Abstract
Susceptibility of proteins and peptides present in immune hemolymph of Galleria mellonella Fabricius (Lepidoptera: Pyralidae) larvae to proteolytic degradation by purified elastase B of Pseudomonas aeruginosa was studied. Results showed that apoLp-III protein was gradually digested by elastase B in vitro. Additionally, polipeptides with molecular mass 6.5 and 4 kDa were degraded after treatment with the studied enzyme. The lack of these peptides and the decrease in anti-Escherichia coli activity could indicate that inducible antimicrobial peptides were digested by elastase B. On the contrary, no change in the lysosome activity level was observed in immune hemolymph incubated with elastase B. Thus, elastase B might contribute to the pathogenesis of P. aeruginosa.
Keywords: antimicrobial peptides, apoLp-III, lysozyme
Introduction
The gram-negative bacterium Pseudomonas aeruginosa is an opportunistic human pathogen responsible for many types of infectious diseases. Different strains of P. aeruginosa secrete several extracellular proteolytic enzymes that have been implicated as virulence factors, namely protease IV, alkaline protease, elastase A, and elastase B (Caballero et al. 2001).
Pseudomonas aeruginosa elastase B is one of the major proteins secreted into the environment by many strains of this opportunistic pathogen. This 33 kDa enzyme (also called LasB protease and pseudolysin) belongs to the thermolysin family of Zndependent neutral metalloendopeptidases (M4) (Morihara et al. 1965; Morihara 1995; Kessler et al. 1998). It has a broad specificity, hydrolyzing internal peptide bonds of proteins and peptides on the amino side of hydrophobic residues in position P1’ (Matthews 1988; Miyoshi and Shinoda 2000). The primary structure of elastase was deduced from a full nucleotide sequence (Bever and Iglewski 1988; Fukushima et al. 1989), and its three-dimensional structure was determined by Thayer et al. (1991). Elastase B is involved in pathogenesis by degradation of human immunologically competent particles. LasB destroys complement components (Schultz and Miller 1974), cytokines (Parmely et al. 1990), immunoglobulins IgA and IgG (Buret and Cripps 1993; Maeda and Yamamoto 1996), human airway lysozyme (Jacquot et al. 1985), proteinase-activated receptors (Dulon et al. 2005), and surfactant protein A and D (Mariencheck et al. 2003).
Insects have a defense mechanisms consisting of cellular and humoral immune response systems (Lavine and Strand 2002; Jiravanichpaisal et al. 2006). The cellular response comprises phagocytosis, encapsulation, and nodulation of non-self bodies. The humoral defense involves production of antimicrobial peptides, reactive oxygen and nitrogen intermediates and complex enzymatic cascades that regulate coagulation and melanization of hemolymph (Lavine and Strand 2002). Antibacterial peptides are mainly produced in the fat body or hemocytes and then released into the hemolymph. Their synthesis is induced (i.e., cecropins, attacins, etc.) or increased (lysozyme) in response to foreign entities (Bulet et al. 1999; Yu et al. 2002 ).
It has been shown that apolipophorin III, a major exchangeable lipid transport protein found in hemolymph, may play an important role in the insect immune response. Recent immune studies indicate that apoLp-III stimulates an increase in hemolymph antibacterial activity (Wiesner et al. 1997; Niere et al. 1999) and may act as a pattern recognition molecule (Dettlof and Wiesner 1999; Whitten et al. 2004). ApoLp-III enhances hemocyte phagocytosis activity (Wiesner et al. 1997) and stimulates cellular encapsulation of foreign material (Whitten et al. 2004).
Andrejko et al. (2005) indicated that proteases IV might be involved in P. aeruginosa pathogenesis by degradation of G. mellonella apoLp-III. On the other hand, another immune protein, lysozyme, seemed to be insensitive to this protease (Andrejko et al. 2005). This raised questions on whether another P. aeruginosa protease, elastase B, is engaged in pathogenesis. This paper presents in vitro studies on the effect of purified elastase B of P. aeruginosa on the activity and level of proteins and peptides in the immune hemolymph of Galleria mellonella Fabricius (Lepidoptera: Pyralidae) larvae.
Materials and Methods
Insect culture and immune challenge
Larvae of the greater wax moth G. mellonella were reared on a natural diet of honeybee nest debris at 30 °C in the dark. Final instar larvae weighing 250–300 mg were selected for this study.
The larvae were immune-challenged by an injection of live Escherichia coli D31 (105 CFU). After the treatment, larvae were kept at 30 °C in the dark on sterile Petri plates, and hemolymph was collected after 24 hours.
Bacteria and enzyme
Escherichia coli K12, strain D31, LPS defective, streptomycin and ampicillin resistant (CGSC 5165) was used (Boman et al. 1974). The bacterial cells were grown in a nutrient broth for 24 hours at 37 °C and pelleted by centrifugation at 20,000 × g for 10 min at 4 °C.
Purified, crystallized elastase B of P. aeruginosa was purchased from Calbiochem (www.emdmillipore.com).
In vivo experiments
For in vivo experiments, G. mellonella larvae were injected with elastase B at concentrations of 0.05 µg, 0.1 µg, and 0.2 µg per larvae. Groups of 12 larvae were used in each case. After challenge, insects were kept on sterile Petri plates at room temperature in the darkness. The percent mortality of larvae 48 hours after enzyme injection was determined.
Hemolymph collection and preparation of hemolymph extract
Prior to hemolymph collection, the insects were chilled for 15 min at 4 °C. Hemolymph samples were obtained by puncturing the larval abdomen with a sterile needle. The outflowing hemolymph was immediately transferred into sterile and chilled Eppendorf tubes containing a few crystals of phenylthiourea (PTU) to prevent melanization. The hemocyte-free hemolymph was obtained by centrifugation at 200 × g for five min and subsequently at 20,000 × g for 10 min at 4 °C. Pooled supernatants were stored at -20 °C until used.
Low molecular mass proteins and peptides were isolated from the hemocyte-free hemolymph by the acidic/methanol extraction method adapted from Schoofs et al. (1990). The hemolymph was diluted 10 times with the extraction solution consisting of methanol: glacial acetic acid: water (90:1:9, v/v/v) and mixed thoroughly. Precipitated proteins were pelleted by centrifugation at 20,000 × g for 30 min at 4 °C. The obtained supernatant was collected, vacuum dried, and the pellet was stored at -20 °C until used. Before used, it was dissolved in an appropriate volume of sterile distilled water. The acidic/methanol hemolymph extract obtained as described above contained proteins and peptides of Mr below 30 kDa.
Antibacterial activity assay
The presence of antibacterial activity in the hemolymph and acidic/methanol extracts of hemolymph was detected by a growth inhibition zone assay using solid agar plates containing viable E. coli cells as described by Hoffmann et al. (1981). To improve the sensitivity of the method, chicken egg white lysozyme (EWL) at a concentration of 2.0 mg × mL-1 of the medium was added (Chalk and Suliaman 1998; Cytryńska et al. 2001). Each well on the Petri dish was filled with 4 µL of hemolymph or hemolymph extract (the amount of protein and other reaction details are presented in Figure legends). The agar plates were then incubated at 37 °C for 24 hours. The diameters of E. coli D31 growth inhibition zones were measured and the level of antimicrobial activity was calculated using the algorithm described by Hultmark et al. (1982). Synthetic cecropin B (Sigma-Aldrich, www.sigmaaldrich.com) was used as a standard for evaluation of antibacterial activity
Lysozyme activity
Lysozyme activity in hemocyte-free hemolymph and in hemocyte-free hemolymph extract was detected using agarose plates containing freeze-dried Micrococcus luteus (Sigma) (Jarosz 1995). Each well on the Petri dish was filled with 4 µL sample, and after 24 hours incubation at 28 °C, the diameters of the M. luteus lytic zones were measured. The activity of lysozyme was calculated from a standard curve made with EWL (Sigma; EC 3.2.1.17) and expressed in µg/mL EWL.
Electrophoresis methods and immunoblotting
Polyacrylamide gel electrophoresis of protein samples was performed by tricine SDS-PAGE (16.5% T, 3% C) according to Schägger and Jagow (1987). Polypeptide bands in gels were stained with Coomassie Brilliant Blue G-250 (Imperial Chemical Industries).
IEF was performed with 100 µg of hemolymph extract protein using the Protean IEF focusing system (Bio-Rad, www.biorad.com) according to the manufacturer's recommendations. The sample was suspended in rehydratation buffer (8.8 mol/L urea, 2%, W/V CHAPS; 70 mmol/L DTT; 0.2 %, W/V, Bio-Lytes) and loaded on 70 mm IPG strips (Bio-Rad). After separation of proteins in the first dimension, strips were equilibrated twice for 15 min in equilibration buffer (6 mol/L urea; 20%, V/V, glycerol; 2%, W/V, SDS; 375 mmol/L Tris-HCL, pH 8.8). The first step was done in equilibration buffer with 130 mmol/L DTT; the second equilibration step contained 135 mmol/L iodoacetamide. Then tricine SDS-PAGE and immunoblotting were performed under the conditions described above.
The samples after tricine and 2D electrophoresis were electroblotted onto Immobilon membranes (Millipore, www.millipore.com) for 90 min at 350 mA. For identification of apolipophorin-III, antiG mellonella apoLp-III antibodies (Agrisera, www.agrisera.com) were used. Alkaline phosphatase-conjugated goat anti-rabbit IgGs were used as secondary antibodies. Immunoreactive bands were visualized by incubation with p-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate.
Protein determination
The protein concentration was estimated by the Bradford (1976) method using bovine serum albumin (BSA) as a standard.
Results
Mortality studies
First, the toxicity of P. aeruginosa elastase B up to the 7th instar larvae of G. mellonella was determined (Figure 1). Three doses of purified elastase B (0.05, 0.1, and 0.2 µg/larvae) were used. Insects were injected through the last pro-leg and mortality rates were determined over a 48 hour period. The mortality rates observed for larvae inoculated with elastase at the concentration of 0.1 and 0.2 µg per larvae after 48 hours incubation were 8 and 14%, respectively. Results indicated that the doses of purified elastase B were sublethal for G. mellonella larvae and these doses were chosen for further experiments.
Figure 1.
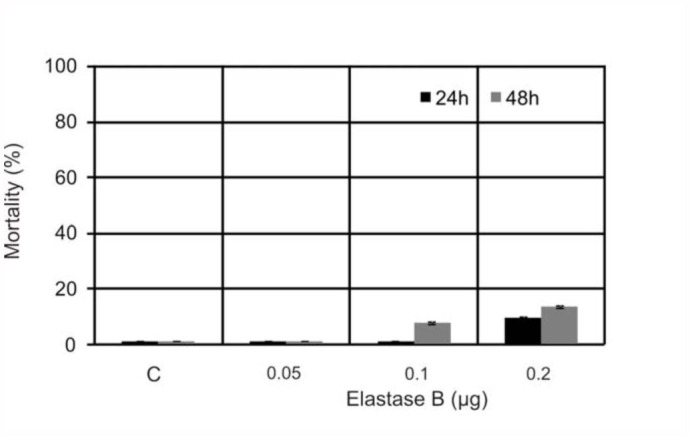
The toxicity of Pseudomonas aeruginosa elastase B to Galleria mellonella larvae. Mortality percentage of insects after injection with indicated elastase B concentrations at 24 (black) and 48 (gray) hours. (C) Control, punctured only larvae. All values represent the mean ± SD of three independent experiments. High quality figures are available online.
Effect of elastase B on hemolymph proteins/peptides profile
Subsequent experiments investigated the susceptibility of proteins and peptides present in immune hemolymph of G. mellonella larvae to proteolytic degradation by purified elastase B of P. aeruginosa. Acidic/methanol extract obtained by the method adopted from Schoofs et al. (1990) was used as the source of proteins and peptides. This procedure allowed elimination of hemolymph proteins of molecular masses above 30 kDa (Cytryńska et al. 2007).
In preliminary studies, the immune hemolymph extract was incubated 60 min at 37 °C with various concentrations of elastase B at 37 °C (optimal for the enzyme) or 30 °C (optimal for the insect). Control extracts were also incubated at both temperatures. All samples were analyzed by Tricine-SDS-PAGE. Polypeptides of molecular mass below 30 kDa had different susceptibility to proteolytic digestion by P. aeruginosa elastase B (Figure 2). The results also showed that heat treatment of the control sample did not result in degradation of insect proteins.
Figure 2.
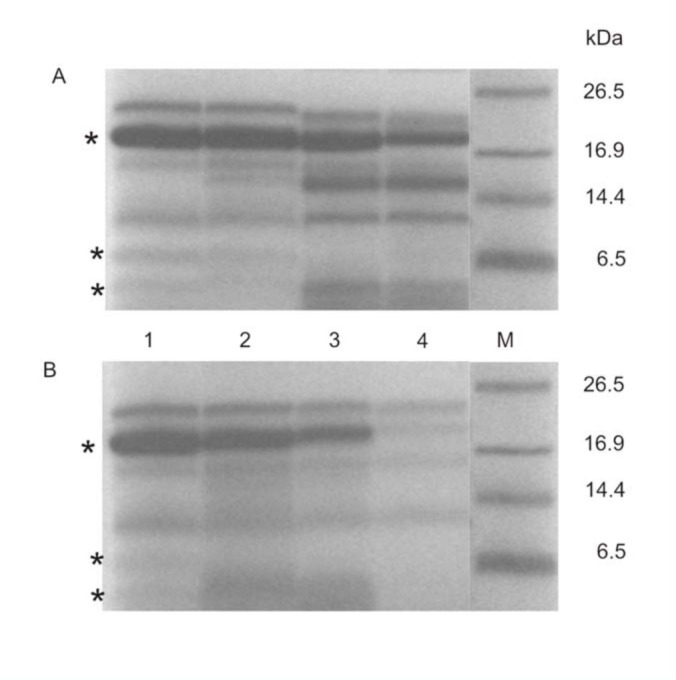
The effect of elastase B on immune hemolymph extract of Galleria mellonella larvae in vitro. Samples containing immune hemolymph extract (20 µg of protein) and purified elastase B (0.05 µg (lane 2), 0.1 µg (lane 3), 0.2 µg (lane 4)) were incubated at 37 °C for 60 min. Then samples were separated by tricine electrophoresis and stained with Commassie Brilliant Blue R-250. (M) Molecular mass markers. Control (hemolymph extract only) in lane 1. (*) Indicates apoLp-III (18 kDa) and antimicrobial peptides (6.5 and 4 kDa). High quality figures are available online.
Identification of apoLp-III as a substrate for elastase B
The major protein band of approximately 18 kDa was effectively digested by the elastase B (Figure 2). It corresponded to the molecular mass of apolipophorin III, which is one of the most abundant polypeptides in G. mellonella hemolymph. Because apoLp-III participates in the induced immunological responses of the larvae of G. mellonella, the question of whether elastase B is responsible for apoLpIII degradation in wax moth hemolymph was addressed.
ApoLp-III protein was almost completely degraded after the incubation of the hemolymph extract with elastase B used at the concentration of 0.2 µg, particularly after incubation at 37 °C (Figure 2B). Therefore, subsequent experiments tested the apoLp-III level using the electrophoretic/immunoblotting technique. The samples containing the hemolymph extract and elastase B (0.05, 0.1, and 0.2 µg/larva) were incubated at 37 °C for 60 min. Next, the samples after tricine SDS-PAGE were electroblotted onto Immobilon membranes. Anti-G. mellonella apoLp-III antibodies were used for identification of apoLp-III. The results of the kinetic studies (Figure 3) revealed that apoLp-III was stepwise degraded to lower molecular mass forms. The amount of apoLp-III protein decreased with the growing concentration of the enzyme. Finally, elastase at the concentration 0.2 µg completely digested apoLp-III protein after 60 min of incubation in vitro (Figure 3A, lane 4).
Figure 3.
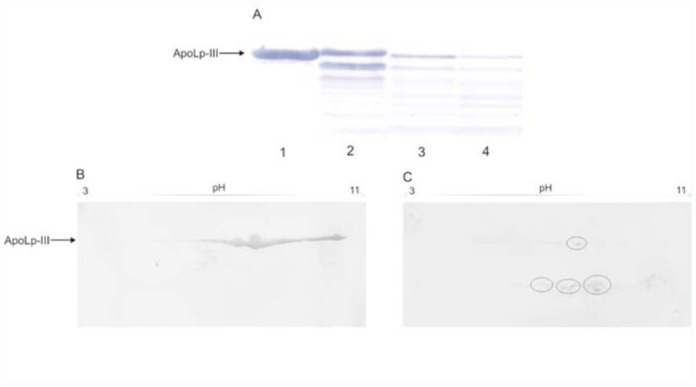
Degradation of apoLp-III from hemolymph extract of Galleria mellonella larvae by elastase B Pseudomonas aeruginosa in vitro. (A) Tricine SDS-PAGE and immunobloting with anti-Galleria mellonella apoLp-III antibodies. The samples containing hemolymph extract (20 µg of protein) and elastase B (0.05 µg (lane 2), 0.1 µg (lane 3), 0.2 µg (lane 4), 0.4 µg (lane 5)) were incubated at 37 °C for 60 min. The reactions were stopped by sample buffer addition. Lane 1 contained hemolymph extract only. (B) and (C) SDS-PAGE 2D electrophoresis. Hemolymph extract ( 100 µg of protein) and elastase B (0.6 µg) were incubated at 37 °C for 60 min. Then samples were loaded on pH 3 to l l isoelectric strips followed by Tricine SDS-polyacrylamide gel electrophoresis. For identification of apoLp-III anti-Golleria mellonella apoLp-III antibodies were used. (B) control sample of apoLp-III. (C) degradation products of apoLp-III are marked in circles. High quality figures are available online.
Samples containing hemolymph extract (100 µg protein) preincubated with elastase B (0.6 µg protein) were also tested by two— dimensional gel electrophoresis. IPG strips that separate proteins with pI values of 3 to 11 were used, as well as 16.5% Tris-Tricine-SDS gel. As revealed by immunoblots (Figure 3B), apoLp-III protein is a predominant protein and exists in different isoforms, which Mr are indistinguishable. The protein isoforms, however differ in the isoelectric point. As presented in Figure 3C, samples incubated with elastase B showed nearly a complete lack of apoLp-III protein in comparison with the control samples (Figure 3B). The trace amount of apoLp-III was observed together with three degradation products with molecular mass approximately 6 kDa, differing in the isoelectric point. The above results suggest that the decline in the 18 kDa protein level may be caused by proteolytic degradation by P. aeruginosa elastase B.
Identification of antimicrobial peptides as substrates for elastase B
It was found that in the extract of immune hemolymph, at least two peptide bands with molecular mass 4–6 kDa displayed antimicrobial activity (Cytryńska et al. 2007).
Tests were run to see whether antimicrobial peptide activity of G. mellonella was abolished by purified elastase B. Samples of cell-free immune hemolymph or hemolymph extract were mixed with P. aeruginosa elastase B, and after 60 min of incubation at 37 °C, they were tested for antibacterial activity against the indicator strain E. coli D31.
Figure 4A shows that the antibacterial activity measured by the diffusion well assay was significantly lower in the samples preincubated with Pseudomonas elastase. Antibacterial activity of acidic/methanol hemolymph extract was completely abolished by elastase used at the concentration of 0.16 µg. However, in the case of hemolymph, complete inactivation of peptides occurred when elastase was added to the sample at the concentration of 0.2 µg/larvae.
The data presented in Figure 2 indicate that peptide bands with molecular mass approximately 6.5 and 4 kDa were degraded by elastase B, used at the concentration of 0.05 µg/larvae.
The synthetic cecropin B of Hyalophora cecropia was used for the comparative study, which showed that cecropin was completely degraded in vitro by 0.02 µg of purified elastase B (Figure 4B). The analysis of cecropin B primary structure revealed four potential cleavage sites for elastase B (Figure 4C).
Figure 4.
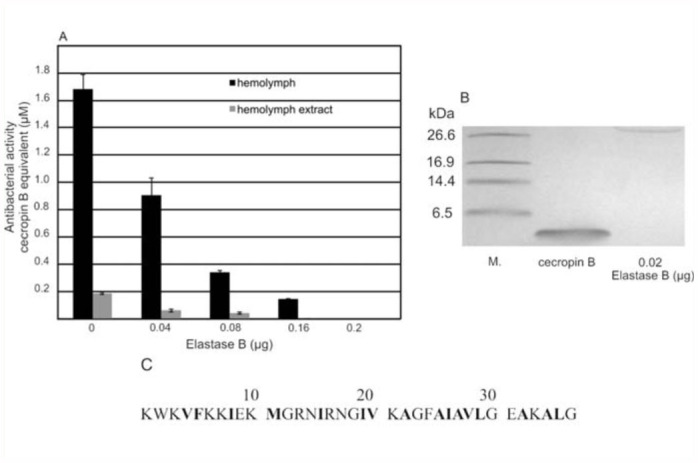
(A) The effect of elastase B on antibacterial activity of Galleria mellonella hemolymph. Samples containing immune hemolymph (black) or immune hemolymph extract (grey) (10 µg of protein) and elastase B were incubated at 37 °C for 60 min. Antibacterial activity was analyzed by diffusion well assay as described in the Materials and Methods section. The bars represent ± SD. (B) Degradation in vitro of synthetic cecropin B by elastase B. Samples containing synthetic cecropin B (2 µg) and elastase B (0.02 µg 0.04 µg) were incubated at 37 °C for 60 min. The reaction was stopped by sample buffer addition. Then the samples were subjected to tricine-SDS-PAGE. (M) Molecular mass markers. (C) Amino acid sequence of cecropin B Hyalophora cecropia. The potential enzyme cleavage sites are indicated in bold. High quality figures are available online.
Studies on lysozyme as a substrate for elastase B
Finally, we investigated whether the level of lysozyme activity changed after treatment with elastase B. It is known that lysozyme plays an important role in humoral defense of G. mellonella.
In immune hemolymph and hemolymph extract incubated with elastase B, the activity of lysozyme activity level was reduced (Figure 5). A near 20% decrease in lysozyme activity both in the case of hemolymph and hemolymph extract was observed in samples containing 0.2 µg elastase B. This indicated that, in contrast to apoLp-III and antimicrobial peptides, lysozyme activity appeared to be insensitive to metaloprotease produced by P. aeruginosa.
Figure 5.
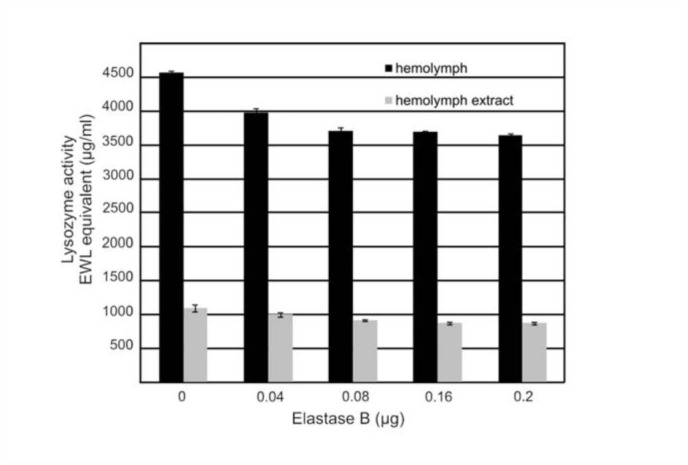
The effect of elastase B on lysozyme activity in Galleria mellonella. Samples containing immune hemolymph (black) or immune hemolymph extract (grey) (10 µg of protein) and elastase B were incubated at 37 °C for 60 min. Lysozyme activity in samples was detected by diffusion assay using agarose plates containing freeze-dried Micrococcus luteus as described in the Materials and Methods section. High quality figures are available online.
Discussion
It is known that thermolysin-like metalloproteinases associated with human or entomopathogenic bacteria and fungi play a predominant role as virulence factors. Pseudomonas aeruginosa virulence is attributed greatly to its ability to secrete metalloproteases (e.g., elastase B) into the environment. The role of elastase as a virulence factor is supported by inactivation or degradation of a variety of biologically important substrates.
Elastase B has been shown to enhance the virulence of P. aeruginosa strains (Morihara and Homma 1985; Nicas and Iglewski 1985). The results of our in vivo tests indicated that elastase injected into hemolymph of G. mellonella larvae was toxic to insects. The cadavers of killed larvae became black, suggesting activation of prophenoloxidase, which mediates melanization (data not presented).
This study demonstrated that proteins and peptides present in immune hemolymph of G. mellonella were susceptible to proteolytic degradation by purified elastase B of P. aeruginosa. In particular, elastase B was responsible for degradation of apoLp-III and antimicrobial peptides with molecular mass 4 and 6.5 kDa. Results also showed that G. mellonella lysozyme activity appeared to be insensitive to elastase B in vitro.
Literature data suggested involvement of apoLp-III in immune response of the greater wax moth (Dunphy and Halwani 1997; Halwani et al. 2000; Pratt and Weers 2004; Whitten et al. 2004). In G. mellonella, apoLpIII has a molecular mass of 18.1 kDa, an isoelectric point of 6.5, and 64–90% amino acid sequence homology with apoLp-III of other Lepidopteran species (Weise et al. 1998). Andrejko et al. (2005) showed that apoLp-III was degraded by extracellular serine protease IV isolated from the entomopathogenic strain of P. aeruginosa in vitro. The increase in the apoLp-III content in vivo was observed during the first 19 hours after injection with P. aeruginosa, and a subsequent decrease after prolonged infection time (Andrejko et al. 2008).
It is known that elastase B hydrolyzes internal peptide bonds of proteins on the amino acid side of the hydrophobic residues with phenylalanine, tyrosine, and leucine, which are the preferred residues in position P1’ (Thayer et al. 1991). The analysis of apoLp-III primary structure (Weise et al. 1998) revealed that among 163 amino acid, 23 residues constitute potential cleavage sites for elastase B.
This study showed that the 18 kDa apoLp-III protein was effectively digested by purified elastase B. As revealed by electrophoretic/immunoblotting technique, the apoLp-III was almost completely degraded after the incubation in vitro with elastase B used at the concentration of 0.2 µg. This result could indicate that a decreasing level of apoLp-III after prolonged time of infection is connected with degradation of this protein by elastase B.
This seemed to be confirmed by our observation in vitro and in vivo that the degradation of apoLp-III was correlated with a significant increase of proteolytical activity produced by P. aeruginosa during late stages of bacterial infection of the host, G. mellonella larvae.
In the extract of immune hemolymph, at least two additional peptide bands with molecular mass 4–6 kDa were detected when compared to the extract prepared from non-immune hemolymph. It was also shown that additional bands displayed antimicrobial activity appearing in the hemolymph in response to immune challenge (Cytryńska et al. 2007). Galleria mellonella can release at least 18 antimicrobial peptides from 10 families to defend itself against invading microbes (Brown et al. 2009).
We determined that peptide bands with molecular mass of approximately 6.5 kDa were not observed in the sample treated with 0.05 µg elastase. The defense peptide with Mr of 6.5 kDa purified from the hemolymph of immune-challenged G. mellonella larvae (Gm anionic peptide 2) is active against certain Gram-positive bacteria and also exhibited antifungal activity (Cytryńska et al. 2007). This could indicate that the studied enzyme was capable of degrading this peptide, causing decreased antimicrobial activity in the hemolymph.
Our results also showed that the peptide band of 4.0 kDa was sensitive to degradation by elastase B. There are at least five different peptides with a molecular mass 4 kDa isolated from immunized G. mellonella larvae: Gm anionic peptide, Gm proline-rich peptide 1, defensin, a defensin—like and cecropin D—like peptide (Mak et al. 2001; Lee et al. 2004; Cytryńska et al. 2007).
The decrease in anti—E. coli activity, lack of peptide bands with molecular mass 6.5 and 4 kDa, and degradation of synthetic cecropin B indicate that inducible antimicrobial peptides were degraded by the P. aeruginosa elastase B.
These results correlate with the studies performed earlier in our laboratory that indicated that elastase B produced by P. aeruginosa entomopathogenic strain digested in vitro inducible antimicrobial peptides of G. mellonella as well as synthetic cecropin B H. cecropia (Andrejko et al. 2009).
Lysozyme plays an important role in humoral defense of G. mellonella. The lysozyme activity in insect hemolymph is maintained on a low level and increases in response to bacterial challenge (Powning and Davidson 1973; Morishima et al. 1995; Lockey and Ourth 1996). Peptidoglycan and lipopolysacharide fragments released by lysozyme from bacterial cell walls act as signal molecules for the activation of a series of immune genes (Dunn et al. 1985; Kanost et al. 1988;Morishima et al. 1992).
Our earlier studies showed that the level of lysozyme was not degraded in vitro by P. aeruginosa crude proteolytic fraction (Andrejko 2004) and in the samples containing protease IV (Andrejko et al. 2005). However, a significant decrease in lysozyme activity exposed to the action of P. aeruginosa proteins was observed (Andrejko 2004).
The results presented here indicated that after incubation of immune hemolymph with purified elastase, the activity of lysozyme was inhibited by 20% in comparison to control samples. The results are in line with in vivo data reported previously (Andrejko et al. 2008). The G. mellonella lysozyme level and activity appeared to be insensitive to extracellular proteinases produced during P. aeruginosa infection (Andrejko et al. 2008)
Hydrolysis of hemolymph proteins by collagenolytic enzymes, such as thermolysin, results in formation of small-sized protein fragments, inducing expression of immune related genes encoding antimicrobial peptides such as galleriomycin, gloverin, IMPI, and lysozyme (Altincicek and Vilcinskas 2006; Altancicek et al. 2007).
Recently, Andrejko et al. (2011) showed that elastase B injected at a sublethal concentration was responsible for eliciting the humoral immune response in G. mellonella larvae. Appearance of antimicrobial peptides, higher level of lysozyme, and apoLp-III were observed in the hemolymph 24 hours after elastase injection, in comparison with control G. mellonella larvae. On the other hand, in the present work, both apoLp-III and antimicrobial peptides were substrates for purified elastase B. It seems that in the first hours of P. aeruginosa infection, elastase B. stimulates the humoral immune response in G. mellonella. Degradation of apoLp-III as with other immune peptides seems to be correlated with a significant increase of proteolytic activity produced by P. aeruginosa during late stages of bacterial infection.
The experimental evidence reported here indicates that in vitro proteolytic degradation proteins/peptides in larval hemolymph may be caused by elastase B from P. aeruginosa. This metalloproteinase of the entomopathogenic strain P. aeruginosa seems to contribute to the virulence against insect immune response. Detailed mechanisms by which P. aeruginosa elastase B influence G. mellonella immune response require further studies.
References
- Altancicek B, Linder M, Linder D, Preissner KT, Vilcinskas A. Microbial metalloproteinass mediate sensing of invading pathogens and activate innate immune responses in the Lepidopteran model host Galleria mellonella. Infection and Immunity. 2007;5:175–183. doi: 10.1128/IAI.01385-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altancicek B, Vilcinskas A. Metamorphosis and collagen-IV-fragments stimulate innate immune response in the greater wax moth, Galleria mellonella. Developmental and Comparative Immunology. 2006;30:1108–1118. doi: 10.1016/j.dci.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Andrejko M. Effect of Pseudomonas aeruginosa crude proteolytic fraction on antibacterial activity of Galleria mellonella haemolymph. Folia Biologica. 2004;52:91. [PubMed] [Google Scholar]
- Andrejko M, Cytryńska M, Jakubowicz T. Apolipophorin III is substrate for protease IV from Pseudomonas aeruginosa. FEMS Microbiology Letters. 2005;243:331–337. doi: 10.1016/j.femsle.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Andrejko M, Mizerska-Dudka M. Elastase B of Pseudomonas aeruginosa stimulates the humoral immune response in the greater wax moth, Galleria mellonella. Journal of Invertebrate Pathology. 2011;107:16–26. doi: 10.1016/j.jip.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Andrejko M, Mizerska-Dudka M, Jakubowicz T. Changes in Galleria mellonella apolipophorin III level during Pseudomonas aeruginosa infection. Journal of Invertebrate Pathology. 2008;97:14–19. doi: 10.1016/j.jip.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Andrejko M, Mizerska-Dudka M, Jakubowicz T. Antibacterial activity in vivo and in vitro in the hemolymph of Galleria mellonella infected with Pseudomonas aeruginosa. Comparative Biochemistry and Physiology. 2009;152:118–123. doi: 10.1016/j.cbpb.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Bever RA, Iglewski BH. Molecular characterization and nucleotide sequence of the Pseudomonas aeruginosa elastase structural gene. Journal of Bacteriology. 1988;170:4309–4314. doi: 10.1128/jb.170.9.4309-4314.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman HG, Nilsson-Faye I, Paul K, Rasmuson T. Insect immunity. I. Characteristic of an inducible cell-free antibacterial reaction in hemolymph of Samia cynthia pupae. Infection and Immunity. 1974;10:136–145. doi: 10.1128/iai.10.1.136-145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–410. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. A peptidomics study reveals the impressive antimicrobial peptide arsenal of the wax moth Galleria mellonella. Insect Biochemistry and Molecular Biology. 2009;39:792–800. doi: 10.1016/j.ibmb.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insect; structure and function. Developmental and Comparative Immunology. 1999;23:329–344. doi: 10.1016/s0145-305x(99)00015-4. [DOI] [PubMed] [Google Scholar]
- Buret A, Cripps AW. The immunoevasive activities of Pseudomonas aeruginosa: relevance for cystic fibrosis. American Review of Respiratory Disease. 1993;148:793–805. doi: 10.1164/ajrccm/148.3.793. [DOI] [PubMed] [Google Scholar]
- Caballero AR, Moreau JM, Engel IS, Marquait ME, Hill JM, O'Callaghan RJ. Pseudomonas aeruginosa protease IV enzyme assay and comparison to other Pseudomonas proteases. Analytical Biochemistry. 2001;290:330–337. doi: 10.1006/abio.2001.4999. [DOI] [PubMed] [Google Scholar]
- Chalk R, Suliaman WY. Antimicrobial peptides from small insects: methods for insect culture and for the detection, visualization, isolation and sequencing of active hemolymph peptides. In: Wiesner A, Dunphy GB, Marmaras VJ, Morishima I, Sugumaran M, Yamakawa M, editors. Techniques in Insect Immunology. SOS Publications; 1998. pp. 109–124. [Google Scholar]
- Cytryńska M, Zdybicka-Barabas A, Jabłonski P, Jakubowicz T. Detection of antibacterial polypeptide activity in situ after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Analytical Biochemistry. 2001;299:274–276. doi: 10.1006/abio.2001.5422. [DOI] [PubMed] [Google Scholar]
- Cytryńska M, Mak P, Zdybicka-Barabas A, Suder P, Jakubowicz T. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides. 2007;28:533–546. doi: 10.1016/j.peptides.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Dettloff M, Wiesner A. Immune stimulation by lipid-bound apolipophorin III. In: Wiesner A, Dunphy GB, Marmaras VJ, Morishima I, Sugumaran M, Yamakawa M, editors. Techniques in Insect Immunology, SOS Publications; 1999. pp. 243–251. [Google Scholar]
- Dulon S, Leduc D, Cottrell GS, D'Alayer J, Hansen KK, Bunnett NW, Hollenberg MD, Pidard D, Chignard M. Pseudomonas aeruginosa elastase disables proteinaseactivated receptor 2 in respiratory epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2005;32:411–419. doi: 10.1165/rcmb.2004-0274OC. [DOI] [PubMed] [Google Scholar]
- Dunn PE, Dai W, Kanost MR, Geng C. Soluble peptidoglycan fragments stimulate antibacterial protein synthesis by fat body from larvae of Manduca sexta. Developmental and Comparative Immunology. 1985;9:559–568. doi: 10.1016/0145-305x(85)90019-9. [DOI] [PubMed] [Google Scholar]
- Dunphy G, Halwani A. Haemolymph proteins of larvae of Galleria mellonella detoxify endotoxins of the insect pathogenic bacteria Xenorhabdus nematophilus (Enterobacteriaceae). Journal of Insect Physiology. 1997;43:1023–1029. doi: 10.1016/s0022-1910(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Yamamoto S, Morihara K, Atsumi Y, Takeuchi H, Kawamoto S, Okuda K. Structural gene and complete amino acid sequence of Pseudomonas aeruginosa IFO 3455 elastase. Journal of Bacteriology. 1989;171:1698–1704. doi: 10.1128/jb.171.3.1698-1704.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halwani AE, Niven DF, Dunphy GB. Apolipophorin-III and the interactions of lipoteichoic acids with the immediate immune responses of Galleria mellonella. Journal of Invertebrate Pathology. 2000;76:233–241. doi: 10.1006/jipa.2000.4978. [DOI] [PubMed] [Google Scholar]
- Hoffman D, Hultmark D, Boman HG. Insect immunity: Galleria mellonella and other Lepidoptera have cecropia-P9-like factors active against gram negative bacteria. Insect Biochemistry. 1981;11:537–548. [Google Scholar]
- Hultmark D, Engström A, Bennich H, Kapur R, Boman HG. Insect immunity: Isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. European Journal of Biochemistry. 1982;127:207–217. doi: 10.1111/j.1432-1033.1982.tb06857.x. [DOI] [PubMed] [Google Scholar]
- Jacquot J, Tournier JM, Puchelle E. In vitro evidence that human airway lysozyme is cleaved and inactivated by Pseudomonas aeruginosa elastase and not by human leukocyte elastase. Infection and Immunity. 1985;47:555–560. doi: 10.1128/iai.47.2.555-560.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz J. Hemolymph immune proteins protect the insect body cavity from invading bacteria. Comparative Biochemistry and Physiology. 1995;111:213–220. [Google Scholar]
- Jiravanichpaisal P, Lee BL, Söderhäll K. Cell-mediated immunity in arthropods: hematopoiesis, coagulation, melanization and opsonization. Immunobiology. 2006;211:213–236. doi: 10.1016/j.imbio.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Dai W, Dunn PE. Peptidoglycan fragments elicit antibacterial protein synthesis in larvae of Manduca sexta. Archives Insect Biochemistry and Physiology. 1988;8:147–164. [Google Scholar]
- Kessler E, Safrin M, Gustin JK, Ohman DE. Elastase and the LasA protease of Pseudomonas aeruginosa are secreted with their propeptides. Journal of Biological Chemistry. 1998;273:30225–0231. doi: 10.1074/jbc.273.46.30225. [DOI] [PubMed] [Google Scholar]
- Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochemistry and Molecular Biology. 2002;32:1295–1309. doi: 10.1016/s0965-1748(02)00092-9. [DOI] [PubMed] [Google Scholar]
- Lee YS, Yun EK, Jang WS, Kim I, Lee JH, Park SY, Ryu KS, Seo SJ, Kim CH, Lee IH. Purification, cDNA cloning and expression of an insect defensin from the great wax moth, Galleria mellonella. Insect Molecular Biology. 2004;13:65–72. doi: 10.1111/j.1365-2583.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- Lockey TD, Ourth DD. Purification and characterization of lysozyme from of Heliothis virescens larvae. Biochemical and Biophysical Research Communications. 1996;220:502–508. doi: 10.1006/bbrc.1996.0434. [DOI] [PubMed] [Google Scholar]
- Maeda H, Yamamoto T. Pathogenic mechanisms induced by microbial proteases in microbial infections. Biological Chemistry. 1996;377:217–226. doi: 10.1515/bchm3.1996.377.4.217. [DOI] [PubMed] [Google Scholar]
- Mak P, Chmiel D, Gacek GJ. Antibacterial peptides of the moth Galleria mellonella. Acta Biochimica Polonica. 2001;48:1191–1195. [PubMed] [Google Scholar]
- Mariencheck WI, Alcorn JF, Palmer SM, Wright JR. Pseudomonas aeruginosa elastase degrades surfactant proteins A and D. American Journal of Respiratory Cell and Molecular Biology. 2003;38:528–537. doi: 10.1165/rcmb.2002-0141OC. [DOI] [PubMed] [Google Scholar]
- Matthews BW. Structural basis of the action of thermolysin and related zinc peptidases. Accounts of Chemical Research. 1988;21:333–340. [Google Scholar]
- Miyoshi S, Shinoda S. Microbial metalloproteases and pathogenesis. Microbes and Infection. 2000;2:91–98. doi: 10.1016/s1286-4579(00)00280-x. [DOI] [PubMed] [Google Scholar]
- Morihara K, Tsuzuki H, Oka T, Inoue H, Ebata M. Pseudomonas aeruginosa elastase. Isolation, crystallization, and preliminary characterization. Journal of Biological Chemistry. 1965;240:3295–3304. [PubMed] [Google Scholar]
- Morihara K, Homma JY. Pseudomonas proteases. In: Holder A, editor. Bacterial Enzymes and Virulence, CRC Press; 1985. pp. 41–79. [Google Scholar]
- Morihara K. Pseudolysin and other pathogen endopeptidases of thermolysin family. Methods in Enzymology. 1995;248:242–253. doi: 10.1016/0076-6879(95)48017-x. [DOI] [PubMed] [Google Scholar]
- Morishima I, Yamada K, Ueno T. Bacterial peptidoglycan as elicitor of antibacterial protein synthesis in larvae of silkworm, Bombyx mori. Insect Biochemistry and Molecular Biology. 1992;22:363–367. [Google Scholar]
- Morishima I, Horiba T, Iketani M, Nishioka E, Yamano Y. Parallel induction of cecropin and lysozyme in larvae of the silk-worm, Bombyx mori. Developmental and Comparative Immunology. 1995;19:357–363. doi: 10.1016/0145-305x(95)00019-p. [DOI] [PubMed] [Google Scholar]
- Nicas T, Iglewski BH. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Canadian Journal of Microbiology. 1985;31:387–392. doi: 10.1139/m85-074. [DOI] [PubMed] [Google Scholar]
- Niere M, Meisslitzer C, Dettloff M, Weise C, Ziegler M, Wiesner A. Insect immune activation by recombinant Galleria mellonella apolipophorin III. Biochimica et Biophysica Acta. 1999;1433:16–26. doi: 10.1016/s0167-4838(99)00148-x. [DOI] [PubMed] [Google Scholar]
- Parmely M, Gale A, Clabaugh M, Horvat R, Zhou WW. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infection and Immunity. 1990;58:3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powning RF, Davidson WJ. Studies on insect bacteriolytic enzymes - I. Lysozyme in hemolymph of Galleria mellonella and Bombyx mori. Comparative Biochemistry and Physiology. 1973;45:669–681. [PubMed] [Google Scholar]
- Pratt CC, Weers PMM. Lipopolysaccharide binding of an exchangeable apolipoprotein, apolipophorin III, from Galleria mellonella. Journal of Biological Chemistry. 2004;385:1113–1119. doi: 10.1515/BC.2004.145. [DOI] [PubMed] [Google Scholar]
- Schultz DR, Miller KD. Elastase of Pseudomonas aeruginosa: inactivation of complement components and complement-derived chemotactic and phagocytic factors. Infection and Immunity. 1974;10:128–135. doi: 10.1128/iai.10.1.128-135.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range 1 to 100 kDa. Analytical Biochemistry. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schoofs L, Holman GM, Hayes TK, Nachman RJ, De Loof A. Locusta tachykinin I and II, two novel insect neuropeptides with homology to peptides from the vertebrate tachykinin family. FEBS Letters. 1990;261:397–401. doi: 10.1016/0014-5793(90)80601-e. [DOI] [PubMed] [Google Scholar]
- Thayer MM, Flaherty KM, McKay DB. Three-dimensional structure of the elastase of Pseudomonas aeruginosa at 1.5-A resolution. Journal of Biological Chemistry. 1991;266:2864–2871. doi: 10.2210/pdb1ezm/pdb. [DOI] [PubMed] [Google Scholar]
- von Hofsten P, Faye T, Kockum K, Lee JY, Xanthopoulos KG, Boman IA, Boman HG, Engström A, Andreu D, Merrifield RB. Molecular cloning, cDNA sequencing, and chemical synthesis of cecropin B from Hyalophora cecropia. Proceedings of the National Academy of Sciences USA. 1985;82:2240–2243. doi: 10.1073/pnas.82.8.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise C, Franke P, Kopaček P, Wiesner A. Primary structure of apolipophorin-III from the greater wax moth, Galleria mellonella. Journal of Protein Chemistry. 1998;17:633–641. doi: 10.1007/BF02780964. [DOI] [PubMed] [Google Scholar]
- Whitten MMA, Tew IF, Lee BL, Ratcliffe NA. A novel role for an insect apolipoprotein (apolipophorin III) in β-1,3-glucan pattern recognition and cellular encapsulation reactions. Journal of Immunology. 2004;172:2177–2185. doi: 10.4049/jimmunol.172.4.2177. [DOI] [PubMed] [Google Scholar]
- Wiesner A, Losen S, Kopaček P, Weise C, Götz P. Isolated apolipophorin III from Galleria mellonella stimulates the immune reactions of this insect. Journal of Insect Physiology. 1997;43:383–391. doi: 10.1016/s0022-1910(96)00113-8. [DOI] [PubMed] [Google Scholar]
- Yu KH, Kim KN, Lee JH, Lee HS, Kim SH, Cho KY, Nam MII, Lee III. Comparative study on characteristics of lysozymes from the hemolymph of three lepidopteran larvae, Galleria mellonella, Bombyx mori, Agrius convolvuli. Developmental and Comparative Immunology. 2002;26:707–713. doi: 10.1016/s0145-305x(02)00027-7. [DOI] [PubMed] [Google Scholar]


