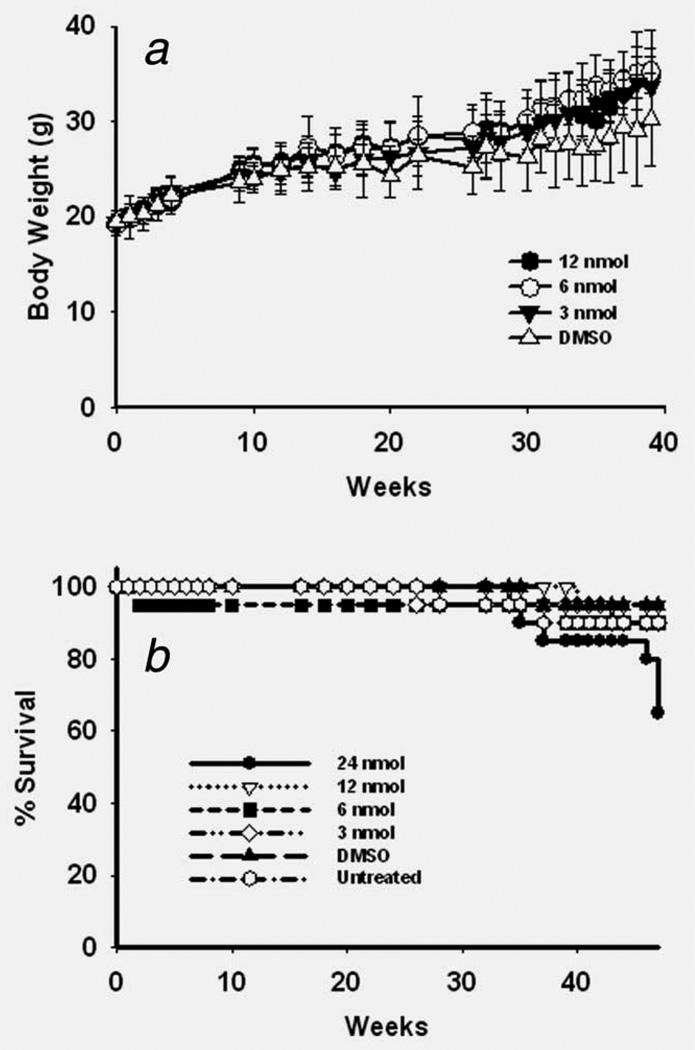
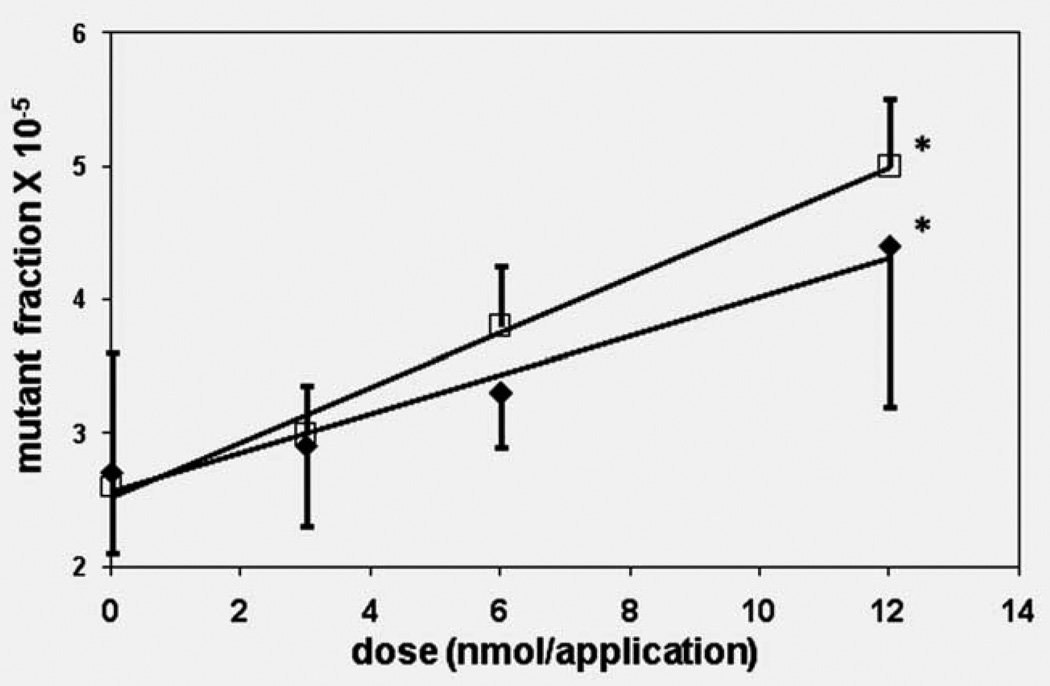
Several trial doses were chosen for the mutagenesis and carcinogenesis assays. At the doses and administrations tested for mutagenesis (3–12 nmol/treatment) DB[a,l]P treatments led to no apparent toxicity as judged by weight gain and physical observation, and no increased mortality (Figs. 1a and 1b). DB[a,l]P was mutagenic in tongue and upper oral mucosa, and exhibited a dose response. The increase in mutant fraction relative to vehicle control reached statistical significance at the high dose (Fig. 2) and there was a statistically significant difference in mutation rates among dosage levels for the mucosa (p = 0.008) and for the tongue (p = 0.018).
Figure 1.
(a) Effect of dose of DB[a,l]P on weights of B6C3F1 and lacZ B6C3F1 mice (total mice for each dose in the two groups). Error bars show SD from mean weights. (b) Survival curves for B6C3F1 and lacZ B6C3F1 mice treated with different doses of DB[a,l]P.
Figure 2.
Dose response for mutagenesis in the cII gene induced by DB[a,l]P in the tongue and other oral tissue of B6C3F1 lacI mice. □, upper oral mucosa; ♦, tongue. ANOVA was conducted on the mutant fractions at the four dosages of DBP (including a control level of zero), separately for the oral mucosa and for the tongue. There was a statistically significant difference in mutation rates among dosage levels for the mucosa (p = 0.008) and for the tongue (p = 0.018). A post hoc comparison of each nonzero dosage against the control was performed using Dunnett’s test (as a 1 tailed test for increased mutant fractions). The mutant fraction for the high dose oral mucosa and the high dose tongue DNA were significantly different from their controls (p = 0.003 and p = 0.004, respectively). *p < 0.05 vs. control.
We then compared the mutational profile of DB[a,l]P with that of BaP in the mouse oral cavity (Table 1). BaP can induce tongue tumors in mice after long-term exposure, although tongue is not the major target.11 The majority of the mutations induced by DB[a,l]P and BaP were base-substitutions, and of these the majority was at GC base pairs. However, the major difference in the mutational profiles of DB[a,l]P and BaP was that DB[a,l]P induced a significantly higher fraction of mutations at AT base pairs than BaP.
Table 1.
Mutational profiles mutations in oral tissue from control, DB[a,l]P and BaP-treated mice and comparison with the reported mutational profile of mutant P53 in head and neck tumors
| Percentage of mutations | ||||
|---|---|---|---|---|
| DB[a,l]P1 | BaP2 | Control3 | P534 | |
| Class | ||||
| GC:AT | 17 | 31 | 63 | 40 |
| GC:TA | 33 | 40 | 14 | 17 |
| GC:CG | 7 | 10 | 3 | 8 |
| AT:GC | 7 | 0 | 3 | 12 |
| AT:CG | 10 | 0 | 3 | 4 |
| AT:TA | 14 | 4 | 6 | 7 |
| in/del5 | 12 | 15 | 9 | 13 |
Percentage based on 42 mutants. Nine redundant mutations were also found and were not included.
Percentage based on 48 mutants. Four redundant mutations were found and were not included.
Percentage based on 35 mutants. Five redundant mutations were found and were not included.
Percentage taken from Ref. 25.
Insertions and deletions.
The distributions of AT containing mutations between the DB[a,l]P and BaP mutational profiles were compared using Fisher’s Exact test which showed that the distributions are statistically significantly different (p = 0.002).
The mutant fractions for DB[a,l]P in upper oral mucosa was 4.9 ± 0.4, and for BaP 18.1 ± 2.1. The total dose of DB[a,l]P was 0.14 µmol and that for BaP was 4 µmol. Mutant fractions for oral tissue controls were 2.7 ± 0.8 and 2.5 ± 0.7, resp.
Carcinogenesis
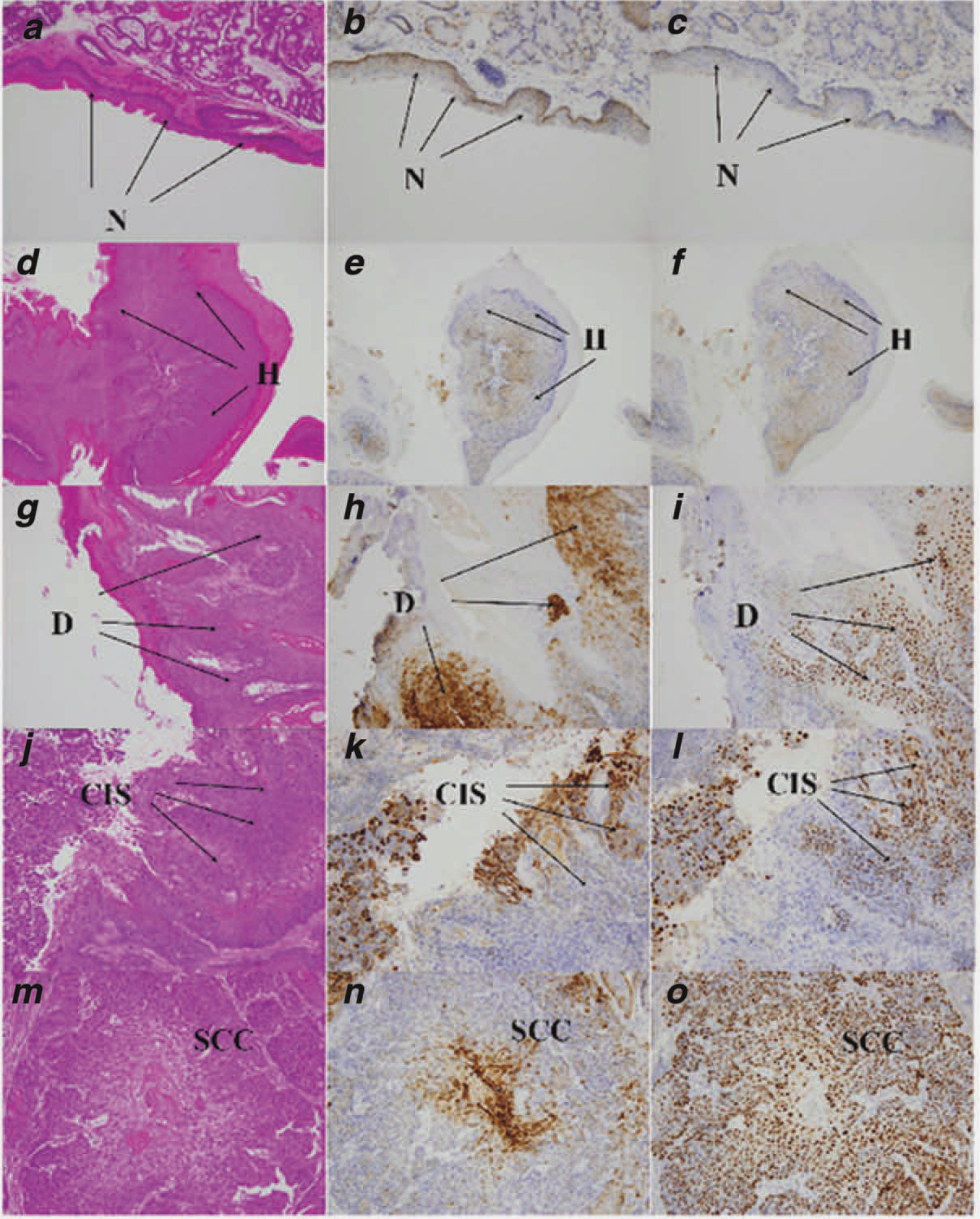
In the carcinogenesis bioassay DB[a,l]P was administered at 3–24 nmol/treatment. Only at the highest dose was increased mortality observed at the later time points (Fig. 1b), which was mostly due to the large size of tumors in the oral cavity. In oral tissues, the 24 nmol dose of DB[a,l]P treatment resulted in neoplasia in 31% of the mice, and another 19% exhibited other oral lesions (papillomas & keratoacanthomas) (Table 2). The other major targets were facial skin (predominantly lips) and ovaries (unpublished data). The oral tumors induced by DB[a,l]P treatment are accompanied by the elevation of p53and COX-2 protein expression (Fig. 3). There was no p53 staining observed in normal and hyperplasia region, and the staining was weaker in dysplasia region than in the region of carcinoma. COX-2 staining was evident in dysplastic and carcinoma tissue, at similar levels and was not observed in normal and hyperplasia region of oral tissues (Fig. 3).
Table 2.
Tumors induced by DB[a,l]P in the oral cavity of female B6C3F1 mice
| DB[a,l]P treatment | Number of mice |
Effective no. of mice1 |
Hyperplasia | Dysplasia | Neoplasia | Others2 | ||
|---|---|---|---|---|---|---|---|---|
| CIS | SCC | Total | ||||||
| Oral tissue | ||||||||
| 24 nmol | 20 | 16 | 15 (94)3 | 11 (69) | 1 (6) | 4 (16)4 | 5 (31)4 | 3 (19)5 |
| 12 nmol | 20 | 19 | 18 (95) | 18 (95) | 1 (5) | 1 (5) | 2 (11) | 1 (5)) |
| 6 nmol | 20 | 18 | 16 (89) | 16 (89) | 0 (0) | 1 (6) | 1 (6) | 1 (6) |
| 3 nmol | 20 | 19 | 19 (100)4 | 17 (89) | 0 (0) | 0 (0) | 0 (0) | 1 (5) |
| DMSO | 20 | 19 | 14 (74) | 18 (95) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Tongue | ||||||||
| 24 nmol | 20 | 17 | 16 (94)5 | 16 (94)4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 12 nmol | 20 | 20 | 20 (20)4 | 19 (95)4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 6 nmol | 20 | 19 | 17 (89)4 | 16 (84)5 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 3 nmol | 20 | 20 | 19 (95)5 | 18 (90)5 | 2 (10) | 0 (0) | 2 (0) | 0 (0) |
| DMSO | 20 | 20 | 12 (60) | 11 (55) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Mice which died before the first tumor appeared in the study or did not reach histology due to cannibalism were not counted.
Others are primarily papillomas.
Number in parentheses, percentage.
Significantly induced more than DMSO, p < 0.01.
Significantly induced more than DMSO, p < 0.05.
Figure 3.
Immunohistochemical analysis of p53 and COX-2 proteins expression in DB[a,l]P-treated oral tissues of B6C3F1 mouse (see “Material and Methods” section). (a) H&E staining of normal squamous epithelium. (b) Expression of COX-2 protein in normal epithelium. (c) Expression of p53 protein in normal epithelium. (d) H&E staining of hyperplasia. (e) Expression of COX-2 protein in hyperplasia. (f) Expression of p53 protein in hyperplasia. (g) H&E staining of dysplasia. (h) Expression of COX-2 protein in dysplasia. (i) Expression of p53 protein in dysplasia. (j) H&E staining of CIS. (k) Expression of COX-2 protein in CIS. (l) Expression of p53 protein in CIS. (m) H&E staining of SCC. (n) Expression of COX-2 protein in SCC. (o) Expression of p53 protein in SCC. All images taken at 20× magnification. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
This pilot study was undertaken to determine whether mutations and cancer could be induced in the oral cavity by a tobacco smoke carcinogen, as current models utilize synthetic chemicals. DB[a,l]P is the most powerful carcinogenic polycyclic aromatic hydrocarbon (PAH) in skin, breast and lung of rodents and consequently seemed like a reasonable candidate for an oral carcinogen. DB[a,l]P is many times more tumorigenic than the more commonly studied tobacco carcinogen, BaP, in several organs.13,15,18 DB[a,l]P administration into the oral cavity might then serve as a highly appropriate model of oral cancer.
DB[a,l]P was mutagenic in the oral cavity, leading to a doubling in mutant fraction at the highest dose tested (Fig. 2). However, this dose was not the maximum tolerated dose and it is likely that even higher levels of mutagenesis could be achieved by employing higher doses of DB[a,l]P, such as those used in the carcinogenesis assay (Table 2). DB[a,l]P induced high fractions of GC>TA and GC>AT substitutions, but also a significant fraction (31%) at AT base pairs (Table 1). We compared its mutational profile with that of BaP, another tobacco carcinogen thought to contribute to smoking-induced cancer.26 The major difference between the mutational profiles of these tobacco carcinogens was the much higher fraction of mutations at AT base pairs induced by DB[a,l]P. These results may be relevant to oncogenic mutations in the p53 gene in head and neck squamous cell carcinoma (HNSCC),27 as the mutational profiles of such tumors exhibit about 60% G:C > A:T + G:C > T:A mutations; and about 30% of the mutations at AT base pairs.25 These observations taken together are consistent with a possible contribution of DB[a,l]P to tobacco-induced carcinogenesis in the oral cavity in humans. The G:C > T:A transversions are characteristic of exogenous DNA-damaging agents derived from bulky carcinogens including tobacco smoke carcinogens and certain other agents.28 A similar difference in the mutational specificities of DB[a,l]P and BaP in the mouse lung has been reported.18 The higher fraction of mutations induced by DB[a,l]P at AT base pairs may result from the fact that DB[a,l]P forms a much higher percentage of depurinating (and total) adducts at adenine than does BaP29 Additionally, fiord-region PAH diol epoxide adenine adducts (such as those from DB[a,l]P) are less likely to be recognized by DNA repair proteins than those from bay region diol epoxides (such as those from BaP) and hence are more persistent.30
At the highest dose tested DB[a,l]P treatment led to oral neoplasia in about one-third of the mice and papillomas in about 20% (Table 2). DB[a,l]P also induced ovarian tumors, as previously reported.31
Immunohistochemical analyses were consistent with the histological progression of oral squamous cell carcinoma (SCC), with the induction of p53 and COX-2 proteins in tumor tissue. The expression/alteration of p53 and COX-2 protein could be valuable for the evaluation of chemotherapeutic or chemopreventive agents in animal models. DB[a,l]P treatment also led to ovarian tumors, which has been previously reported.
The DB[a,l]P model for oral carcinogenesis has an advantage over most other models in that it uses a tobacco smoke carcinogen, since tobacco smoke is a major cause of oral cancer.2 Also, all of the metabolic steps involved in the oxidative metabolic activation of tobacco PAHs are represented. This is important for studies on modulators of carcinogenesis. One study reported that long-term high dose feeding administration of BaP can induce tongue tumors in mice, but the mortality is high at doses inducing tongue tumors, and the forestomach is the major target organ.11 We have previously reported that BaP is mutagenic in the mouse oral cavity when administered by gavage,22 but under conditions do not yield tumors of the oral cavity.32 Hence, relationships between BaP-induced mutagenesis and carcinogenesis in the oral cavity in that model are uncertain. A method of carcinogen administration utilizing a surgical canal into the oral cavity has been reported,12 but this would be very difficult for routine carcinogenesis studies and would be complicated by wound healing effects.
Conclusion
The mutagenic and carcinogenic effects of DB[a,l]P in the oral cavity of female B6C3F1 lacI transgenic mice were determined and indicate the protocol described here represents a new model for oral cancer. The transgene is not expressed and hence is neutral,33 so that effects of DB[a,l]P on both strains of mice should be identical. Several doses were employed, the higher of which were mutagenic and carcinogenic. Since mutagenesis precedes carcinogenesis it seems likely that DB[a,l]P-induced mutagenesis will be observable before DB[a,l]P-induced carcinogenesis. Hence DB[a,l]P-induced mutagenesis in the oral cavity should provide a shorter-term assay that predicts DB[a,l]P carcinogenesis. Further study under different conditions will be necessary to establish this, but the current results demonstrate that orally instilled DB[a,l]P is a mutagen and carcinogen in the oral cavity.
Acknowledgements
The DB[a,l]P was synthesized in Synthesis Core. We thank Dr. Robert G. Norman (NYU Dental College) for discussions, and statistical evaluation of the mutational profiles.
Grant sponsor: NCI; Grant numbers: R01-CA100924, NO2-CB-81013-74; Grant sponsor: Penn State Cancer Institute Seed Funds
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Suzui M, Inamine M, Kaneshiro T, Morioka T, Yoshimi N, Suzuki R, Kohno H, Tanaka T. Indole-3-carbinol inhibits the growth of human colon carcinoma cells but enhances the tumor multiplicity and volume of azoxymethane-induced rat colon carcinogenesis. Int J Oncol. 2005;27:1391–1399. [PubMed] [Google Scholar]
- 4.Tanaka T. Chemoprevention of oral carcinogenesis. Oral Oncol Eur J Cancer Res B. 1995;31:3–15. doi: 10.1016/0964-1955(94)00026-z. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T, Kohno H, Sakata K, Yamada Y, Hirose Y, Sugie S, Mori H. Modifying effects of dietary capsaicin and rotenone on 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Carcinogenesis. 2002;23:1361–1367. doi: 10.1093/carcin/23.8.1361. [DOI] [PubMed] [Google Scholar]
- 6.Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–313. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 7.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 8.Rodolfo C, Lanza A, Tornaletti S, Fronza G, Pedrini AM. The ultimate carcinogen of 4-nitroquinoline 1-oxide does not react with Z-DNA and hyperreacts with B–Z junctions. Nucleic Acids Res. 1994;22:314–320. doi: 10.1093/nar/22.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shklar G. Development of experimental oral carcinogenesis and its impact on current oral cancer research. J Dent Res. 1999;78:1768–1772. doi: 10.1177/00220345990780120101. [DOI] [PubMed] [Google Scholar]
- 10.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 11.Culp SJ, Gaylor DW, Sheldon WG, Goldstein LS, Beland FA. A comparison of the tumors induced by coal tar and benzo[a]pyrene in a 2-year bioassay. Carcinogenesis. 1998;19:117–124. doi: 10.1093/carcin/19.1.117. [DOI] [PubMed] [Google Scholar]
- 12.Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, Hoffmann D. Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res. 1986;46:4162–4166. [PubMed] [Google Scholar]
- 13.Prahalad AK, Ross JA, Nelson GB, Roop BC, King LC, Nesnow S, Mass MJ. Dibenzo[a,l]pyrene-induced DNA adduction, tumorigenicity, and Ki-ras oncogene mutations in strain A/J mouse lung. Carcinogenesis. 1997;18:1955–1963. doi: 10.1093/carcin/18.10.1955. [DOI] [PubMed] [Google Scholar]
- 14.Cavalieri EL, Rogan EG, Higginbotham S, Cremonesi P, Salmasi S. Tumor-initiating activity in mouse skin and carcinogenicity in rat mammary gland of dibenzo[a]pyrenes: the very potent environmental carcinogen dibenzo[a, l]pyrene. J Cancer Res Clin Oncol. 1989;115:67–72. doi: 10.1007/BF00391602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalieri EL, Higginbotham S, RamaKrishna NV, Devanesan PD, Todorovic R, Rogan EG, Salmasi S. Comparative dose-response tumorigenicity studies of dibenzo[α,l]pyrene versus 7,12-dimethylbenz[α]anthracene, benzo[α]pyrene and two dibenzo[α,l]pyrene dihydrodiols in mouse skin and rat mammary gland. Carcinogenesis. 1991;12:1939–1944. doi: 10.1093/carcin/12.10.1939. [DOI] [PubMed] [Google Scholar]
- 16.Higginbotham S, RamaKrishna NV, Johansson SL, Rogan EG, Cavalieri EL. Tumor-initiating activity and carcinogenicity of dibenzo[a,l]pyrene versus 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene at low doses in mouse skin. Carcinogenesis. 1993;14:875–878. doi: 10.1093/carcin/14.5.875. [DOI] [PubMed] [Google Scholar]
- 17.Snook ME, Severson RF, Arrendale RF, Higman HC, Chortyk OT. The identification of high molecular weight polynuclear aromatic hydrocarbons in a biologically active fraction of cigarette smoke condensate. Beitrag Tabakforsch. 1977;9:79–101. [Google Scholar]
- 18.Leavitt SA, George MH, Moore T, Ross JA. Mutations induced by benzo[a]pyrene and dibenzo[a,l]pyrene in lacI transgenic B6C3F1 mouse lung result from stable DNA adducts. Mutagenesis. 2008;23:445–450. doi: 10.1093/mutage/gen033. [DOI] [PubMed] [Google Scholar]
- 19.Sharma AK, Kumar S, Amin S. A highly abbreviated synthesis of dibenzo[def,p]chrysene and its 12-methoxy derivative, a key precursor for the synthesis of the proximate and ultimate carcinogens of dibenzo[def,p]chrysene. J Org Chem. 2004;69:3979–3982. doi: 10.1021/jo0303822. [DOI] [PubMed] [Google Scholar]
- 20.Ansell LL, Rangarajan T, Burgess WM, Eisenbraun EJ, Keen GW, Hamming MC. The synthesis of 1,2,3,7,8,9-hexahydrodibenzo[def, mno]chrysene and the use of hydriodic acid-red phosphorus in the deoxygenation of ketones. Org Prep Proced Int. 1976;8:133–140. [Google Scholar]
- 21.Leininger J, Jokinen M. Tumours of the oral cavity, pharynx, oesophagus and stomach. In: Turusov VS, Mohr U, editors. Pathology of tumours in laboratory animals. 2nd Edn. vol. 111. Lyon, France: IARC Sci Publ; 1994. pp. 167–193. [PubMed] [Google Scholar]
- 22.Guttenplan JB, Spratt TE, Khmelnitsky M, Kosinska W, Desai D, El-Bayoumy K. Effects of 3H-1,2-dithiole-3-thione, 1,4-phenylenebis(methylene)selenocyanate, and selenium-enriched yeast individually and in combination on benzo[a]pyrene-induced mutagenesis in oral tissue and esophagus in lacZ mice. Mutat Res. 2004;559:199–210. doi: 10.1016/j.mrgentox.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Guttenplan JB, Zhao ZL, Kosinska W, Norman RG, Krzeminski J, Sun YW, Amin S, El-Bayoumy K. Comparative mutational profiles of the environmental mammary carcinogen, 6-nitrochrysene and its metabolites in a lacI mammary epithelial cell line. Carcinogenesis. 2007;559:199–210. doi: 10.1093/carcin/bgm142. [DOI] [PubMed] [Google Scholar]
- 24.Guttenplan J, Chen KM, Khmelnitsky M, Kosinska W, Hennessy J, Bruggeman R, Desai D, Amin S, Sun YW, Spratt TE, El-Bayoumy K. Effects of 1,4-phenylenebis(methylene)selenocyanate on mutagenesis and p53 protein expression in the tongue of lacI rats treated with 4-nitroquinoline-N-oxide. Mutat Res. 2007;634:146–155. doi: 10.1016/j.mrgentox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hjortsberg L, Rubio-Nevado JM, Hamroun D, Claustres M, Béroud C, Soussi T. The p53 Handbook. 2008;2 http://p53.free.fr/Database/p53_database.html. [Google Scholar]
- 26.Denissenko MF, Pao A, Tang M, Pfeifer GP. Preferential formation of benzo[a]pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274:430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 27.Fuller CD, Wang SJ, Thomas CR, Jr, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973–1998. Cancer. 2007;109:1331–1343. doi: 10.1002/cncr.22563. [DOI] [PubMed] [Google Scholar]
- 28.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a001008. a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakravarti D, Pelling JC, Cavalieri EL, Rogan EG. Relating aromatic hydrocarbon-induced DNA adducts and c-H-ras mutations in mouse skin papillomas: the role of apurinic sites. Proc Natl Acad Sci USA. 1995;92:10422–10426. doi: 10.1073/pnas.92.22.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buterin T, Hess MT, Luneva N, Geacintov NE, Amin S, Kroth H, Seidel A, Naegeli H. Unrepaired fjord region polycyclic aromatic hydrocarbon-DNA adducts in ras codon 61 mutational hot spots. Cancer Res. 2000;60:1849–1856. [PubMed] [Google Scholar]
- 31.Buters JT, Mahadevan B, Quintanilla-Martinez L, Gonzalez FJ, Greim H, Baird WM, Luch A. Cytochrome P450 1B1 determines susceptibility to dibenzo[a,l]pyrene-induced tumor formation. Chem Res Toxicol. 15:1127–1135. doi: 10.1021/tx020017q. 1127; [DOI] [PubMed] [Google Scholar]
- 32.Hakura A, Tsutsui Y, Sonoda J, Kai J, Imade T, Shimada M, Sugihara Y, Mikami T. Comparison between in vivo mutagenicity and carcinogenicity in multiple organs by benzo[a]pyrene in the lacZ transgenic mouse (Muta Mouse) Mutat Res. 1998;398:123–130. doi: 10.1016/s0027-5107(97)00248-0. [DOI] [PubMed] [Google Scholar]
- 33.Cosentino L, Heddle JA. A test for neutrality of mutations of the lacZ transgene. Environ Mol Mutagen. 1996;28:313–316. doi: 10.1002/(SICI)1098-2280(1996)28:4<313::AID-EM3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]