Abstract
Spinocerebellar ataxia (SCA) types 1, 2, 3, 6, 7, and 17 as well as Huntington's disease are a group of neurodegenerative disorders caused by expanded CAG repeats encoding a long polyglutamine (polyQ) tract in the respective proteins. Evidence has shown that the accumulation of intranuclear and cytoplasmic misfolded polyQ proteins leads to apoptosis and cell death. Thus suppression of aggregate formation is expected to inhibit a wide range of downstream pathogenic events in polyQ diseases. In this study, we established a high-throughput aggregation screening system using 293 ATXN3/Q75-GFP cells and applied this system to test the aqueous extract of Paeonia lactiflora (P. lactiflora) and its constituents. We found that the aggregation can be significantly prohibited by P. lactiflora and its active compound paeoniflorin. Meanwhile, P. lactiflora and paeoniflorin upregulated HSF1 and HSP70 chaperones in the same cell models. Both of them further reduced the aggregation in neuronal differentiated SH-SY5Y ATXN3/Q75-GFP cells. Our results demonstrate how P. lactiflora and paeoniflorin are likely to work on polyQ-aggregation reduction and provide insight into the possible working mechanism of P. lactiflora in SCA3. We anticipate our paper to be a starting point for screening more potential herbs for the treatment of SCA3 and other polyQ diseases.
1. Introduction
Spinocerebellar ataxias (SCAs) are a large, complex group of heterogeneous autosomal dominant neurodegenerative disorders characterized by cerebellar dysfunction alone or in combination with other neurological abnormalities [1]. Among them, the expansions of CAG trinucleotide repeats encoding a polyglutamine (polyQ) stretch have been shown to cause dominantly inherited SCA1, SCA2, SCA3, SCA6, SCA7, SCA17, and dentatorubropallidoluy-sianatrophy (DRPLA) [2–8]. These polyQ-mediated genetic disorders in SCAs have shown selective progressive degeneration of the cerebellum, brainstem, and spinal tract, with prominent pathological hallmark of intranuclear and cytoplasmic accumulation of aggregated polyQ proteins inside degenerated neurons [9]. Different polyQ tract-containing proteins ultimately lead to the dysfunction and degeneration of specific neuronal subpopulations [10]. The aggregated polyQ proteins may cause dysfunction of mitochondria, chaperone, and ubiquitin proteasome system, leading to apoptosis and cell death [11–13]. As misfolding of the polyQ protein is likely the initial event in the pathogenic cascade, suppression of protein misfolding is expected to inhibit a wide range of downstream detrimental events, and to rescue neuronal dysfunction.
Increasing evidence suggests that some herbs may potentially attenuate the deterioration of neurodegenerative diseases. Paeonia lactiflora (P. lactiflora), belonging to the Paeoniaceae family, is a perennial herb frequently used as an important ingredient in many traditional prescriptions. It has been commonly used for nourishing blood, alleviating pain, reducing irritability, as well as treating liver disease and cancer [14]. Paeoniflorin, one of the main compounds extracted from P. lactiflora, has been reported to ameliorate neurodegenerative process in Parkinson's disease (PD) and Alzheimer's disease (AD) models [15–17]. However, the effect of P. lactiflora herb extract and paeoniflorin in treating SCA remains unraveled.
In the present study, we firstly built up an aggregation screening cell model by overexpressing CAG-expanded ATXN3, the causative mutation in SCA3 [2], in 293 cells, and then examined the anti-aggregation effect of P. lactiflora aqueous extract and paeoniflorin. We further demonstrated that the anti-aggregation activity of P. lactiflora extract and paeoniflorin was contributed by the enhancement of heat shock transcription factor 1 (HSF1)-heat shock protein (HSP) 70 chaperone system. These findings provide evidence that P. lactiflora and paeoniflorin may be a novel alternative therapeutic agent for the treatment of SCAs.
2. Materials and Methods
2.1. P. lactiflora Extract Preparation and HPLC Analysis
Aqueous extract from P. lactiflora was provided by Sun-Ten Pharmaceutical Company (Taipei, Taiwan). Briefly, 100 g of dried P. lactiflora was boiled with 1500 mL of water at 100°C for 30 min and was sieved using a 100-mesh sieve. The extract was concentrated to 100 mL and filtered through a 200-mesh sieve. The extract was then dried by speed vacuum concentration and then stored at −20°C until used.
High pressure liquid chromatography (HPLC) was performed using a LaChrom Elite HPLC system (Hitachi), consisting of a photo diode array detector. The chromatographic separation of P. lactiflora extract (50 μL, 1 mg/mL) was carried out on a Hypersil ODS (C18) column (250 × 4.6 mm, 5 μm), eluted with the mixture of 0.1% formic acid in water (A) or acetonitrile (B). The linear gradient elution program for A : B (v/v) was set as follows: 95 : 5 (0–10 min), 95 : 5–70 : 30 (10–40 min), 70 : 30–15 : 85 (40–55 min), 15 : 85–95 : 5 (55–60 min), 95 : 5 (60–75 min) with a flow rate of 1 mL/min. Absorbance was monitored at 230, 250, 270 nm and the scan range for photo diode array was 190∼400 nm. Paeoniflorin, gallic acid, and albiflorin (2∼10 µL, 20 mM) were used as reference compounds for P. lactiflora [18, 19].
2.2. Cell Culture and Cell Proliferation Assay
Human embryonic kidney HEK-293 cells (ATCC No. CRL-1573) were cultivated in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). Human neuroblastoma SH-SY5Y cells (ATCC No. CRL-2266) were maintained in DMEM F12 supplemented with 10% FBS. Cells were cultivated at 37°C incubator containing 5% CO2 and cell proliferation was measured based upon the reduction of the tetrazolium salt, 3,[4,5-dimethylthiazol-2-yL]-2,5-diphenyl-tetrazolium bromide (MTT). Cells were plated into 48-well (5 × 104/well) dishes, grown for 20 hr and treated with different concentrations of the P. lactiflora extract (5∼30 mg/mL) or pure compound (100 nM∼1 mM). After one day, 20 μL MTT (5 mg/mL in PBS, Sigma) was added to cells and incubated for 2 hr. The absorbance of the purple formazan dye was measured at 570 nm by a Bio-Tek μQuant Universal Microplate Spectrophotometer.
2.3. Flp-In-293 Triple Fluorescent Reporter Cells and Fluorescent Assay
A triple fluorescent reporter plasmid with mCherry, ZsYellow1, and AmCyan1 was first constructed in pAmCyan1-N1. A proximal promoter (−360∼+2, with the translation initiation site as +1) from HSF1 gene was used, as 331 bp upstream of the murine HSF1 translation start site is required for maximal basal expression [20]. Promoter fragments from HSF1 (enhancing chaperone expression), heat shock cognate protein (HSPA8, −1140∼+38, driving constitutively expressed HSP70) [21] and heat-inducible HSP70 chaperone (HSPA1A, −273∼+215, driving heat-inducible HSP70) [22] are placed upstream of the three fluorescent reporters. The fragment containing the HSF1, HSPA8, and HSPA1A driven reporters was excised with AseI and NotI restriction enzymes and used to replace an AseI-NotI fragment in pcDNA5/FRT/TO plasmid (Invitrogen). The resulting triple fluorescent reporter plasmid was used to generate Flp-In triple fluorescent reporter cells and maintained according to the supplier's instructions (Invitrogen). Geranylgeranylacetone (GGA, Sigma) or paeoniflorin (100 nM~100 µM) was added to the medium for 24 hr. The three fluorescence colors were analyzed simultaneously using high-content analysis (HCA) system (ImageXpressMICRO, Molecular Devices), with excitation/emission wavelengths at 453/486 (mCherry), 531/540 (ZsYellow1) and 587/610 nm (AmCyan1).
2.4. ATXN3 and HSF1 cDNA Constructs
Polyadenylated RNA (200 ng) isolated from neuroblastoma SK-N-SH cells was reverse transcribed using the SuperScript III reverse transcriptase (Invitrogen). The sense and antisense primers used for ATXN3/Q14 cDNA (+826∼+1152, NM_004993) amplification were 5′-ATTCAGCTAAGTATGCAAGGTAGTTCCA (codon for Met257 underlined) and 5′-CATGCCATGGCATGTTTTTTTCCTTCTGTT (NcoI site underlined). The amplified 3′ polyQ-containing cDNA fragment (translated into amino acids 257∼361) was cloned into pGEM-T Easy (Promega) and sequenced. The ATXN3/Q14 cDNA was excised with EcoRI (in pGEM-T Easy vector) and NcoI and subcloned into pEGFP-N1 (Clontech). Then DNA fragment containing in-frame ATXN3/Q14-EGFP was excised with HindIII-NotI and subcloned into the pcDNA5/FRT/TO. The ATXN3/Q75 cDNA was made by replacing an 88 bp ATXN3/Q14 BsmBI-BsmFI fragment with a 271 bp ATXN3/Q75 fragment from the cDNA clone of a SCA3 patient. The HSF1 cDNA (BC014638) in pOTB7 was obtained from Bioresource Collection and Research Center (BCRC), Food Industry Research and Development Institute, Taiwan. The cDNA was excised with EcoRI and XhoI and subcloned into pcDNA3 (Invitrogen).
2.5. Isogenic 293 and SH-SY5Y Cell Lines
Human 293-derived Flp-In-293 cells (Invitrogen) were cultivated in DMEM containing 10% FBS as described. The cloned pcDNA5/FRT/TO-ATXN3/Q14 and Q75 plasmids were used to generate the isogenic ATXN3/Q14∼75 cell lines by targeting insertion into Flp-In-293 cells, according to the supplier's instructions. The repeats in these ATXN3 cell lines were examined by PCR and sequencing. These cell lines were grown in medium containing 5 µg/mL blasticidin and 100 µg/mL hygromycin (InvivoGen). Human SH-SY5Y-derived Flp-In host cell line was constructed as described [23]. The SH-SY5Y host cells were used to generate isogenic ATXN3/Q14∼75 lines and maintained as described above.
2.6. ATXN3/Q75 Aggregation Assay
293ATXN3/Q75-GFP cells were plated into 96-well (2 × 104/well) dishes, grown for 24 hr and treated with different concentrations of the P. lactiflora extract (2∼200 μg/mL) or suberoylanilide hydroxamic acid (SAHA, Cayman Chemical), paeoniflorin (Sigma), gallic acid, and albiflorin (Chromadex) (100 nM∼5 µM) for 8 hr. Then doxycycline (10 μg/mL, BD) was added to the medium to induce ATXN3/Q75-GFP expression for 6 days. Oxaliplatin (5 μM, Sigma) was also added for aggregate accumulation through inhibition of cell division [24]. Then cells were stained with Hochest 33342 (0.1 μg/mL, Sigma) and aggregation percentage was assessed by HCA system, with excitation/emission wavelengths at 482/536 (GFP).
SH-SY5Y ATXN3/Q75-GFP cells were seeded in 6-well (2 × 105/well) plate, with all trans retinoic acid (10 µM, Sigma) added at seeding time. At day 2, cells were treated with paeoniflorin (100 nM) or the P. lactiflora extract (10 µg/mL) for 8 hr, and then doxycycline (5 μg/mL) was added to induce ATXN3/Q75-GFP expression. The cells were kept in the medium containing 10 μM trans retinoic acid, doxycycline and paeoniflorin/P. lactiflora extract for 7 days. After that, cells were stained with Hochest 33342 (0.1 μg/mL) and aggregation percentage was assessed as described.
2.7. Real-Time PCR
Total RNA from 293 ATXN3 lines was extracted using Trizol reagent (Invitrogen). The RNA was DNase (Stratagene) treated, quantified, and reverse-transcribed to cDNA as described. Real-time quantitative PCR experiments were performed in the ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Amplification was performed on 100 ng cDNA with gene-specific TaqMan fluorogenic probes Hs0024525_ml for ATXN3 and 4326321E for HPRT1 (endogenous control) (Applied Biosystems). Fold change was calculated using the formula 2ΔCt, ΔCt = Ct(control) − Ct(target), in which Ct indicates cycle threshold.
2.8. Western Blot Analysis
Total proteins were prepared using lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.1% SDS and 0.5% sodium deoxycholate, 1% Triton X-100, protease inhibitor cocktail (Calbiochem). Proteins (25 μg) were separated on 10% SDS-polyacrylamide gel electrophoresis and blotted on to nitrocellulose membranes by reverse electrophoresis. After blocking, the membrane was probed with HSF1 (1 : 1000 dilution, Abnova), HSPA8 (1 : 500 dilution, Santa Cruz), HSPA1A (1 : 500 dilution, Santa Cruz), H3F3B (1 : 3000 dilution, GeneTex), GFP (1 : 500 dilution, Santa Cruz), β-actin (1 : 5000 dilution, Millipore) or GAPDH (1 : 1000 dilution, MDBio) at 4°C overnight. Then the immune complexes were detected by horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit IgG antibody (1 : 5000 dilution, GeneTex) and chemiluminescent substrate (Millipore).
2.9. ATXN3/Q75 and HSF1 cDNA Co-Transfection
Human embryonic kidney HEK-293T cells (ATCC No. CRL-11268) were cultivated in DMEM containing 10% FBS as described. For transient overexpression, cells were plated into 12-well (1 × 105/well) dishes, grown for 20 hr, and transfected using T-Pro reagent (JF Biotechnology, Taiwan) with pcDNA5/FRT/TO-ATXN3/Q75 and pcDNA3-HSF1 or pcDNA3 vector plasmids (1.5 μg each). The cells were grown for 48 hr for ATXN3/Q75 aggregation assay as described.
2.10. Statistical Analysis
For each set of values, data were expressed as the means ± standard deviation (SD). Three independent experiments were performed and non-categorical variables were compared using the Student's t-test. All P values were two-tailed, with values of P < 0.05 considered significant.
3. Results
3.1. Construction of 293 Cells Expressing ATXN3/Q75 Aggregates
For therapies toward the polyQ diseases, we aimed to screen herbs/compounds potentially inhibiting polyQ aggregation. As removal of the N-terminus of polyQ-expanded ATXN3 is required for aggregation in vitro and in vivo [25], we cloned GFP-tagged ATXN3 C-terminal Q14∼75-containing fragment to establish Flp-In 293 cells with ATXN3/Q14∼75-GFP expression in an inducible fashion. As shown in Figure 1(a), the GFP antibody detected 40 kDa ATXN3/Q14-GFP and 57 kDa ATXN3/Q75-GFP proteins in doxycycline (Dox) induced ATXN3 cells. ATXN3-RNA levels were then examined by real-time PCR using ATXN3-specific probe and primers. As shown in Figure 1(b), in the presence of Dox, the two ATXN3 lines expressed ∼20 times more ATXN3 RNA than in the absence of Dox. While the expressed ATXN3/Q14 was mainly diffused, the expressed ATXN3/Q75-GFP formed aggregates (Figure 1(c)).
Figure 1.
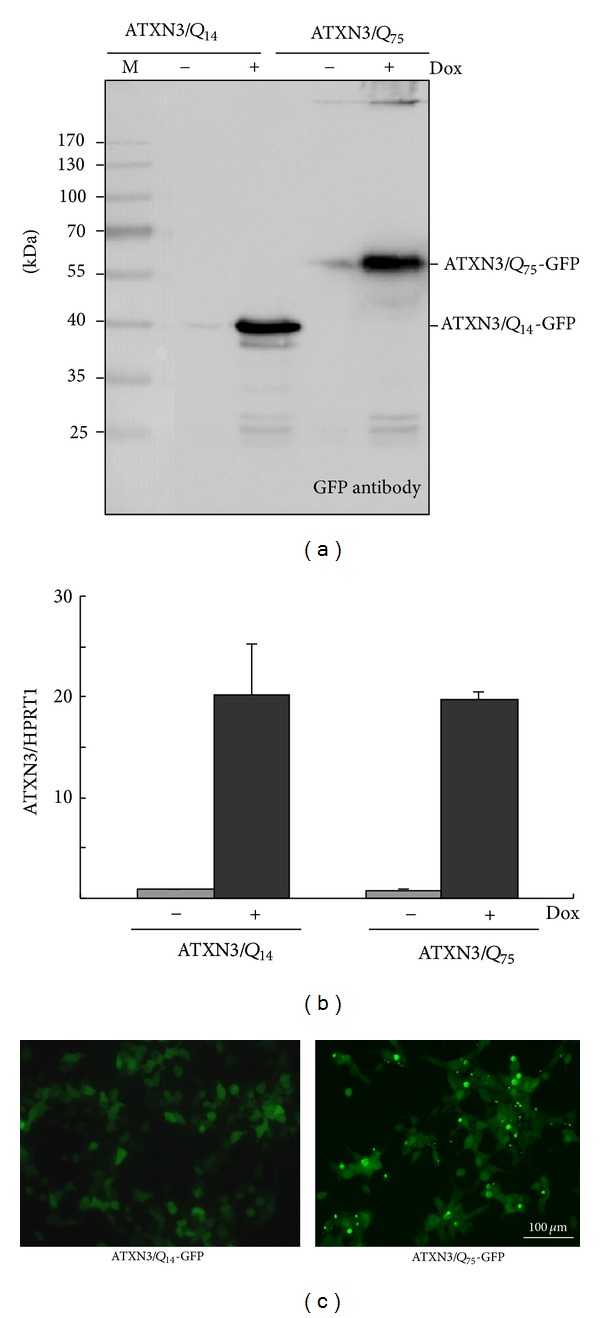
Flp-In 293 cells with ATXN3/Q14∼75-GFP expression in an inducible fashion. (a) Western blot analysis of ATXN3/Q14∼75-GFP protein expression using GFP antibody after two days of induction (+Dox). (b) Real-time PCR quantification of ATXN3/Q14∼75-GFP RNA expression relatively to HPRT after two days of induction (+Dox). (c) Fluorescence microscopy images of ATXN3/Q14∼75-GFP expression after six days of induction. The scale bar = 100 µm.
3.2. Aqueous Extract of P. lactiflori and Constituents
To examine the potential active compounds in P. lactiflori, the chemical profile of aqueous extract was analyzed and quantified by full-spectrum analytic HPLC. Chromatographic patterns showed peaks in 230 nm corresponding to the retention time compatible with paeoniflorin, garlic acid and albiflorin (Figure 2(a)). The amounts of paeoniflorin, garlic acid, and albiflorin in aqueous extract of P. lactiflori were 2.27%, 0.30%, and 0.73%, respectively, corresponding to 47.33 mM, 18.06 mM, and 15.16 mM, respectively, in 1 g/mL aqueous extract (Figure 2(b)).
Figure 2.
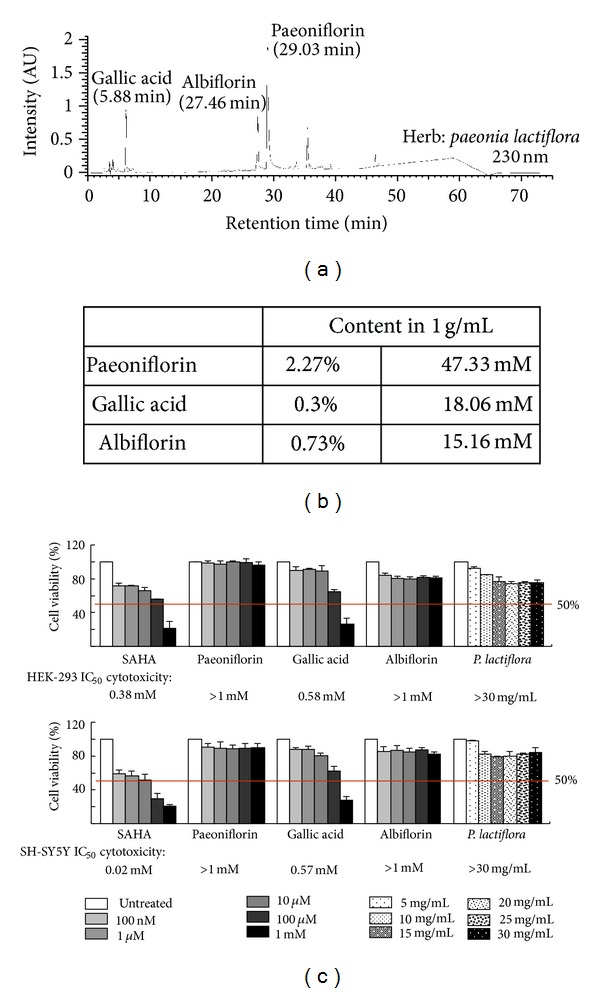
Chemical profile and cytotoxicity of the aqueous extract of P. lactiflora. (a) Chromatographic patterns from HPLC analysis (230 nm) showed peaks compatible with paeoniflorin, albiflorin and garlic acid. (b) The relative amount of above molecules in the extract. (c) Cytotoxicity of the aqueous extract of P. lactiflora, paeoniflorin, garlic acid, albiflorin, and SAHA against HEK-293 and SH-SY5Y cells using MTT viability assay. The IC50 of each herb/compound was shown under the columns. To normalize, the relative viability in untreated cells is set as 100%. The red line represents 50% viability.
MTT assays were performed with human embryonic kidney 293 and human neuroblastoma SH-SY5Y cells after treatment with extract of P. lactiflora and the its three constituents, respectively, for 24 hr. The histone deacetylase inhibitor suberoylanilide suberoylanilide hydroxamic acid (SAHA) known to reduce SDS-insoluble polyQ aggregate [26] was included for comparison. The IC50 of the herb and compounds were calculated using the interpolation method. Both P. lactiflora extract and its constituents paeoniflorin and albiflorin had an IC50 higher than the highest concentration tested ( >30 mg/mL for P. lactiflora and >1 mM for paeoniflorin and albiflorin), suggesting their very low cytotoxicity (Figure 2(c)).
3.3. P. lactiflori Extract and Paeoniflorin Reduce ATXN3/Q75 Aggregation on 293 Cell Model
To screen if herb/compounds potentially inhibit aggregation, we used ATXN3/Q75-GFP cells to examine aqueous extract of P. lactiflora and its constituents for their potentials to reduce the ATXN3/Q75 aggregation. The experiment flow chart is shown in Figure 3(a) and representative fluorescence microscopy images of aggregation after treatment with paeoniflorin and the aqueous extract of P. lactiflora are shown in Figure 3(b). As a positive control, HDAC inhibitor SAHA reduced the ATXN3/Q75 aggregation to 85% (at 100 nM) as compared to untreated cells (Figure 3(c)). While garlic acid did not display good aggregation-inhibitory potential (90∼95% at 100 nM∼1 μM), P. lactiflora (81∼82% at 2∼50 μg/mL), paeoniflorin (73% at 100 nM) and albiflorin (78% at 5 μM) had greater aggregation reduction potential than SAHA (Figure 3(c)). The IC50 cytotoxicity/effective (reduced the ATXN3/Q75 aggregation to 85% or lower) dose ratio of SAHA, paeoniflorin, albiflorin, and extract of P. lactiflora were 3800, >10000, >200, and >15000, respectively. Considering 2 µg/ml of P. lactiflora extract contained 95 nM paeoniflorin and 30 nM albiflorin and tested greatest aggregation reduction potential of 100 nM for paeoniflorin and 5 µM for albiflorin, paeoniflorin was regarded as a major active component for the aggregation inhibition in P. lactiflora.
Figure 3.
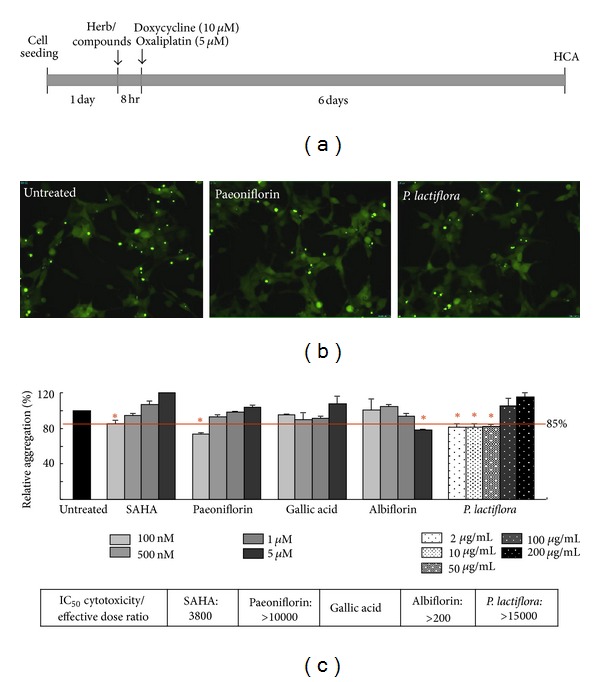
High-content compound screen using Flp-In 293 cells with inducible ATXN3/Q75-GFP expression. (a) Experiment flow chart. ATXN3/Q75-GFP 293 cells were plated into 96-well dishes, grown for 24 hr and treated with different concentrations of the herb or compound for 8 hr. Then doxycycline and oxaliplatin was added to the medium to for 6 days and aggregation percentage was assessed by HCA system. (b) Representative fluorescence microscopy images of ATXN3/Q75-GFP cells untreated or treated with P. lactiflora (10 µg/mL) or paeoniflorin (100 nM) for 6 days. (c) Aggregation analysis of ATXN3/Q75-GFP cells untreated or treated with aqueous extract of P. lactiflora (2∼200 µg/mL), paeoniflorin, garlic acid, albiflorin and SAHA (100 nM∼5 µM). To normalize, the relative aggregation level in untreated cells is set as 100%. The red line represents 85% aggregation with SAHA treatment (100 nM).
3.4. Paeoniflorin Enhanced HSF1 and HSP70 Chaperone Expression on 293 Cells
To screen the potential of herb/compounds to enhance HSF1 and HSP70 chaperone expression, we established a triple fluorescent reporter 293 cell model with mCherry, ZsYellow1 and AmCyan1 reporters downstream of HSF1, HSPA8 and HSPA1A promoters (Figure 4(a)). The cloned promoters effectively drove the expression of red, yellow, and blue fluorescent reporters (Figure 4(b)). As shown in Figure 4(c), treatment of GGA (100 nM~100 µM), a potent HSP inducer, for one day significantly increased HSF1 (110%~112%, P = 0.010~0.002), HSPA8 (106%∼116%, P = 0.024~0.000) and HSPA1A (108%∼118%, P = 0.019~0.001) promoter activity. This was also true for paeoniflorin (100 nM∼100 µM) treatment, with 108%∼120% HSF1 (P = 0.028~0.001), 112%∼120% HSPA8 (P = 0.044∼0.000) and 110%∼119% HSPA1A (P = 0.025∼0.001) promoter activities compared to no treament. The enhancement of paeoniflorin (100 nM) on HSF1 (158%, P = 0.027), HSPA8 (140%, P = 0.011) and HSPA1A (137%, P = 0.007) expression was confirmed by the Western blot in HEK-293 cells after two days treatment (Figure 4(d)).
Figure 4.
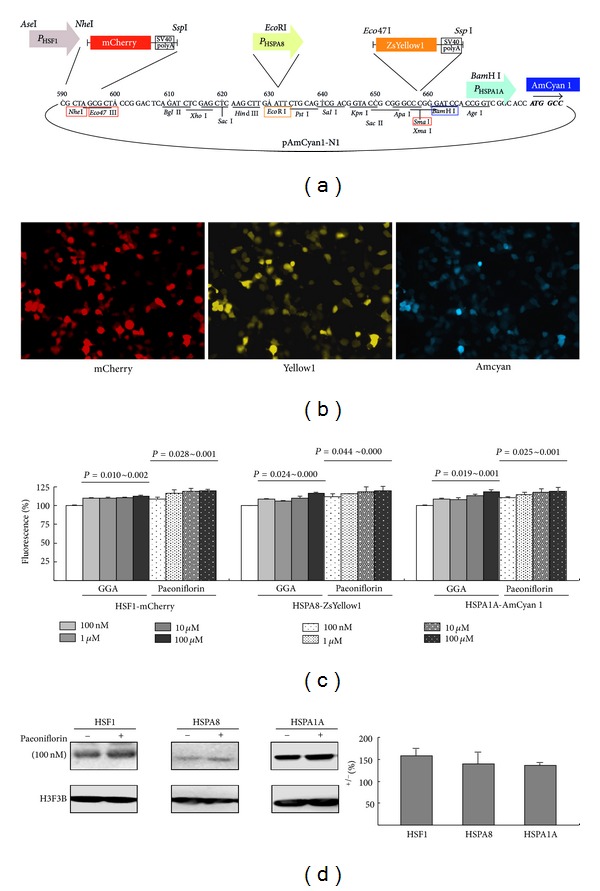
Enhancement of chaperone expression by paeoniflorin in 293 cells. (a) Triple fluorescent reporter plasmid with HSF1, HSPA8, and HSPA1A promoter fragments upstream of mCherry, ZsYellow1, and AmCyan1 fluorescent reporters, respectively. (b) Microscopic images of the triple fluorescent reporter cells. (c) Effect of GGA and paeoniflorin (100 nM∼100 µM) on HSF1, HSPA8, and HSPA1A reporters. To normalize, the fluorescence level in untreated cells is set as 100%. Three independent experiments were performed with P < 0.05 considered significant. (d) Representative western blot image of paeoniflorin-(100 nM) treated 293 cells for two days using HSF1, HSPA8, HSPA1A, and H3F3B antibodies. Levels of HSF1, HSPA8, HSPA1A, were normalized with a loading control (H3F3B). Data are expressed as the mean ± SEM values from three independent experiments.
3.5. P. lactiflori Extract and Paeoniflorin Enhanced HSF1 and HSP70 Chaperone Expression on 293 ATXN3/Q75 Cell Model
To examine if paeoniflorin and P. lactiflora extract also up-regulated HSF1 and HSP70 chaperone expression in ATXN3/Q75 293 cells, we compared the expression levels of HSF1, HSPA8 and HSPA1A between with and without paeoniflorin/P. lactiflora and/or Dox treatment. As shown in Figure 5, induced expression of ATXN3/Q75 for 6 days attenuated the expression of HSF1 (78%∼67%), HSPA8 (86%) and HSPA1A (82%). This reduction can be rescued by the addition of paeoniflorin (100 nM) or P. lactiflora (10 µg/mL), with significantly increased HSF1 (113%∼126%, P = 0.005∼0.001), HSPA8 (123%∼124%, P = 0.018∼0.005) and HSPA1A (118%∼119%, P = 0.046∼0.009) expression. These findings suggested that P. lactiflora and paeoniflorin up-regulated HSF1 and HSP70 chaperon expression to reduce ATXN3/Q75 aggregation in this cell model.
Figure 5.
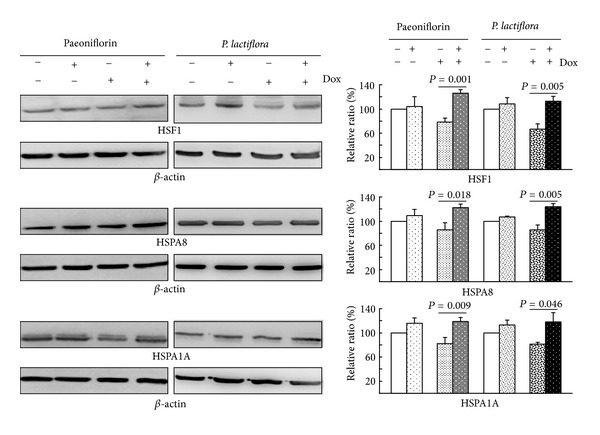
Enhancement of HSF1 and chaperone expression by paeoniflorin and the aqueous extract of P. lactiflora in ATXN3/Q75-GFP 293 cells. Cells were pre-treated with paeoniflorin (100 nM) or herb (10 μg/mL) for 8 hours and ATXN3/Q75-GFP expression induced for 6 days. Relative HSF1, HSPA8, and HSPA1A expressions were analyzed by western blot analysis using β-actin as a loading control.
3.6. HSF1 Overexpression to Reduce ATXN3/Q75 Aggregation
To determine whether HSF1 could suppress aggregation of mutant ATXN3, we transiently co-expressed HSF1 with ATXN3/Q75 in HEK-293T cells. As shown in Figure 6, with HSF1 co-transfection, visible aggregates significantly decreased in ATXN3/Q75 cells (13.8% versus 24.0%, P = 0.024).
Figure 6.
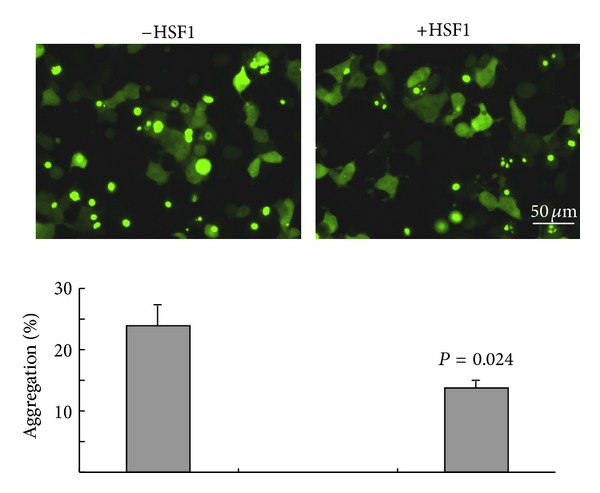
HSF1 overexpression in ATXN3/Q75-GFP transient cell models. HEK-293T cells were co-transfected with plasmids encoding ATXN3/Q75-GFP and plasmid with (+HSF1) or without (−HSF1) HSF1 cDNA. After 2 days, aggregation percentage was assessed by HCA system. (The scale bar = 50 μm). The percentage of aggregate formation counted among three random fields.
3.7. P. lactiflori Extract and Paeoniflorin Reduced ATXN3/Q75 Aggregation on SH-SY5Y Cell Model
To test the aggregation reduction potential of P. lactiflori extract and paeoniflorin in neuronal cells, we constructed Flp-In SH-SY5Y cells with N-terminal truncated ATXN3/Q14∼75-GFP expression in an inducible fashion. GFP-tagged 40∼57 kDa ATXN3/Q14∼75 protein in Dox-induced SH-SY5Y cells can be seen in Western blot (Figure 7(a)). Then we differentiated ATXN3/Q14∼75 SH-SY5Y cells using retinoic acid and found that the induced ATXN3/Q75 formed aggregates in ∼1% neuronal cells (Figure 7(b)). The treatment of paeoniflorin or P. lactiflora leaded to 21%∼16% of aggregation reduction (P = 0.013∼0.035) in ATXN3/Q75 expressed neuronal cells (Figure 7(c)). These results confirmed the aggregation-inhibitory effect of paeoniflorin and P. lactiflora in differentiated neurons.
Figure 7.
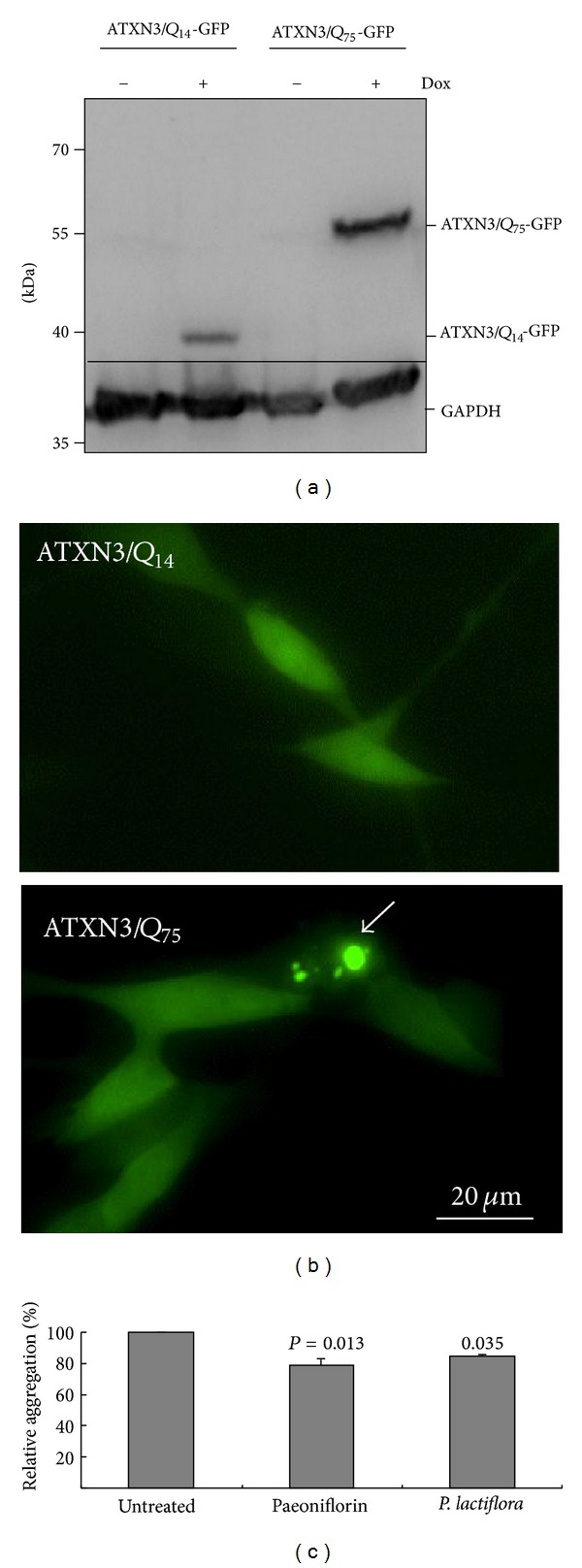
Reduction of aggregation by paeoniflorin and the aqueous extract of P. lactiflora in ATXN3/Q75-GFP SH-SY5Y cells. (a) Western blot analysis using GFP and GAPDH antibodies after two days induction (+Dox). (b) Microscopic images of differentiated SH-SY5Y cells expressing ATXN3/Q14/75-GFP for 6 days (the scale bar = 20 μm). (c) Relative aggregation after treatment with paeoniflorin (100 nM) or the aqueous extract of P. lactiflora (10 μg/mL) for 6 days. To normalize, the relative aggregation level in untreated cells is set as 100%.
4. Discussion
Although Chinese herbs have been reported to reduce pneumonia risk in elderly patients with dementia [27] and regarded as a potential treatment of Huntington's disease (HD) [28], the attempts to apply this alternative treatment in SCA are still few. Okabe et al. (2007) reported a patient with SCA6 was treated with a mixture of 18 medical herbs (modified Zhengan Xifeng Tang) and then the patient's ataxia was remarkably reduced [29]. However, the therapeutically effective compound(s) in this remedy remains unknown. In this study we identified the aqueous extract of P. lactiflora that reduced ATXN3-aggregates mainly via its active compound paeoniflorin (Figures 2 and 3). The reporter gene assay and Western blotting further indicated the aggregation-reduction effect of paeoniflorin was modulated by the up-regulation of HSF1 and its targets, HSPA8 and HSPA1A chaperone expressions (Figure 4).
P. lactiflora, with immunomodulatory and anti-inflammatory effects [30], has been widely used as a component of traditional Chinese prescriptions to relieve pain and to treat rheumatoid arthritis, systemic lupus erythromatosus, dysmenorrhea, hepatitis, muscle spasm, and fever with a long history. Its main bioactive component, paeoniflorin, possesses wide pharmacological effects in the nervous system. It has been reported to decrease the death of rat cortical cells while exposed to H2O2-induced oxidative stress [31]. Subcutaneous injection of paeoniflorin has shown functional protection in the 6-OHDA lesion rodent model of PD [16], and reduce the MPTP-induced toxicity by activation of the adenosine A1 receptor to inhibit neuroinflammation [15]. In Aβ (1–42)-injected AD rat model, paeoniflorin attenuates the neurotoxicity and cognitive decline by regulating calcium homeostasis and ameliorating oxidative stress [17]. Our results demonstrate both extract of P. lactiflora and paeoniflorin up-regulating HSF1 and HSP70 expressions to inhibit aggregate formation in ATXN3/Q75 293 cells (Figure 5). This aggregation-inhibitory effect can also be seen in neuronal differentiated SH-SY5Y cells expressing ATXN3/Q75 (Figure 7), providing a novel mechanism of P. lactiflora and paeoniflorin to slow down the neurodegenerative process by inducing the expression of heat shock proteins.
Peoniflorin was the first reported active component of herbal medicines to induce expression of heat shock proteins in HeLa, IMR-32, and normal rat kidney cells [32]. Upon stress, HSF1 is released from the chaperone complex, self-trimerizes, and then is transported into the nucleus as a transcription factor. It activates chaperones, which play an important role in preventing unwanted protein aggregation. Overexpression of HSF1 significantly improved the life span of R6/2 Huntington's disease mouse [33]. Up-regulation of chaperone expression by HSF1 and its activating compounds 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) demonstrate a strong inhibitory effect on HD aggregate formation [34]. We also showed aggregation-inhibitory effect of HSF1 overexpression in 293 cells expressing ATXN3/Q75 (Figure 6). Overexpression of its downstream target gene HSPA1A suppresses polyQ-mediated neurodegeneration in Drosophila and mouse models [35, 36]. Reduced expression of HSPA8, another target genes of HSF1 [37], has been shown in the 293 cells overexpressing expanded TBP-Q61 [38] and SCA17 lymphoblastoid cells [39]. The therapeutic potential of P. lactiflora and paeoniflorin in the treatment of SCA is strongly supported by the up-regulated HSF1 and HSP70 expressions.
In conclusion, our in vitro study provides strong evidence that P. lactiflora and paeoniflorin could be novel therapeutics for SCA3 and other polyQ diseases. Future application of P. lactiflora and paeoniflorin to SCA animal models would solidify their effects on aggregation reduction and disease improvement. Since the pathogenesis of the polyQ diseases is not completely clear and effective treatment is not available, our cell models are extremely valuable for identifying potential therapeutic targets in polyQ diseases. A systemic high-throughput screening of herbal and chemical compounds using ATXN3/Q75 293 cell model is undergoing.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgments
This work was supported by Grants NSC100-2325-B-003-001 and NSC101-2325-B-003 -002 from the National Science Council, Executive Yuan, NTNU100-D-02 from National Taiwan Normal University, Taipei, and Aim for the Top University Project–NTNU, Taiwan. K.-H. Chang, W.-L. Chen, and L.-C. Lee equally contributed to this work.
References
- 1.Matilla-Dueñas A, Corral-Juan M, Volpini V, Sanchez I. The spinocerebellar ataxias: clinical aspects and molecular genetics. Advances in Experimental Medicine and Biology. 2012;724:351–374. doi: 10.1007/978-1-4614-0653-2_27. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi Y, Okamoto T, Taniwaki M, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nature Genetics. 1994;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- 3.Orr HT, Chung MY, Banfi S, et al. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nature Genetics. 1993;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- 4.Pulst SM, Nechiporuk A, Nechiporuk T, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinooerebellar ataxia type. Nature Genetics. 1996;14(3):269–276. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- 5.Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the α (1A)-voltage-dependent calcium channel. Nature Genetics. 1997;15(1):62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 6.David G, Abbas N, Stevanin G, et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nature Genetics. 1997;17(1):65–70. doi: 10.1038/ng0997-65. [DOI] [PubMed] [Google Scholar]
- 7.Koide R, Kobayashi S, Shimohata T, et al. A neurological disease caused by an expanded CAG trinucleotide repeat in the TATA-binding protein gene: a new polyglutamine disease? Human Molecular Genetics. 1999;8(11):2047–2053. doi: 10.1093/hmg/8.11.2047. [DOI] [PubMed] [Google Scholar]
- 8.Koide R, Ikeuchi T, Onodera O, et al. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA) Nature Genetics. 1994;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- 9.Zoghbi HY, Orr HT. Polyglutamine diseases: protein cleavage and aggregation. Current Opinion in Neurobiology. 1999;9(5):566–570. doi: 10.1016/S0959-4388(99)00013-6. [DOI] [PubMed] [Google Scholar]
- 10.Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nature Reviews Genetics. 2005;6(10):743–755. doi: 10.1038/nrg1691. [DOI] [PubMed] [Google Scholar]
- 11.Solans A, Zambrano A, Rodríguez M, Barrientos A. Cytotoxicity of a mutant huntingtin fragment in yeast involves early alterations in mitochondrial OXPHOS complexes II and III. Human Molecular Genetics. 2006;15(20):3063–3081. doi: 10.1093/hmg/ddl248. [DOI] [PubMed] [Google Scholar]
- 12.Bennett EJ, Shaler TA, Woodman B, et al. Global changes to the ubiquitin system in Huntington’s disease. Nature. 2007;448(7154):704–708. doi: 10.1038/nature06022. [DOI] [PubMed] [Google Scholar]
- 13.Chafekar SM, Duennwald ML. Impaired heat shock response in cells expressing full-length polyglutamine-expanded Huntingtin. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0037929.e37929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ou TT, Wu CH, Hsu JD, Chyau CC, Lee HJ, Wang CJ. Paeonia lactiflora Pall inhibits bladder cancer growth involving phosphorylation of Chk2 in vitro and in vivo. Journal of Ethnopharmacology. 2011;135(1):162–172. doi: 10.1016/j.jep.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Liu HQ, Zhang WY, Luo XT, Ye Y, Zhu XZ. Paeoniflorin attenuates neuroinflammation and dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease by activation of adenosine A1 receptor. British Journal of Pharmacology. 2006;148(3):314–325. doi: 10.1038/sj.bjp.0706732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu DZ, Zhu J, Jin DZ, et al. Behavioral recovery following sub-chronic paeoniflorin administration in the striatal 6-OHDA lesion rodent model of Parkinson’s disease. Journal of Ethnopharmacology. 2007;112(2):327–332. doi: 10.1016/j.jep.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Zhong SZ, Ge QH, Li Q, Qu R, Ma SP. Peoniflorin attentuates Aβ (1-42)-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. Journal of the Neurological Sciences. 2009;280(1-2):71–78. doi: 10.1016/j.jns.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Jeon MH, Kwon HJ, Jeong JS, Lee YM, Hong SP. Detection of albiflorin and paeoniflorin in Paeoniae Radix by reversed-phase high-performance liquid chromatography with pulsed amperometric detection. Journal of Chromatography A. 2009;1216(21):4568–4573. doi: 10.1016/j.chroma.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 19.Li SL, Song JZ, Choi FFK, et al. Chemical profiling of Radix Paeoniae evaluated by ultra-performance liquid chromatography/photo-diode-array/quadrupole time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2009;49(2):253–266. doi: 10.1016/j.jpba.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Koushik S, Dai R, Mivechi NF. Structural organization and promoter analysis of murine heat shock transcription factor-1 gene. Journal of Biological Chemistry. 1998;273(49):32514–32521. doi: 10.1074/jbc.273.49.32514. [DOI] [PubMed] [Google Scholar]
- 21.He M, Guo H, Yang X, et al. Genetic variations in HSPA8 gene associated with coronary heart disease risk in a Chinese population. PloS One. 2010;5(3):p. e9684. doi: 10.1371/journal.pone.0009684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y-R, Wang C-K, Chen C-M, et al. Analysis of heat-shock protein 70 gene polymorphisms and the risk of Parkinson's disease. Human Genetics. 2004;114(3):236–241. doi: 10.1007/s00439-003-1050-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee L-C, Chen C-M, Wang H-C, et al. Role of the CCAAT-binding protein NFY in SCA17 pathogenesis. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035302.e35302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flis S, Spławiński J. Inhibitory effects of 5-fluorouracil and oxaliplatin on human colorectal cancer cell survival are synergistically enhanced by sulindac sulfide. Anticancer Research. 2009;29(1):435–441. [PubMed] [Google Scholar]
- 25.Haacke A, Broadley SA, Boteva R, Tzvetkov N, Hartl FU, Breuer P. Proteolytic cleavage of polyglutamine-expanded ataxin-3 is critical for aggregation and sequestration of non-expanded ataxin-3. Human Molecular Genetics. 2006;15(4):555–568. doi: 10.1093/hmg/ddi472. [DOI] [PubMed] [Google Scholar]
- 26.Mielcarek M, Benn CL, Franklin SA, et al. SAHA decreases HDAC 2 and 4 levels in vivo and improves molecular phenotypes in the R6/2 mouse model of Huntington's disease. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027746.e27746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwasaki K, Kato S, Monma Y, et al. A pilot study of Banxia Houpu Tang, a traditional Chinese medicine, for reducing pneumonia risk in older adults with dementia. Journal of the American Geriatrics Society. 2007;55(12):2035–2040. doi: 10.1111/j.1532-5415.2007.01448.x. [DOI] [PubMed] [Google Scholar]
- 28.Satoh T, Takahashi T, Iwasaki K, et al. Traditional Chinese medicine on four patients with Huntington’s disease. Movement Disorders. 2009;24(3):453–455. doi: 10.1002/mds.22447. [DOI] [PubMed] [Google Scholar]
- 29.Okabe T, Fujisawa M, Sekiya T, Ichikawa Y, Goto J. Successful treatment of spinocerebellar ataxia 6 with medicinal herbs. Geriatrics & Gerontology International. 2007;7(2):195–197. [Google Scholar]
- 30.He D-Y, Dai S-M. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Frontiers in Pharmacology. 2011;2, article 10 doi: 10.3389/fphar.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim SH, Lee MK, Lee KY, Sung SH, Kim J, Kim YC. Chemical constituents isolated from Paeonia lactiflora roots and their neuroprotective activity against oxidative stress in vitro. Journal of Enzyme Inhibition and Medicinal Chemistry. 2009;24(5):1138–1140. doi: 10.1080/14756360802667977. [DOI] [PubMed] [Google Scholar]
- 32.Yan D, Saito K, Ohmi Y, Fujie N, Ohtsuka K. Paeoniflorin, a novel heat shock protein-inducing compound. Cell Stress and Chaperones. 2004;9(4):378–389. doi: 10.1379/CSC-51R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimoto M, Takaki E, Hayashi T, et al. Active HSF1 significantly suppresses polyglutamine aggregate formation in cellular and mouse models. Journal of Biological Chemistry. 2005;280(41):34908–34916. doi: 10.1074/jbc.M506288200. [DOI] [PubMed] [Google Scholar]
- 34.Fujikake N, Nagai Y, Popiel HA, Okamoto Y, Yamaguchi M, Toda T. Heat shock transcription factor 1-activating compounds suppress polyglutamine-induced neurodegeneration through induction of multiple molecular chaperones. Journal of Biological Chemistry. 2008;283(38):26188–26197. doi: 10.1074/jbc.M710521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warrick JM, Chan HYE, Gray-Board GL, Chai Y, Paulson HL, Bonini NM. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nature Genetics. 1999;23(4):425–428. doi: 10.1038/70532. [DOI] [PubMed] [Google Scholar]
- 36.Cummings CJ, Sun Y, Opal P, et al. Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Human Molecular Genetics. 2001;10(14):1511–1518. doi: 10.1093/hmg/10.14.1511. [DOI] [PubMed] [Google Scholar]
- 37.Kim SA, Yoon JH, Kim DK, Kim SG, Ahn SG. CHIP interacts with heat shock factor 1 during heat stress. FEBS Letters. 2005;579(29):6559–6563. doi: 10.1016/j.febslet.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 38.Lee LC, Chen CM, Chen FL, et al. Altered expression of HSPA5, HSPA8 and PARK7 in spinocerebellar ataxia type 17 identified by 2-dimensional fluorescence difference in gel electrophoresis. Clinica Chimica Acta. 2009;400(1-2):56–62. doi: 10.1016/j.cca.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Chen CM, Lee LC, Soong BW, et al. SCA17 repeat expansion: mildly expanded CAG/CAA repeat alleles in neurological disorders and the functional implications. Clinica Chimica Acta. 2010;411(5-6):375–380. doi: 10.1016/j.cca.2009.12.002. [DOI] [PubMed] [Google Scholar]


