Abstract
Fossilised arthropod compound eyes have frequently been described. Among the oldest known are those from the lower Cambrian of the Chengjiang Lagerstätte (China, c 525 Ma). All these compound eyes, though often excellently preserved, however, represent just the outer shells, because soft tissues, or even individual cells, usually do not fossilise. Using modern techniques, including μct-scanning and synchrotron radiation analysis we present the discovery of the sensory cell system of compound eyes, belonging to trilobites around 400 million years old, which allows their description and analysis. They are interpreted as forming part of an apposition-like ommatidium, which is a basic functional type of compound eye present in arthropods of today. Considered in greater detail, it is similar to the compound eye of the horseshoe crab Limulus, generally regarded as a ‘living fossil’, which probably retained this ancient basal system successfully until today.
Excellently preserved fossilised compound eyes have been reported recently, such as those of the Emu Bay, Australia1,2 or the Chengjiang Lagerstätte, China3,4. All these systems, however, are fossilised only as their outer shells. They are retained most often even just as fine networks, the frames of the facets, because soft tissues or even cells usually do not fossilise. Only in Eocene amber (~50 Ma) has the cellular analysis of the visual system of a fly been possible and successful5. It proved to be a modern-type of superposition eye as in flies living today, and remarkable as it was, the results did not offer insights to the earlier history of the evolution of vision. This must be sought in ancient arthropods, especially in trilobites, which possess well developed compound eyes. These are among the oldest invertebrates in the fossil record and which dominated the marine arthropod fauna of the Palaeozoic6.
Amongst modern arthropods, the most basic and most common kind of compound eye is the so-called apposition eye7. With up to 20.000 facets, each capping a so-called ommatidium, they form a visual system of identically repeated units. A dioptric apparatus focuses the light onto a central light-guiding structure, the rhabdom, which commonly possesses 6 to 9 sensory cells. Each of the sensory cells has a slender nerve, which combines to an optical nerve connecting the sensory cells with the central nervous system. This sensory unit is isolated by numerous pigment cells from the neighbouring ommatidia. Because the rhabdom combines all incident light which enters an individual facet, an apposition eye thus forms a mosaic-like image, and its resolution depends inter alia on the number of facets.
The eyes of the extinct trilobites, which lived between c 522 and 251 million years ago (Ma) were compound, like those of crustaceans and insects living today, but, in contrast to recent arthropods, their lenses were formed of primary calcite6,8,9. Only these calcite lenses and adjacent parts of the exoskeleton are normally preserved and the sublensar structures have remained largely unknown until now. Most trilobite eyes are holochroal, that is they possess many small contiguous lenses set on a curving surface; this is the original kind of trilobite eye. One group of trilobites, however, the Suborder Phacopina (c 488–359 Ma: Lower Ordovician to Late Devonian) has schizochroal eyes, in which the lenses are much larger, fewer and separated one from another. The lenses are also internally differentiated, allowing light to focus sharply10. Each visual unit has its own thin cornea, prolonged below into a capsule with a flattish or rounded lower termination, though such thin membraneous capsules are seldom preserved11,12,13,14. These eyes originated by paedomorphosis from a holochroal precursor15.
Up till now, the only parts of the original sublensar elements documented were the membraneous capsules. The use of μ-ct-scanning (high resolution computer tomography), and synchrotron radiation, for the first time, has given much more information about the cellular sensory structures that lay within these ancient compound eyes, not far from being half a billion years old. Devonian phacopids investigated here include Geesops, Barrandeops, Phacops and Chotecops; the latter was investigated using light microscopy alone.
The basic concept of the investigation undertaken here is that there exist three possibilities of sensory systems which could be found in fossilised Palaeozoic compound eyes: 1. There exist numerous types of functionally differentiated compound eyes in recent arthropods, such as many forms superpose light from adjacent facets to exploit light more effectively. They derive from the most basal and widest represented visual concept in the arthropod realm. This is the apposition eye, and all more specialised forms are younger than Devonian16. The apposition eye consists of optically isolated units, the ommatidia, containing the sensory cells with a central rhabdom in common as explained above. 2. There exists a functional alternative, a small capsule floored by a tiny retina, forming a so-called ocellus. Several of these may form an aggregate eye similar to compound eyes. They are represented for example in myriapods. The ocelli are also the typical main visual system of chelicerata, except Xiphosura as Limulus (lateral compound eyes), onychophora17,18 and the related Palaeozoic lobopodians19. 3. The third alternative would be to find a new concept not known so far.
No cellular sensory structures have been found so far in the fossil record older than the relative young Eocene amber, and a discovery of such a kind would open insights to the early evolution of vision, especially of arthopod vision.
Results
Geesops
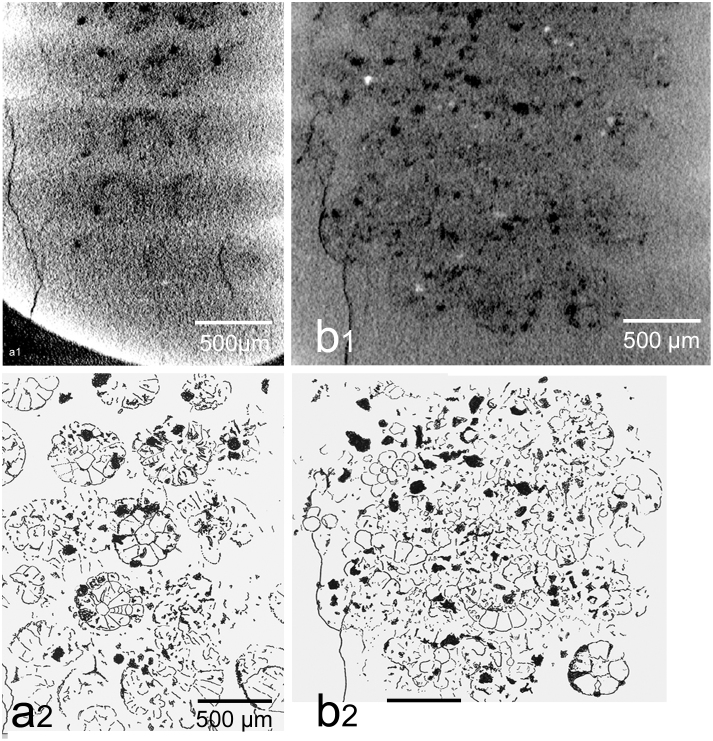
A single Geesops schlotheimi (Bronn, 1835) specimen, one of very many investigated, reveals remarkable details of internal structure. These phacopids originate from the Middle Devonian (Ahrdorf Formation, Eifelian, c 390 Ma) of the Gees-Gerolstein district, Eifel, Germany. The trilobites are excellently preserved in a very fine matrix, and the sensory structures have been preserved by diagenetic ‘seeding’ of a mineral film by bacteria. When the original structures decayed, the mineral shell remained. Because sensory cells decay easily, this must have taken place very soon after the death of the animal. There are two tangential ct-scans, taken at Phoenix x-ray, Munich. They show different levels within the eye, and are here clarified by black and white drawings, made from photographic enlargements, using a light table. Both scans reveal circular structures, each lying below a lens. They are of constant form and arranged in a regular pattern; it is thus unlikely that they result from an overall diagenesis. The higher scan (Fig. 1a) shows at least twelve complete or partial rosettes, with distinct cellular structures arranged in two circlets around an irregularly star-shaped unit with a central ring. In the lower scan (Fig. 1b) the rosettes are generally rather disordered and crushed together, they were probably more decayed, and somewhat displaced from the lenses before mineralisation. Figure 2 shows the capsule (Fig. 2a) and the internal structures of these rosettes in more detail. There is an inner circlet, which consists of six or seven wedge-shaped cells (Fig. 2b1, 2b2), with an extra thin kite-shaped cell (Fig. 2b1, 2b2, 2c2, 5), usually not touching the centre. The outer circlet comprises separate small black spots (Fig. 2b1, 2b2, 1; 2c1, 2c2, 1), each set at the outer margin, and at the junction between two cells of the inner circlet. Even so several rosettes are distinct and one of these shows the original structure particularly well, though obviously affected by extra mineralisation. The inner circlet is interpreted as the remains of original sensory cells, surrounding a rhabdom with a central rod (Fig. 2b1, 2b2 3, 4; 2c1, 2c2 3, 4) whereas the outer circlet is likely to represent the original pigment cells (Fig. 2b1, 2b2, 1; 2c1, 2c2, 1), now preserved as hollow spaces. The narrow kite-shaped cell (Fig. 2b1, 2b2, 5; 2b1, 2c2, 5) may be equivalent to the eccentric cell in the lateral eyes of Limulus20,21.
Figure 1. Two cross sections of the (schizochroal) compound eye of Geesops schlotheimi (Bronn, 1835), μct-scanning.
Age and location: Flesten Mb, Ahrdorf Fm, Middle Devonian, Gees/Gerolstein, Eifel, Germany, (a1) Cross section (slightly oblique) through the upper third of the compound eye, (a2) schematic drawing of a1. (b1) Crossection (slightly oblique) through the lower third of the compound eye. (b2) schematic drawing of b1. Both show the regular patterns of the sublensar sensory elements.
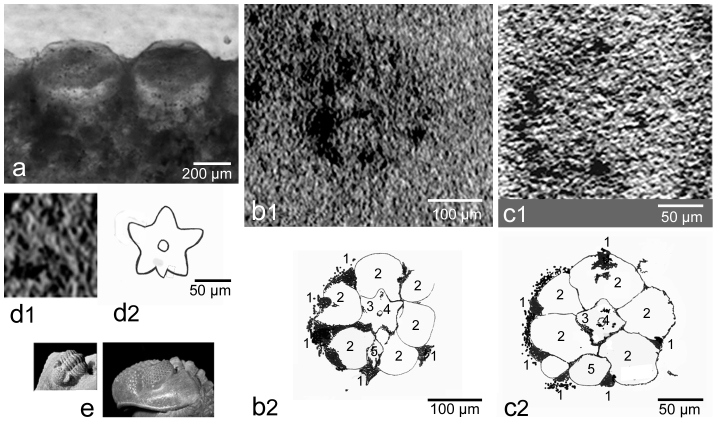
Figure 2. ~390 Mio year old visual unit of Geesops schlotheimi (Bronn, 1835).
Age and location: Flesten Mb, Ahrdorf Fm, Middle Devonian, Gees/Gerolstein, Eifel, Germany. (a) Light microscopic lateral aspect of the visual unit showing lens and capsule. (b1) Cross section through the upper third of the visual unit. (b2) Schematic drawing of b1. (c1) Cross section through a visual unit, slightly deeper than b. (c2) Schematic drawing of c1. (b, c, μct-scannings). (d1) Presumed rhabdomeric structure. (d2) Schematic drawing of d1, note the central circular structure (presumed process of the eccentric cell). (e) Geesops schlotheimi (Bronn, 1835), size of the specimen c 1 cm if stretched. Speciman and photographs B. S. 1 presumed pigment cell, 2 presumed sensory cell, 3 star-shaped central element, presumed rhabdomeric structure, 4 central element inside of the presumed rhabdomeric structures, interpreted as the process opf the excentric cell, 5 presumed excentric cell, not reaching the central axis, b–d x-ray tomography, ct-scanning.
Barrandeops cf. granulops
(Chatterton et al. 2006), Emsian, lower Devonian (391.9 ± 3.4 Ma), Hamar Laghdad, Morocco.
In the silicified specimens the capsules themselves are preserved by a silicified film. μ-ct analysis, undertaken at the Steinmann Institute, University of Bonn and at Phoenix x-ray, Munich, revealed clearly the lower part of the sensory system, as seen from within the eye. Here (Fig. 3a) are visible some thirteen ‘cells’, like segments of an orange, arranged radially. By comparison with the ‘Geesops’ specimen, these must represent the original outer ring of pigment cells, like those of ‘Geesops’ but appreciably larger than those of Geesops.
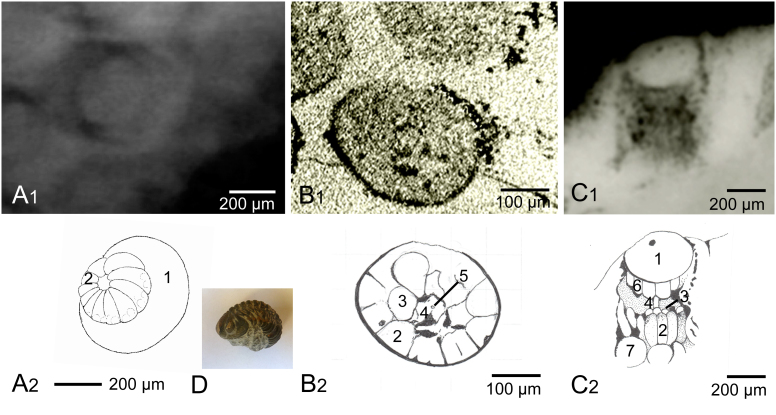
Figure 3. The visual unit of Barrandeops cf. granulops Chatterton et al. 2006, Age and location: Emsian, lower Devonian (391.9 ± 3.4 Ma), Hamar Laghdad, Morocco.
(a1) Three dimensional reconstruction of an individual visual unit from the rear, showing a shielding cup of ~11 cells, headed by the wider margin of the lower rim of the lens, which covers the visual unit (x-ray-ct). (a2) Schematic drawing of a1. (b1) Cross section of the visual unit. (b2) Schematic drawing of b1. (c) Synchrotron image of the lateral section of he visual unit of B. cf. granulops. (d) Barrandeops cf. granulops Chatterton et al 2006, size of the specimen c 3.5 cm if streched. (Specimen and photograph B. S.) 1 lens. 2 presumed pigment cells covering the more central sensory cells. 3 presumed sensory cells, 4 rhabdomeric structure, 5 central element inside of the rhabdomeric structure (presumed process of the excentric cell). 6. ?lens-building cells.
Barrandeops (II)
Here again, there is a star-shaped central unit with a ‘core’ (Fig. 3b1, 3b2 4,5) and an outer ring of radially arranged, wedge-shaped cells, originally about 10–12 in number, presumably pigment cells, and 5 or 6 more irregularly arranged cells between the centre and the outer ring. The center is typically star-shaped as in living arthropods, and may consequently be interpreted as the central rhabdomeric structure.
Barrandeops (III)
This specimen was investigated using synchrotron radiation, undertaken at ESRF, Grenoble. The sublensar structures here are seen in lateral view, parallel with the main axis of the capsule (Fig. 3c). Although the lens has been diagenetically converted to featureless calcite the contents of the capsule are clear. Directly below the lens are several more or less square cells with rounded lower terminations (Fig. 3c1, 3c2, 6), and a central vertical rod (Fig. 3c1, 3c2, 4) connecting the lens to an array of vertically elongated bodies (Fig. 3c1, 3c2, 2), with a number of large rounded ‘balloons’ (Fig. 3c1, 3c2, 7) below. A reasonable interpretation would be that the structures (Fig. 3c1, 3c2, 6) are the equivalent of lens-building cells, whereas the rod (Fig. 3c1, 3c2, 4) represents the rhabdom. This rod seems to preserve well and has been seen in several other instances. The elongated bodies (Fig. 3c1, 3c2, 2) are likely to be either pigment cells (Fig. 3b1, 3b2, 1; 3c, 3c2, 1), originally surrounding sensory cells (Fig. 3b, c 4), or sensory cells themselves. As yet the nature of the ‘balloons’ (Fig. 3c1, 3c2 7) remains uncertain.
Chotecops
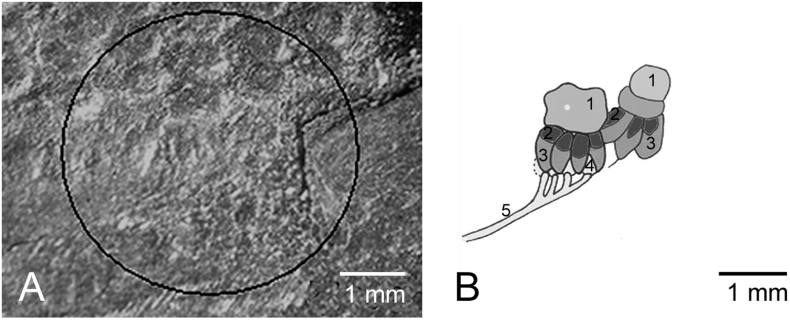
An exceptionally well-preserved specimen of Chotecops ferdinandi (Kayser, 1880) from the Bundenbach Schiefer (Lower Emsian, Lower Devonian), Germany, largely confirms ct-scanning observations on Geesops and Barrandeops. Here the fossils are lightly pyritised. In this specimen the outermost surface of the eye has split off revealing in tangential light a slightly squeezed capsule below the lens. This partially pyritised lens (Fig. 4a, b 1) is attached to several darker petal-shaped structures below (Fig. 4b 2, 3). In turn, and set underneath are light-coloured elements alternating with the ‘petals’ (Fig. 4b 4). These are connected by an oblique filament (Fig. 4b 5). We interpret the ‘petals’ as sheeting pigment cells (Fig. 4b 3), and the lighter elements as sensory cells (Fig. 4b 4), each connected to a minor nerve (Fig. 4b 5). The almost squared elements (Fig. 4b 2) correspond to those of Geesops (Fig. 2a, right capsule) and Barrandeops (Fig. 3c1, 3c2, 6). Relicts of a second and similar cellular system lie adjacent to the first. These are of similar dimensions to those in Geesops (Fig. 2).
Figure 4. Visual unit, slightly, obliquely squeezed, of Chotecops ferdinandi Struve,1985, Age and location: Siegenian/Emsian Stage (~407 million years) lower Devonian, Bundenbach, Hunsrück, Germany.
(a) Obliquely ‘opened’ compound eye of B. cf. granulops. (b) schematic drawing of a. 1 lens, 2 ?lens-building cells 3 shielding pigment cells, 4 sensory cells, 5 efferent nerves of the sensory cells. Insert: Chotecops ferdinandi Struve,1985.
Discussion
Previous models for the phacopid sensory system presupposed an ocellar system, with a retina of many cells flooring the capsule12,13,22,23,24,25, as it could be a basic visual system in arthropods generally. Our present investigation, on the other hand, indicates an evident resemblance between the phacopid sensory system and that of ommatidia in the apposition eyes of euarthropods, which was unexpected. The phacopid sensory system, however, especially the sensory cells, is very much larger than that of any insects or crustaceans (sensory cells of phacopid compound eyes: ~80 μm in diameter, as opposed to 2–10 μm common in modern euarthropods). In dimensions and structure, however, there is a strong resemblance between the phacopid system and that of the lateral eyes of the living Limulus. In the latter each ommatidium consists of 4–20 sensory cells, while the rhabdomers form a star-shaped pattern round a vertical process of an eccentric cell, itself a modified photoreceptor. Pigment cells form a sheet around the photoreceptors. In phacopids, Geesops in particular, but in Barrandeops also, there are 6 or 7 sensory cells, arranged around a star-shaped element possibly comparable to rhabdomers within a Limulus ommatidium, while the central circular structure could be the process of an eccentric cell. The narrow kite-shaped cell (Fig. 2b1, 2b2 5; 2c1, 2c2 5) could be a section of the body of an eccentric cell. If these indications prove realistic then the lateral eyes of Limulus could have retained a fundamental archaic system which has continued to function successfully today, amongst so many other kinds of compound eye in living arthropods. This may also hold significant interest for phylogeny.
Compound eyes are a typical character for all recent Mandibulata (insects and crustaceans), and Xiphosura among the Chelicerata. In Myriapoda it is only in the Scutigeromorpha that a kind of apposition eye, different from the aggregate eyes of other myriapoda, exists. Only the Xiphosura possess an excentric cell, rather similar to the phacopid trilobites investigated here.
The sophisticated internal structure of the lenses of some phacopid trilobites10 had suggested that there may be a small retina flooring the capsules below the lenses12,13,22,23,24,25, and that the double lens system improved image formation. The same qualities, however, support in the same way an apposition eye, providing well focused light onto the rhabdomeres. It had been shown that the schizochroal eyes of phacopid trilobites derived by paedomorphosis from the holochroal eyes of earlier trilobites15. The holochroal eyes are the most common type of trilobite eyes and consist very often of several hundreds or even thousands of sometimes tiny facets. If the schizochroal eye derived from the holochroal eye this suggests that the holochroal eye also was an apposition eye. The sensory cells, however, must have been smaller, resulting in sizes comparable to most of the receptor cells of many modern apposition eyes. Because the lateral eyes of Limulus, as a “living fossil” show an excentric cell as the phacopid trilobite eyes do, it is rather probable, that Limulus is equipped with an archaic system, which might have been realised in the holochroal eyes also. An alternative was, that especieally dense holochroal eyes with tiny lenses had even simpler systems, comparable to such as had been suggested recently for a kind of prototype of a crustacean compound eye from the upper Cambrian with just one receptor below each lens, which was forced by physical reasons in a miniscule three dimensionally preserved compound eye system26. This result is in agreement with ideas about the evolution of eyes formulated by Darwin27 and Gehring28, where the development of visual units derive from one-cellular systems.
A third possibility was that bearers of small facetted holochroal eyes possessed ommatidia specialised to be small as in Collembola and tiny insects29, or, lastly, that they had another still unknown principle.
The enormous size of the sensory cells in the phacopid trilobites found here, comparable to those of Limulus, indicates a convergent evolution. Because phacopid trilobite eyes show a system similar to the Limulus-type of apposition eye with its excentric cell, rather probably this is an archaic and basic type of compound still represented today. In the competition between prey and predator about the most effective visual system as formulated in the Light Switch Theory30,31 it has continued to function successfully today.
Author Contributions
B.S. and E.C. conducted the analyses, wrote the main manuscript text, and prepared the figures. All authors reviewed the manuscript.
Acknowledgments
We are gratefull to C. Adams, Phoenix|x-ray Munich and A. Bergmann, Bonn for their help with the x-ray tomography, P. Tafforeau, W. Ludwig, and especially the European Synchrotron Radiation Facility (ESRF) in Grenoble for enabling the synchrotron analyses which were so important here. Greatest thanks also to C. Klug, Zürich, H. Prescher, Kerpen, and W. Südkamp, Bundenbach for supporting us with excellent specimens for this investigation.
References
- Lee M. S. Y. et al. Modern Optics in exceptionally preserved eyes of the Early Cambrian arthropods from Australia. Nature 474, 631–634 (2011). [DOI] [PubMed] [Google Scholar]
- Paterson J. R. et al. Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature 480, 237–240 (2011). [DOI] [PubMed] [Google Scholar]
- Schoenemann B. & Clarkson E. N. K. Eyes and vision in the Chengjiang arthropod Isoxys indicating adaptation to habitat. Lethaia 44, 223–230 (2010). [Google Scholar]
- Schoenemann B. & Clarkson E. N. K. The eyes of Leanchoilia. Lethaia 45, 524–531 (2012). [Google Scholar]
- Tanaka G., Parker A. R., Siveter D. J., Maeda, H. & Furutani, M. An exceptionally well-preserved Eocene dolichopodiod fly eye: function and evolutionary significance. Proc. Roy. Soc. B. 276, 1015–1019 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson E. N. K., Horváth G. & Levi-Setti R. The eyes of trilobites; the oldest preserved visual system. Arthropod Struct. Dev. 35, 247–259 (2006). [DOI] [PubMed] [Google Scholar]
- Land M. F. & Nilsson D.-E. Animal Eyes. (Oxford University Press, 2002), pp. 221. [Google Scholar]
- Towe K. M. Trilobite eyes; calcified lenses in vivo. Science 197, 1007–1009 (1973). [DOI] [PubMed] [Google Scholar]
- Clarkson E. N. K. The eye: morphology, function and evolution. In Treatise on Invertebrate Paleontology, part O. Arthropoda 1.Trilobite, revised (eds. Kaesler, R. L. et al.) 114–132 (The University of Kansas Press and Geological Society of America, 1997). [Google Scholar]
- Clarkson E. N. K. & Levi-Setti R. Trilobite eyes and the optics of Des Cartes and Huygens. Nature 254, 663–667 (1975). [DOI] [PubMed] [Google Scholar]
- Clarkson E. N. K. Fine structure of the eye in two species of Phacops (Trilobita). Palaeontology 10, 603–616 (1967). [Google Scholar]
- Clarkson e N. K. The evolution of the eye in trilobites. Fossils & Strata 4, 7–31 (1975). [Google Scholar]
- Campbell K. S. W. The functional anatomy of trilobites: musculature and eyes. J. Proc. Roy. Soc. New South Wales 108, 168–188 (1975). [Google Scholar]
- Bruton D. J. & Haas W. The puzzling eye of Phacops. Spec. Pap. Palaeontol. 70, 349–361 (2003). [Google Scholar]
- Clarkson E. N. K. & Zhang X.-g. Ontogeny of the Carboniferous trilobite Paladin eichwaldi shunnerensis (King 1914). Trans. Roy. Soc. Edinburgh, Earth. Sci. 82, 277–296 (1991). [Google Scholar]
- Gaten E. Eye structure and phylogeny: is there an insight? The evolution of superposition eyes in the Decapoda (Crustacea). Contrib. Zool. 67, 223–235 (1998). [Google Scholar]
- Eakin R. M. & Westfall J. A. Fine structure of the eye of Peripatus (Onychophora). Z. Zellforsch. 68, 278–300 (1965). [DOI] [PubMed] [Google Scholar]
- Mayer G. Structure and development of onychophoran eye: what is the ancestral visual organ in arthropods? Arthropod Struct. Dev. 35, 231–245 (2006). [DOI] [PubMed] [Google Scholar]
- Schoenemann B., Liu J.-n., Shu D.-g., Han J.-a. & Zhang Z.-f. A Miniscule Optimised Visual System in the Lower Cambrian. Lethaia 42, 265–273 (2009). [Google Scholar]
- Fahrenbach W. H. The morphology of the eyes of Limulus II. Ommatidia of the compound eye. Z. Zellforsch. 93, 451–483 (1969). [DOI] [PubMed] [Google Scholar]
- Barlow R. B. & Powers M. Seeing at night and finding mates: the role of vision 83–102 in The American Horseshoe Crab (eds. Shuster, C. N., Barlow, R. B. & Brockman, H. J.) 1–427 (Harvard University Press 2003). [Google Scholar]
- Clarkson E. N. K. The visual system of trilobites. Palaeontology 22, 1–22 (1979). [Google Scholar]
- Buschbeck E., Ehmer B. & Hoy R. Chunk Versus Point Sampling: Visual Imaging in a Small Insect. Science, 86 (5442), 1178–1180 (1999). [DOI] [PubMed] [Google Scholar]
- Fordyce D. & Cronin T. W. Comparison of fossilized schizochroal compound eyes of phacopid trilobites with eyes of modern marine crustaceans and other arthropods. Journal of Crustacean Biology, 9(4), 554–569 (1989). [Google Scholar]
- Fordyce D. & Cronin T. W. (1993).Trilobite vision; a comparison of schizochroal and holochroal eyes with the compound eyes of modern arthropods. Paleobiology, 19, 288–303. [Google Scholar]
- Schoenemann B. The eyes of a tiny ‘Orsten’ crustacean – A compound eye at receptor level? Vision Research 76, 89–93 (2013). [DOI] [PubMed] [Google Scholar]
- Darwin C. On the origin of Species by means of natural selection. John Murray, London (1859).
- Gehring W. J. The evolution of vision. Wires Developmental Biology. 10.1002/wdev.96 (2012).
- Paulus H. F. Eye Structure and the Monophyly of the Arthropoda in Arthropod Phylogeny (ed. Gupta, A. P.), 299–383 (Van Nostrand Reinhold Co., New York (1979), pp. 762. [Google Scholar]
- Parker A. R. Colour in Burgess Shale animals and the effect of light on evolution in the Cambrian. Proc. Roy. Soc. B. 217, 177–189 (1998). [Google Scholar]
- Parker A. R. In the Blink of an Eye. Perseus, New York (2003). [Google Scholar]