To the Editor
Exposure to high altitude elicits integrated physiological responses to permit survival during hypoxia. Natives of Tibet living at high altitude have adapted in part through the generation of high levels of nitric oxide and circulating nitrogen oxide species that enable greater blood flow and oxygen delivery to offset hypoxia.1 Therefore, we hypothesized that in lowlanders acclimatizing to high altitude, levels of circulating vasoactive nitrogen oxides would increase to counteract hypoxia. To test this hypothesis, we assessed levels of extracellular and erythrocytic nitrogen oxide species in 15 persons living in low-altitude areas as they ascended in altitude during a 19-day trek in Nepal (Fig. 1A).
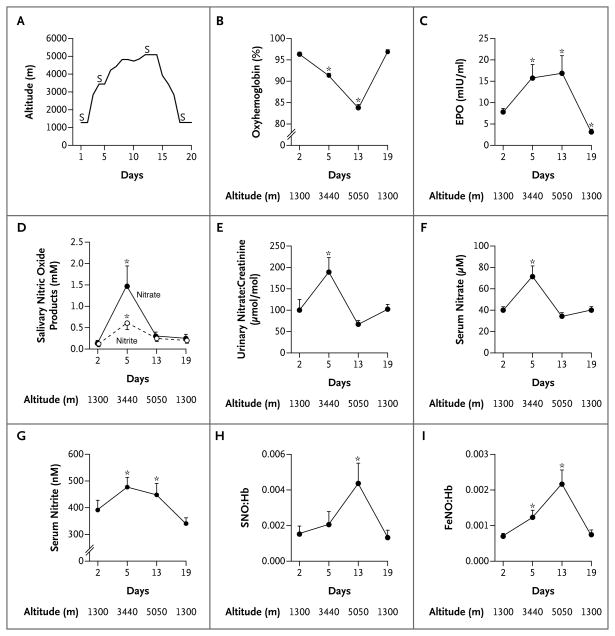
Figure 1. Acclimatization Responses at High Altitude.
Panel A shows the altitudes reached on each of the 19 days in the study; the letter S signifies the days on which samples were collected (days 2, 5, 13, and 19). Panel B shows that the arterial oxygen saturation decreased with ascent, Panel C that erythropoietin (EPO) levels increased at 3440 m and remained elevated at 5050 m before returning to baseline levels at 1300 m. In Panels D, E, and F, levels of salivary, urinary, and serum nitrogen oxides, respectively, increase at 3440 m before falling to baseline values at 5050 m. In contrast, as shown in Panel G, serum levels of nitrite continued to increase with ascent, remained elevated, and then returned to baseline levels at 1300 m. In Panels H and I, the molar ratios of S-nitrosohemoglobin (SNO:Hb) and iron nitrosyl hemoglobin (FeNO:Hb) increased with ascent and returned to baseline values at 1300 m. (Methods of measurement are provided in the Supplementary Appendix.) Asterisks indicate that P<0.05 for the comparison with baseline values by pairwise t-test. T bars indicate standard errors.
Oxyhemoglobin saturation fell progressively during ascent (Fig. 1B), whereas arterial oxygen content decreased at 3440 m but did not decrease further at 5050 m because of the increase in hemoglobin content (Table 1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). In a finding that is consistent with this phenomenon, the levels of two proteins regulated by hypoxia-inducible factor (HIF), endothelin-1 and erythropoietin, increased at high altitude (Fig. 1C, and Table 1 in the Supplementary Appendix).
Levels of serum, urinary, and salivary nitrate and nitrite increased in all participants at 3440 m (Fig. 1D through 1G). On further ascent, levels of salivary, urinary, and serum nitrate decreased while serum nitrite levels plateaued (Fig. 1D through 1G). In contrast, levels of intracellular red-cell forms of nitric oxide, S-nitrosohemoglobin and iron nitrosyl hemoglobin, which at 1300 m were similar to those reported elsewhere in healthy persons,2 increased strikingly during ascent to 5050 m (Fig. 1H and 1I). Although the fact that blood samples were collected in the field but assayed at sea level may have led to some degree of sample oxygenation that may have affected levels of S-nitrosohemoglobin, uniform handling of the samples permits a valid comparison of relative changes across samples. To assess the effects of acclimatization on function, we used the 6-minute walk test. The average distance walked at peak altitude was 86% of the distance walked at baseline (P<0.001). With the use of stepwise regression, we identified the levels of S-nitrosohemoglobin, endothelin-1, and hemoglobin as making positive contributions to the distance walked in 6 minutes (R2= 0.81; P = 0.01).
Although the number of study participants was small, the results clearly identify nitrogen oxides as integral components in acclimatization to hypobaric hypoxia. The reaction of nitric oxide with hemoglobin greatly limits the lifetime of nitric oxide in blood; thus redox-activated nitrogen oxides, such as nitrite and S-nitro-sothiols, have been proposed as enablers of hypoxia-associated vasodilation.3,4 In this context, levels of circulatory, oral, and urinary nitrate decreased at maximal altitude, whereas erythrocytic species associated with the nitrogen oxide bioactivation plateaued or increased. These findings suggest that nitric oxide–hemoglobin interactions that occur parallel to genetic HIF-controlled protein responses contribute to acclimatization and potentially to the survival of persons with acute or chronic hypoxia that is related to disease.
Acknowledgments
Supported by the Nepal Health Research Council, the Explorers Club Exploration Fund, and the Ev-K2-CNR Committee and by grants from the National Institutes of Health (HL60917, to Dr. Erzurum; HL59337, to Dr. Gaston; and HL0101871, to Drs. Gaston and Doctor), the American Heart Association (GIA 0950133G to Dr. Doctor), the National Center for Research Resources (1UL1RR024989 and 1UL1RR024992, to Dr. Doctor), and the Public Health Service (T32HL083823).
The study was carried out within the framework of the Everest-K2 National Research Council Project in collaboration with the Nepal Academy of Science and Technology, as required by the Memorandum of Understanding between Nepal and Italy.
We thank the Italian National Research Council and the Italian Ministry of Foreign Affairs.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Allison J. Janocha, Cleveland Clinic, Cleveland, OH
Carl D. Koch, Cleveland Clinic, Cleveland, OH
Mauro Tiso, National Institutes of Health, Bethesda, MD
Andrea Ponchia, University of Padua, Padua, Italy
Allan Doctor, Washington University, St. Louis, MO
Lindsey Gibbons, Washington University, St. Louis, MO
Benjamin Gaston, University of Virginia School of Medicine, Charlottesville, VA
Cynthia M. Beall, Case Western Reserve University, Cleveland, OH
Serpil C. Erzurum, Email: erzurus@ccf.org, Cleveland Clinic, Cleveland, OH
References
- 1.Erzurum SC, Ghosh S, Janocha AJ, et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci U S A. 2007;104:17593–8. doi: 10.1073/pnas.0707462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta B, Tufnell-Barrett T, Bleasdale RA, et al. Red blood cell nitric oxide as an endocrine vasoregulator: a potential role in congestive heart failure. Circulation. 2004;109:1339–42. doi: 10.1161/01.CIR.0000124450.07016.1D. [DOI] [PubMed] [Google Scholar]
- 3.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci U S A. 2006;103:8366–71. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladwin MT, Grubina R, Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res. 2009;42:157–67. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]