Abstract
Recent advances in genomic and post-genomic technologies have facilitated a genome-wide analysis of the insecticide resistance-associated genes in insects. Through bed bug, Cimex lectularius transcriptome analysis, we identified 14 molecular markers associated with pyrethroid resistance. Our studies revealed that most of the resistance-associated genes functioning in diverse mechanisms are expressed in the epidermal layer of the integument, which could prevent or slow down the toxin from reaching the target sites on nerve cells, where an additional layer of resistance (kdr) is possible. This strategy evolved in bed bugs is based on their unique morphological, physiological and behavioral characteristics and has not been reported in any other insect species. RNA interference-aided knockdown of resistance associated genes showed the relative contribution of each mechanism towards overall resistance development. Understanding the complexity of adaptive strategies employed by bed bugs will help in designing the most effective and sustainable bed bug control methods.
Insecticide resistance is considered to be a condensed model of natural selection. The extensive use of insecticides accelerated the accumulation of resistance related factors in survivors. Therefore, studies on the molecular basis of these adaptive traits are of theoretical and applied importance in understanding the evolution of insecticide resistance and devising the most effective and sustainable resistance management tactics1. The physiological and biochemical mechanisms of insecticide resistance may evolve along several trajectories2,3. When insects come in contact with or consume insecticides, they may develop resistance by modification in the insect cuticle or digestive tract linings that prevent or reduce the rate of penetration (termed reduced penetration)4. Once insecticide enters the organism, enhanced metabolic detoxification could decrease the concentration of insecticides before they reach the target site2,4. In some instances, the insecticide may be excreted from the organism at an accelerated rate in resistant populations5. Resistant insects may also evolve target site insensitivity mechanisms which reduce or eliminate the binding affinity of insecticides to their target proteins3,6. Additionally, behavioral resistance helps to avoid the lethal effects of insecticides through behaviors that reduce exposure3,4. A common phenomenon of insecticide resistance is that multiple mechanisms operate simultaneously in resistant insects such as the house fly7,8,9, mosquito10,11,12,13, cockroach14, and cotton bollworm15. Typically, a combination of diverse mechanisms provides significantly higher levels of resistance than one individual mechanism4.
Pyrethroid insecticides are the mainstay for bed bug control due to their safety, effectiveness, longevity of their residual activity and low cost. However, ubiquitous development of resistance to pyrethroids and the fact that pyrethroid resistance generally confers cross-resistance to other insecticides make bed bug management a difficult task16. Recent advances in insect genomics and development of post-genomic technologies have facilitated a genome wide analysis of the resistance associated genes in many medically or agriculturally important insect species17,18,19,20. In the current study, molecular markers related to pyrethroid resistance were identified based on thorough transcriptome analysis. The identified markers were then used to examine the contribution of different resistance mechanisms in 21 field-collected bed bug populations. Interestingly, bed bugs express most of their pyrethroid resistance associated genes in the integument which serves as the first cellular barrier for pyrethroids to cross before reaching target sites. RNA interference-aided knockdown in the expression of genes coding for proteins involved in mechanisms of pyrethroid resistance in the insecticide-resistant bed bugs showed relative contribution of each mechanism towards overall resistance.
Results
Resistant population selection and residual bioassays
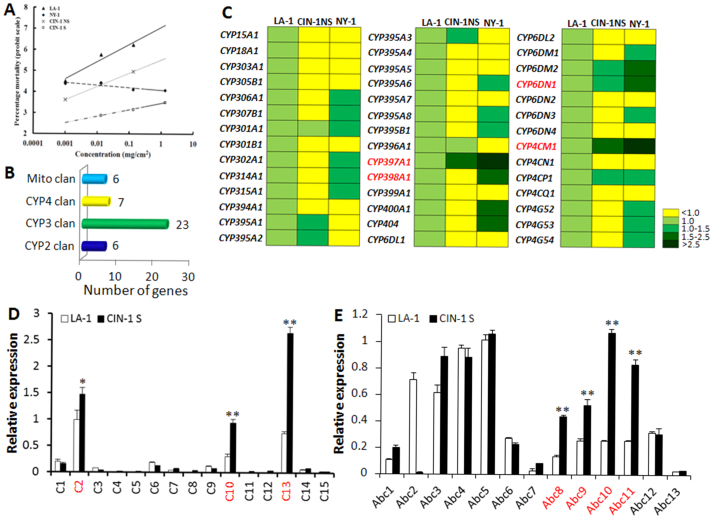
A bed bug population from Cincinnati, CIN-1 NS (the CIN-1 strain before selection) collected in 2007 showed >10,000-fold deltamethrin resistance16 but the resistance levels decreased after rearing them for multiple generations. To enhance the pyrethroid resistance level of this population, CIN-1 S (the selected strain) was selected with commercial insecticide, Temprid® (a commercial insecticide that includes β-cyfluthrin and imidacloprid as active ingredients). Bioassays showed dramatic differences in the susceptibility to deltamethrin between the susceptible strain, LA-1 and resistant strains, NY-1, CIN-1 NS and CIN-1 S (Fig. 1A). LA-1 was susceptible to deltamethrin (n = 240, slope = 0.355, LC50 = 0.003 mg/cm2). However, NY-1 did not show higher percentage of mortality when the concentration of deltamethrin increased from 0.001 mg/cm2 to 1 mg/cm2 (n = 280). Before selection, the resistance ratio of CIN-1 NS relative to LA-1 was 51 (n = 240, slope = 0.275, LC50 = 0.153 mg/cm2). After selection, the resistance ratio of CIN-1 S compared with LA-1 increased drastically (n = 300, slope = 0.135, LC50 = 98,100 mg/cm2).
Figure 1. Identification of target genes associated with insecticide resistance.
(A) Dose-response curves (log dose versus mortality on a probit scale) for C. lectularius female adults exposed to deltamethrin. LA-1 ( ), a susceptible strain; NY-1 (
), a susceptible strain; NY-1 (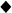 ), a deltamethrin resistant strain; CIN-1 NS (x), a deltamethrin resistant strain without selection; and CIN-1 S (
), a deltamethrin resistant strain; CIN-1 NS (x), a deltamethrin resistant strain without selection; and CIN-1 S ( ), a deltamethrin resistant strain after selection were exposed to serially diluted deltamethrin and the mortality was recorded and graphed. (B) Cytochrome P450 genes in C. lectularius. Totally 42 Cytochrome P450 genes (P450s) were identified through assembling of Cimex transcriptome and named by the P450 nomenclature committee. These genes fall into 4 clans, Mito CYP clan, CYP4 clan, CYP3 clan, and CYP2 clan. The number of P450s in each clan was labeled on the top of the column. (C) mRNA levels of 42 C. lectularius P450s in LA-1, CIN-1 NS, and NY-1. mRNA levels were shown as mean fold relative to their levels in LA-1. P450s highlighted in red showed the significant increase in CIN-1 NS and/or NY-1 compared to their levels in LA-1 (Student t-test, P < 0.05). (D) Relative mRNA levels of cuticular protein genes. Total RNAs were extracted from one week-old female adults were used in qRT-PCR to quantify relative mRNA levels in susceptible LA-1 as compared with the pyrethroid-resistant CIN-1 S. The data shown are mean + SEM (n = 3). Genes highlighted in red showed significant difference between LA-1 and CIN-1 S (Student's t test. * P < 0.05, ** P < 0.01). (E) Same as B except the mRNA levels of Abc transporter genes were quantified.
), a deltamethrin resistant strain after selection were exposed to serially diluted deltamethrin and the mortality was recorded and graphed. (B) Cytochrome P450 genes in C. lectularius. Totally 42 Cytochrome P450 genes (P450s) were identified through assembling of Cimex transcriptome and named by the P450 nomenclature committee. These genes fall into 4 clans, Mito CYP clan, CYP4 clan, CYP3 clan, and CYP2 clan. The number of P450s in each clan was labeled on the top of the column. (C) mRNA levels of 42 C. lectularius P450s in LA-1, CIN-1 NS, and NY-1. mRNA levels were shown as mean fold relative to their levels in LA-1. P450s highlighted in red showed the significant increase in CIN-1 NS and/or NY-1 compared to their levels in LA-1 (Student t-test, P < 0.05). (D) Relative mRNA levels of cuticular protein genes. Total RNAs were extracted from one week-old female adults were used in qRT-PCR to quantify relative mRNA levels in susceptible LA-1 as compared with the pyrethroid-resistant CIN-1 S. The data shown are mean + SEM (n = 3). Genes highlighted in red showed significant difference between LA-1 and CIN-1 S (Student's t test. * P < 0.05, ** P < 0.01). (E) Same as B except the mRNA levels of Abc transporter genes were quantified.
Transcriptome analysis and resistance marker selection
RNA isolated from CIN-1 NS strain was sequenced by 454 GS FLX pyrosequencing. The sequences obtained from this strain and other sequences available in the NCBI database (SRX028107, SRX013985, SRX013984 and 7131 ESTs) were assembled by Roche de novo Assembler program (Newbler) (Fig. S1). These data resulted in a total of 2,197,566 aligned reads constituting 756,568,733 bases producing 129,294 ESTs (25,935 contigs and 103,359 singletons). The length of the contigs varied from 100 bp to 8,249 bp with an average length of 850 bp (Fig. S2A). The length of singletons ranged from 50 bp to 511 bp with an average length of 261 bp (Fig. S2B). Gene Ontology (GO) analysis was performed based on BLAST matches to proteins with known functions. Each GO category, the molecular function (ontology level 5), biological process (ontology level 2), and cellular components (ontology level 5) are shown in the Figs. S3A, B, C, respectively. Based on our previous studies21,22 and recent publications18,20, several categories of genes were identified for the potential association with pyrethroid resistance in the bed bug.
Metabolic enzymes
Increased metabolic detoxification by cytochrome P450s, esterases, and/or glutathione S-transferases (GSTs)23,24,25 is one of the major mechanisms involved in pyrethroid resistance. Our previous studies suggested that P450-mediated metabolic detoxification may serve as one of the resistant mechanisms in bed bugs21,22. Typically, each insect genome contains a variable number of P450 genes varying from tens to more than one hundred24. In the current study, 42 cytochrome P450s were annotated from 129,294 ESTs and named by the P450 nomenclature committee (Dr. D. Nelson, personal communication) (Tables S1 and S2). Of these 42 P450 genes, six (CYP15, 18, 303, 305, 306, 307 families) derived from CYP2 clan, six (CYP301, 302, 314, 315, 394 families) belong to Mito clan, 23 (CYP6, 395–400, 404 families) derived from CYP3 clan and seven CYP4 genes belong to CYP4 clan (Tables S1 and S2, Fig. 1B). The relative expression of these 42 P450s were examined among insecticide susceptible strain LA-1 and resistant strains, CIN-1 NS and NY-1, comparing with the expression of the most stable housekeeping gene rpl822 (Fig. 1C). Four P450s, CYP397A1, CP398A1, CYP6DN1, CYP4CM1 were chosen as the target markers on the basis of their significant up-regulation in resistant strain(s) when compared to their expressions in susceptible strains as well as their relative higher expression when compared to that of rpl8 (Fig. 1C). The same criteria were used to select other genes associated with insecticide resistance as described below. As regards to esterases and GSTs, the expression of three genes identified previously18 was compared between LA-1 and CIN-1 S strains. Only esterase, ClCE21331 showed significant increase in the resistant strain (Table S3) and this gene was selected for further validation.
Cuticular proteins
Cuticular proteins are the major components of insect cuticle which serves as the first line of defense to insecticides26. Recent studies reported that cuticle thickening was associated with pyrethroid resistance in Anopheles funestus26. In Colorado potato beetle, the mRNA levels of three putative cuticular proteins were higher in azinphosmethyl resistant beetles than in susceptible beetles27. To determine the role of cuticular proteins in the pyrethroid resistance of bed bugs, the expression of 15 genes coding for putative cuticular proteins was examined in LA-1 and CIN-1 S strains (Fig. 1D, Table S3) and three genes (C2, C10, and C13) that showed increased expression in resistant strains were selected for further studies.
Abc transporters
ATP-binding cassette (Abc) transporters constitute one of the largest classes of transporters that are responsible for the ATP-powered translocation of many substrates across membranes28. These substrates include ions, sugars, amino acids, vitamins, peptides, polysaccharides, hormones, lipids and xenobiotics29. Recent RNA-Seq studies showed that eight out of 27 Abc transporters were up-regulated in insecticide resistant bed bugs when compared to susceptible insects20. We selected 13 contigs annotated as Abc transporters with significant expression levels in adult bed bugs and the mRNA levels of these were quantified (Fig. 1E, Table S3). Genes coding for four Abc transporters, Abc8, Abc9, Abc10, Abc11 showed an increase in expression in resistant strain and selected for further analysis.
Kdr mutations
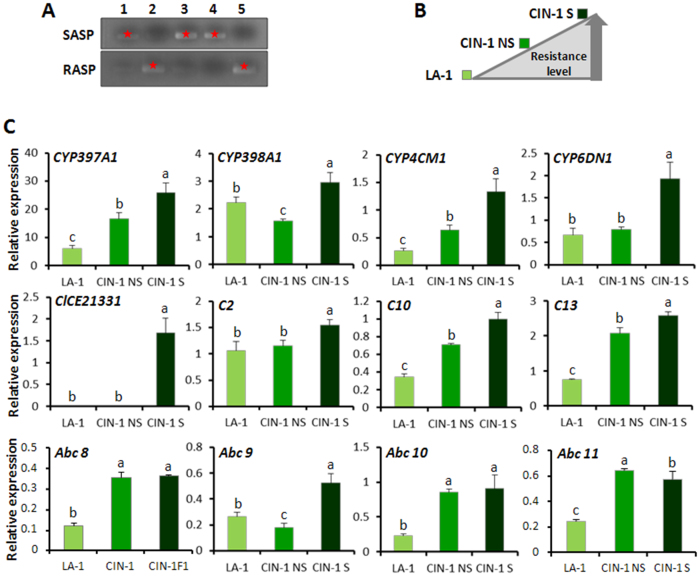
Pyrethroid insecticides target the sodium channels within the insect nervous system. Point mutations in the sodium channels, termed the kdr mutations, reduce or eliminate the binding affinity of insecticides to sodium channels causing insecticide resistance6. Two mutations, V419L and L925I, in voltage-gated sodium channel α-subunit gene had been identified as very important substitutions responsible for deltamethrin resistance in bed bugs21,30. A causal link between one or both mutations and deltamethrin resistance was reported21. A dual-primer Allele-Specific PCR (dASPCR) approach was developed to identify these two kdr mutations. Two PCR reactions performed with Susceptible Allele-Specific Primer (SASP) and Resistant Allele-Specific Primer (RASP) primers conclusively show status of kdr mutations (Fig. 2A).
Figure 2. Differential expressions of 12 target genes in susceptible and resistant C. lectularius laboratory populations.
(A) Gel showing dASPCR results. Lanes 1–5 show 5 independent DNA samples. Two PCRs were performed with different primers, SASP or RASP, under the same reaction conditions. For each DNA sample, there was only one band (highlighted with red star) shown on the gel illustrating this sample either has mutation (band shown on the gel beneath) or has no mutation (band shown on the gel above). (B) Relative levels of resistance to deltamethrin in LA-1, CIN-1 NS and CIN-1 S drawn based on bioassay data. (C) The relative mRNA levels of 12 target genes were quantified by qRT-PCR in susceptible LA-1 and resistant CIN-1 NS as well as CIN-1 S populations and normalized using the mRNA levels of rpl8. The data represent mean + SEM (n = 4 – 12). Statistical significance of the gene expression among samples was calculated using one-way ANOVA followed by Duncan multiple mean separation techniques (SAS v9.4). There was no significant difference among relative expression within samples with the same alphabetic letter.
Validation of selected markers in resistant and susceptible strains
To validate the relative expression of 12 genes selected as those that play important roles in pyrethroid resistance, the mRNA levels of these genes were quantified in LA-1, CIN-1 NS and CIN-1 S strains that showed different levels of resistance to deltamethirn (Fig. 2B). All of these target genes are significantly overexpressed in resistant strain(s) when compared to their expression in the susceptible population (Fig. 2C). Moreover, most of these genes showed an increase in expression following the selection from CIN-1 NS to CIN-1 S. The causal link between the overexpression of target genes and deltamethrin resistance suggest that these 12 genes might be involved in the pyrethroid resistance of bed bugs and could serve as molecular markers to monitor the pyrethroid resistance in field populations.
Integument affiliated expression of selected genes
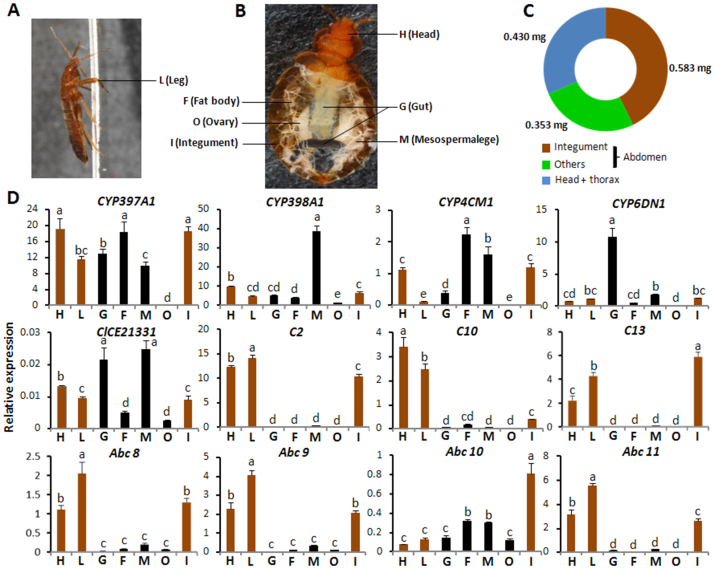
The tissue specific expression of a gene normally is related to its function in that tissue31. Bed bug has an extremely flat body prior to blood meal (Fig. 3A). The functional significance of the integument in regards to bed bugs is especially important, as the integument constitutes the major part of their body (Fig. 3A–C). In order to investigate the tissue-specific expression, the mRNA levels of 12 target genes were quantified by qRT-PCR in the head, leg, gut (foregut and midgut), fat body, mesospermalege, ovary, and integument (epidermis + cuticle) isolated from one week-old female adult NY-1 (Fig. 3D) and CIN-1 NS (Fig. S4) bed bugs. Interestingly, most of the genes coding for metabolic enzymes, cuticular proteins and Abc transporters are expressed higher in the head, legs and integument dissected from NY-1 bed bugs (Fig. 3D). All these three tissues contain epidermis and cuticle. In addition, higher levels of mRNAs when compared to the levels in other tissues were detected for CYP397A1 in gut, fat body and mesospermalege; CYP398A1 in the mesospermalege; CYP4CM1 in the fat body and mesospermalege; CYP6DN1 in the gut and the esterase ClCE21331 in the gut and mesospermalege (Fig. 3D). Quantification of mRNA levels of genes associated with resistance in CIN-1 NS strain showed similar patter as those in NY-1 strain (Fig. S4).
Figure 3. Spatial expression of resistance associated genes in NY-1 strain.
(A) Representative profile of a bed bug. L, leg. (B) Representative anatomic structure of 1 week old female bed bug. H, Head; G, gut including foregut and midgut; F, fat body; M, mesospermalege; O, ovary and I, integument. (C) Average weight of body parts in 1-week-old female bed bugs. The abdomen of bed bug was split to integument and others. The average weights of abdomen integument (0.583 mg/individual), abdomen other parts (0.353 mg/individual), head + thorax (0.430 mg/individual) were calculated from 30 individuals within 3 replicates. (D) The mRNA levels of 12 target genes were quantified by qRT-PCR. The head (H), leg (L), gut (foregut and midgut) (G), fat body (F), mesospermalege (M), ovary (O), and integument (I) were dissected from 1 week-old female adult NY-1 bed bugs. Relative mRNA levels were normalized using the mRNA levels of rpl8. The data shown are mean + SEM (n = 3). There was no significant difference among relative expression within samples with the same alphabetic letter (i.e. a, b and c) (One-way ANOVA followed by Duncan multiple mean separation, SAS v9.4).
Multiple mechanisms of resistance exist in field collected bed bug populations
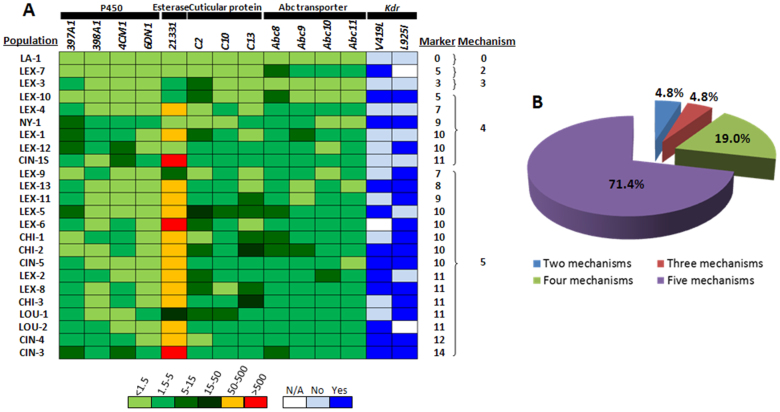
In order to inspect mechanisms of insecticide resistance in field populations, we collected 21 bed bug populations from dwellings in four cities located in the Midwestern United States during November 2011 to February 2012 (Table S4, Fig. S5). The relative expression of 12 target genes and the presence of two kdr mutations were investigated by qRT-PCR and dASPCR, respectively. On an average more than three molecular markers showed a difference in each of the 21 field populations tested (Fig. 4A). P450, esterase, and cuticular protein mediated resistance were identified in all the field populations except LEX-7. An Abc transporter associated mechanism was also detected in all the populations except LEX-5. One or both kdr mutations are present in 18 populations tested (Fig. 4A). Out of the marker genes studied, ClCE21331 showed the maximum increase (>50 fold) of gene expression in most field populations (76.2%). A majority (57.1%) of the populations tested showed >5 fold overexpression of genes coding for cuticular proteins. The overexpression of P450 and Abc transporter markers in field populations was less than 5-fold in most of the populations tested (Fig. 4A). The geographic distribution analysis of molecular mechanisms showed that seven out of 13 Lexington populations, two Louisville populations, three Cincinnati populations, and three Chicago populations showed overexpression of multiple resistance genes (Figs. 4A and 4B). Moreover, among 21 field bed bug populations tested, 100% of populations were associated with at least two molecular mechanisms (not including LA-1 the reference population), implying the prevalent involvement of multiple mechanisms of pyrethroid resistance in the field bed bug populations.
Figure 4. Multiple mechanisms of resistance in field-collected bed bug populations (n = 21) and laboratory strains (LA-1, NY-1, and CIN-1 S).
(A) Analysis of transcription profile of 12 molecular target genes and two kdr mutations among 24 field-collected and laboratory populations. The left 12 rectangles are colored on the basis of results of relative mRNA levels compared with that of LA-1 and normalized by the expression of rpl8. The data shown are mean + SEM (n = 3 – 12). The two columns of rectangles on the right represent two kdr mutations identified by dual-primer allele specific PCR. (B) A pie chart showing the percentage of populations with two, three, four or five mechanisms of resistance.
RNAi studies
To investigate the relative contribution of each mechanism towards overall resistance development, we exploited RNAi to knockdown the expression of insecticide resistance associated genes in CIN-1 S bed bugs and examined the susceptibility of these bed bugs to pyrethroid insecticide. By mimicking traumatic insemination, dsRNAs were injected through the mesospermalege into the body of female bed bugs as described in our recent publication22. Bed bugs injected with dsRNA of malE or target genes suffered <10% mortality within 6 days after injection. In order to investigate comprehensive contribution of each category of target genes to the pyrethroid resistance, dsRNAs synthesized for each target gene from the same category were pooled and injected into one week-old female bed bugs. The target genes tested were successfully suppressed by RNAi (Fig. 5A–C). The expression of closely related non-target P450 genes, CYP399A1, CYP4CN1, and CYP6DM1 were unaffected in the bed bugs injected with dsRNA targeting select P450s (Fig. S6). Expression of esterase ClCE21331 is quite variable in CIN-1 S population, therefore the RNAi analysis was not performed for this gene.
Figure 5. Knockdown in the expression of insecticide resistance associated genes reduced the resistance to pyrethroid insecticide.
(A–C) Injection of dsRNA decreases mRNA levels of target genes. Relative expression of 4 target P450s (A), 3 cuticular proteins (B) and 2 Abc transporters (C) in control (malE dsRNA) and dsRNA of target genes injected bed bugs. The relative mRNA levels are shown as a ratio in comparison with the levels of rpl8 mRNA. The data shown are mean + SEM (n = 3). (D) The percent survival of CIN-1 S bed bugs treated with 5 μg β-cyfluthrin on 6th day after injection of dsRNA. Mortality was recorded after 72 h exposure to β-cyfluthrin (3 replicates, 30 individuals for each replicate).
Six days after injection of dsRNAs, the survivors were exposed to β-cyfluthrin topical application. CIN-1 S bed bugs are highly resistant to deltamethrin, with a high topical dose leading to no mortality. Therefore, we decided to use a more effective synthetic pyrethroid, β-cyfluthrin, to assay insecticide susceptibility of RNAi insects. The P450s or Abc8 and Abc9 knockdown in pyrethroid resistant CIN-1 S bed bugs showed a consistent increase in their susceptibility to β-cyfluthrin compared with control bed bugs injected with malE dsRNA (Fig. 5D). In contrast, there was no significant difference in the susceptibility to β-cyfluthrin between cuticular proteins knockdown and control bed bugs (Fig. 5D).
Discussion
The overall goal of this study is to understand the molecular basis of bed bugs adaption to insecticides which will be used to devise the most effective and sustainable resistance management strategies. To accomplish this goal, we took advantage of the recent advances in bed bug genomics and post-genomic technologies to carry out a genome wide analysis of the pyrethroid resistance associated genes. Previous studies reported that reduced cuticle permeability is one of the modes of insect resistance to insecticides32,33. Reduced penetration can affect a broad range of insecticides resulting in cross resistance2. Our study revealed that resistance-associated genes belonging to diverse categories (metabolic enzyme, cuticular protein, Abc transporter) are all expressed in the epidermal layer of the integument, which prevents or slows down the toxin from reaching the target sites on nerve cells, where an additional layer of resistance (kdr) is common. This unique resistance strategy employed by bed bugs is based on their distinctive morphological, physiological and behavioral characteristics. The bodies of bed bugs are extremely flat prior to taking a blood meal (Fig. 3A); this allows them to adapt well to their habitats by moving efficiently and hiding easily in concealed locations. The integument covered body and appendages constitute majority of their body weight (Fig. 3B and 3C). Since bed bugs are obligate blood feeders at all post embryonic stages, insecticide-laced baits used to control pestiferous ants, cockroaches and termites are ineffective in bed bugs. Therefore contact remains the major route of exposure of bed bugs to insecticides. However, integument affiliated expression of resistance-associated genes could reduce insecticide penetration, increase toxin transport, and enhance detoxification in the integument before reaching target sites. Better understanding of the adaptive strategy evolved by bed bugs to resist insecticides may help with developing more efficient delivery of insecticides to target sites by selective synergism or enhancement of penetration. Expression of genes coding for metabolic enzymes in the mesospermalege (Fig. 3D) is also interesting and further research is required to understand functional significance of this observation.
The major goal of the current study is to identify sound molecular markers associated with pyrethroid resistance and use them to investigate relative contribution of different resistance mechanisms in field bed bug populations. kdr mutations mediated target-site insensitivity has been identified as a very important mechanism responsible for pyrethroid resistance in bed bugs21,30. Several recent studies suggested enhanced metabolic detoxification as one of resistance mechanisms in different laboratory bed bug populations18,20,22,34,35. Additionally, the RNA-Seq study by Mamidala et al.20 revealed the possible contribution of cuticular proteins and Abc transporters in insecticide resistant laboratory strains. Our data showed that multiple mechanisms involved in pyrethroid resistance is not only a phenomenon occurring in laboratory bed bug strains but also is widespread in naturally occurring bed bug populations (Figs. 4B).
Functional studies by knocking down the expression of target genes provided direct evidence for contribution of multiple mechanisms in pyrethroid resistance in bed bugs. Inactivation of four P450 target genes significantly enhanced the susceptibility of resistant bed bugs to pyrethroid insecticide, which further confirmed the involvement of P450-mediated metabolic detoxification in pyrethroid resistance of bed bugs. This result is consistent with our prior functional studies on NADPH-cytochrome P450 reductase22. Typically Abc transporters function as either an importer or an exporter, transporting molecules in or out of cells, respectively, in regards to cellular demand. In insects, Abc transporters play vital roles in metabolism, development, and insecticide resistance29,36,37. An Abc transporter in the tobacco hornworm Manduca sexta was suggested to confer resistance to nicotine38. In tobacco budworm Heliothis virescens, the expression of Abc transporter in various resistant populations was higher than in the susceptible larvae33,39. Our studies, for the first time, verified that Abc transporter related mechanism is one of the mechanisms involved in pyrethroid resistance of bed bugs. Although inactivation of target cuticular proteins did not lead to significant enhancement in the susceptibility to pyrethroid insecticides, the potential function of these genes involved in pyrethroid resistance could not be ruled out. The function of target cuticular proteins will be further studied in the future experiments.
Methods
Bed bug populations
The insecticide-susceptible colony, LA-1, collected in 2006 in Los Angeles, CA34 was maintained in the laboratory without insecticide exposure. The deltamethrin resistant populations of bed bugs, CIN-1 (Cincinnati, OH) and NY-1 (Plainview, NY) were collected from human dwellings during 2006–2008 and maintained in the laboratory by using a parafilm-membrane feeder40. These three bed bug populations were kept in screened containers and fed with 37°C heparinized chicken blood or rabbit blood with sodium citrate through a thinly stretched parafilm membrane. Blood was purchased from Hema Resource and Supply Company (Aurora, OR). Bed bugs were reared at 27°C, 65 ± 5% RH, and a photoperiod of 14:10 (L:D) h. Twenty-one populations of bed bugs (adults) were collected from human dwellings in Cincinnati (OH), Lexington (KY) and Louisville (KY) (Table S4 and Fig. S5 show the sampling sites and collection dates for all populations). Samples were frozen with liquid nitrogen and kept in −80°C freezer until use.
Resistant population selection
Filter paper disks (Whatman#2; 4.25 cm diam.) were treated with Temprid® (Bayer CropScience, Kansas City, MO, USA) diluted in water to a concentration of 0.075% a.i. This insecticide was applied until the paper was uniformly wetted using a fine mist sprayer (118 ml SQB.4FMS, ProChemical and Dye, Somerset, MA). Disks were allowed to air dry overnight. Adults from CIN-1 strain16 were exposed for 1 h to this substrate in 6 well plates. After this exposure they were removed from the treated surface and placed individually in wells of a 24-well plate lined with untreated filter paper (Whatman #2, cut to 2.27 cm2 [1.7 cm diam]). After 24 h, survivors were removed and placed in 75 ml jars (feeders) with organza covered lids at a sex ratio of 5 females to 2 males (more males would result in extra female mortality because of damage caused by traumatic insemination). Parental females were allowed to oviposit on blotter paper in the feeder. Adults were transferred to a new feeder weekly leaving a group of 0 to 7 day-old eggs behind. When F1 offspring (CIN-1 S) molted into the adult stage they were used in molecular studies. The CIN-1 strain without selection was then named as CIN-1 NS.
Residual bioassays
In order to evaluate susceptibility to deltamethrin, a residual bioassay as described in Romero et al.16,41 was utilized. Technical grade deltamethrin was serially diluted in acetone to concentrations of 0.006, 0.060, 0.600 and 6.00% (w/v). An aliquot of each solution (50 μl) was applied to filter paper disks (Whatman #2, cut to 2.27 cm2 [1.7 cm diam]) and allowed to dry overnight before being placed in the bottom of individual cells of 24-well cell culture plates, which were then covered. Control discs received acetone only. Mortality was then evaluated after 24 h, and log-dose probit analysis was performed using the collected data41.
Pyrosequencing and sequence analysis
Total RNA isolated from insecticide resistant CIN-1 NS strain was used to prepare cDNA libraries and the libraries were sequenced following 454 sequencing manual. The adapter and primer sequences were removed and the C. lectularius transcriptome was assembled using the newbler software. The files used in the assembly were as follows: NCBI Accession number 1) SRX028107; 2) SRX013985; 3) SRX013984; 4) EST C. lectularius (#7131) University of Kentucky CIN-1 strain. All the contigs and singletons thus obtained were analyzed using Blast2go. BLASTx was then performed against NCBI NR database. Blast2go software was used to predict the function of sequences. GO ID, Enzyme ID and Interpro accession numbers were obtained for all the sequences in order to functionally characterize them.
RNA extraction, cDNA preparation, and qRT-PCR
Total RNA was isolated from three adult bed bugs using the TRI reagent (Molecular Research Center Inc., Cincinnati, OH) and the RNA was treated with DNase I (Ambion Inc., Austin, TX). cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA) with DNase I treated total RNA as a template. qRT-PCR was performed in Applied Biosystems StepOnePlus™ Real-Time PCR System (Life technologies™, Carlsbad, CA). Each qRT-PCR reaction (10 μl final volume) contained 5 μl FastStart SYBR Green Master (Roche Diagnostics, Indianapolis, IN), 1.0 μl of cDNA, 3.6 μl ddH2O, and 0.4 μl each of forward and reverse gene specific primers (Table S5, stock 10 μM). An initial incubation of 95°C for 3 min, followed by 40 cycles of 95°C for 10 s, 55°C for 60 s settings were used. A fluorescence reading determined the extension of amplification at the end of each cycle. Each experiment was repeated at least three times using independent biological samples.
Dual-primer allele specific PCR (dASPCR)
One Susceptible Allele-Specific Primer (SASP) was based on the specific sequence of voltage-gated sodium channel α–subunit gene in LA-1 (susceptible strain without mutation) and the other one, Resistant Allele-Specific Primer (RASP) was based on the sequence of voltage-gated sodium channel α–subunit gene in NY-1 (resistant strain with two mutations) by placing a specific nucleotide polymorphism at the 3′ end of each primer to permit preferential amplification of the gene allele without or with mutation. Two rounds of PCR reactions were performed as described as our previous study21. The primers used for dASPCR were listed in Table S5.
RNA interference (RNAi)
The double-stranded RNA (dsRNA) synthesis and injection method in bed bugs was as described by Zhu et al22 through mimicking traumatic insemination.
Bioassays with β-cyfluthrin after dsRNA injection
Bed bug adults were treated with serial dilutions of technical grade β-cyfluthrin prepared in acetone in the preliminary studies. An LD30 of 3.9 micrograms (causing approximately 30% of mortality) of β-cyfluthrin (5 μg) was applied for the bioassays. Acetone was used as a control. The solution was dropped on the thorax of the bugs (0.5 μl/drop) using a PB-600 repeating dispenser (Hamilton Co., Reno). The mortality was determined at 72 h after treatment. Mean and standard errors for each time point were obtained from at least three independent bioassays.
Statistical analysis
Statistical analyses were carried out using SAS software (v9.4, SAS Institute Inc., Cary, NC). Student's t-test (two-tailed paired t-test) was used to compare the gene expression difference between two samples. The differences among samples were analyzed by One-way ANOVA, followed by Duncan multiple mean separation techniques. The level of significance was set at P < 0.05.
Author Contributions
Conceived and designed the experiments: F.Z. K.F.H. M.F.P. S.R.P. Performed the experiments: F.Z. J.G. H.G. Analyzed the data: F.Z. J.G. H.G. Contributed reagents/materials/analysis tools: F.Z. J.G. H.G. K.F.H. M.F.P. S.R.P. Wrote the paper: F.Z. J.G. K.F.H. S.R.P.
Supplementary Material
Supplementary information
Acknowledgments
The authors are grateful to Shelby Stamper, Scott Bessin, and Mark Goodman (University of Kentucky) for collecting bed bug samples. We thank Dr. David R. Nelson (University of Tennessee) for help in naming Cimex lectularius P450s. We thank Timothy Walter Moural for his help with language editing. Supported by grants from National Research Initiative of the USDA-NIFA (2011-67013-30143) and Bayer Environmental Science. The paper is contribution (13-08-43) from Kentucky Agricultural Experiment Station.
References
- Ffrench-Constant R. H., Daborn P. J. & Le Goff G. The genetics and genomics of insecticide resistance. Trends Genet 20, 163–70 (2004). [DOI] [PubMed] [Google Scholar]
- McKenzie J. A. Ecological and evolutionary aspects of insecticide resistance. (Academic Press, Austin, 1996).
- Liu N., Zhu F., Xu Q., Pridgeon J. W. & Zhang L. Behavioral change, physiological modification, and metabolic detoxification: mechanisms of insecticide resistance. Acta Entomologica Sinica 48, 672–680 (2006). [Google Scholar]
- Scott J. G. in Handbook of Pest Management in Agriculture. (ed. Pimentel D.) 663 (CRC Press, Boca Raton, 1991).
- Argentine J. A., Zhu K. Y., Lee S. H. & Clark J. M. Biochemical-Mechanisms of Azinphosmethyl Resistance in Isogenic Strains of Colorado Potato Beetle. Pestic Biochem Physiol 48, 63–78 (1994). [Google Scholar]
- Dong K. Insect sodium channels and insecticide resistance. Invert Neurosci 7, 17–30 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N. N. & Pridgeon J. W. Metabolic detoxication and the kdr mutation in pyrethroid resistant house flies, Musca domestica (L.). Pestic Biochem Physiol 73, 157–163 (2002). [Google Scholar]
- Wen Z. M. & Scott J. G. Genetic and biochemical mechanisms limiting fipronil toxicity in the LPR strain of house fly, Musca domestica. Pestic Sci 55, 988–992 (1999). [Google Scholar]
- Scott J. G. & Georghiou G. P. Mechanisms responsible for high levels of permethrin resistance in the house fly. Pest Manag Sci 17, 195–206 (1986). [Google Scholar]
- Xu Q., Liu H., Zhang L. & Liu N. Resistance in the mosquito, Culex quinquefasciatus, and possible mechanisms for resistance. Pest Manag Sci 61, 1096–1102 (2005). [DOI] [PubMed] [Google Scholar]
- Hardstone M. C., Leichter C. A. & Scott J. G. Multiplicative interaction between the two major mechanisms of permethrin resistance, kdr and cytochrome P450-monooxygenase detoxification, in mosquitoes. Evol Bio 22, 416–423 (2009). [DOI] [PubMed] [Google Scholar]
- Awolola T. S. et al. Evidence of multiple pyrethroid resistance mechanisms in the malaria vector Anopheles gambiae sensu stricto from Nigeria. Trans R Soc Trop Med Hyg 103, 1139–1145 (2009). [DOI] [PubMed] [Google Scholar]
- Perera M. D., Hemingway J. & Karunaratne S. P. Multiple insecticide resistance mechanisms involving metabolic changes and insensitive target sites selected in anopheline vectors of malaria in Sri Lanka. Malar J 7, 168 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anspaugh D. D., Rose R. L., Koehler P. G., Hodgson E. & Roe R. M. Multiple Mechanisms of Pyrethroid Resistance in the German Cockroach, Blattella germanica (L). Pestic Biochem Physiol 50, 138–148 (1994). [Google Scholar]
- Martin T., Chandre F., Ochou O. G., Vaissayre M. & Fournier D. Pyrethroid resistance mechanisms in the cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae) from West Africa. Pestic Biochem Physiol 74, 17–26 (2002). [Google Scholar]
- Romero A., Potter M. F., Potter D. A. & Haynes K. F. Insecticide resistance in the bed bug: a factor in the pest's sudden resurgence? J Med Entomol 44, 175–8 (2007). [DOI] [PubMed] [Google Scholar]
- Ranson H. et al. Evolution of supergene families associated with insecticide resistance. Science 298, 179–181 (2002). [DOI] [PubMed] [Google Scholar]
- Adelman Z. N. et al. Deep Sequencing of pyrethroid-resistant bed bugs reveals multiple mechanisms of resistance within a single population. PloS One 6, e26228 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatolos N. et al.. Pyrosequencing the transcriptome of the greenhouse whitefly, Trialeurodes vaporariorum reveals multiple transcripts encoding insecticide targets and detoxifying enzymes. BMC Genomics 12, 56 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidala P. et al. RNA-Seq and molecular docking reveal multi-level pesticide resistance in the bed bug. BMC Genomics 13, 6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F. et al. Widespread distribution of knockdown resistance mutations in the bed bug, Cimex lectularius (Hemiptera: Cimicidae), populations in the United States. Arch Insect Biochem Physiol 73, 245–257 (2010). [DOI] [PubMed] [Google Scholar]
- Zhu F. et al. RNA interference of NADPH-cytochrome P450 reductase results in reduced insecticide resistance in the bed bug,. Cimex lectularius. PloS One 7, e31037 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enayati A. A., Ranson H. & Hemingway J. Insect glutathione transferases and insecticide resistance. Insect Mol Biol 14, 3–8 (2005). [DOI] [PubMed] [Google Scholar]
- Feyereisen R. in Insect Molecular Biology and Biochemistry. (ed. Gilbert L. I.) (Elsevier B. V., 2011).
- Hemingway J., Hawkes N. J., McCarroll L. & Ranson H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol 34, 653–665 (2004). [DOI] [PubMed] [Google Scholar]
- Wood O. R., Hanrahan S., Coetzee M., Koekmoer L. L. & Brooke B. D. Cuticle thickening assoicated with pyrethroid resistance in the major malaria vector Anopheles funestus. Parasites & Vectors 3, 1–7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Goyer C. & Pelletier Y. Environmental stresses induce the expression of putative glycine-rich insect cuticular protein genes in adult Leptinotarsa decemlineata (Say). Insect Mol Biol 17, 209–216 (2008). [DOI] [PubMed] [Google Scholar]
- Rees D. C., Johnson E. & Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol 10, 218–227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe R., Caveney S. & Donly C. Genetic analysis of the xenobiotic resistance-associated ABC gene subfamilies of the Lepidoptera. Insect Mol Biol 20, 243–256 (2011). [DOI] [PubMed] [Google Scholar]
- Yoon K. S. et al. Biochemical and molecular analysis of deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae). J Med Entomol 45, 1092–1101 (2008). [DOI] [PubMed] [Google Scholar]
- Regard J. B., Sato I. T. & Coughlin S. R. Anatomical profiling of G protein-coupled receptor expression. Cell 135, 561–571 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad M., Denholm I. & Bromilow R. H. Delayed cuticular penetration and enhanced metabolism of deltamethrin in pyrethroid-resistant strains of Helicoverpa armigera from China and Pakistan. Pest Manag Sci 62, 805–810 (2006). [DOI] [PubMed] [Google Scholar]
- Lanning C. L., Ayad H. M. & Abou-Donia M. B. P-glycoprotein involvement in cuticular penetration of [14C ]thiodicarb in resistant tobacco budworms. Toxicol Lett 85, 127–133 (1996). [DOI] [PubMed] [Google Scholar]
- Romero A., Potter M. F. & Haynes K. F. Evaluations of piperonyl butoxide as a deltamethrin synergist for pyrethroid resistant bed bugs. J Econ Entomol 102, 2310–2315 (2009). [DOI] [PubMed] [Google Scholar]
- Bai X., Mamidala P., Rajarapu S. P., Jones S. C. & Mittapalli O. Transcriptomics of the bed bug (Cimex lectularius). PloS One 6, e16336 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycz J., Borycz J. A., Kubow A., Lloyd V. & Meinertzhagen I. A. Drosophila ABC transporters mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J Exp Biol 211, 3454–3466 (2008). [DOI] [PubMed] [Google Scholar]
- Richardo S. & Lehmann R. An ABC transportercontrols export of a Drosoohila germ cell attractant. Science 323, 943–946 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. L., Quaglia M., Arnason J. T. & Morris C. E. A Putative Nicotine Pump at the Metabolic Blood-Brain-Barrier of the Tobacco Hornworm. Neurobiol 25, 23–34 (1994). [DOI] [PubMed] [Google Scholar]
- Lanning C. L. et al. Tobacco budworm P-glycoprotein: biochemical characterization and its involvement in pesticide resistance. Biochim Biophys Acta 1291, 155–162 (1996). [DOI] [PubMed] [Google Scholar]
- Montes C., Cuadrillero C. & Vilella D. Maintenance of a laboratory colony of Cimex lectularius (Hemiptera: Cimicidae) using an artificial feeding technique. Medi Entomol 39, 675–679 (2002). [DOI] [PubMed] [Google Scholar]
- Finney D. J. Probit analysis. (Cambridge University Press, Cambridge, 1971).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information