Abstract
Impulsivity describes the tendency of an individual to act prematurely without foresight and is associated with a number of neuropsychiatric co-morbidities, including drug addiction. As such, there is increasing interest in the neurobiological mechanisms of impulsivity, as well as the genetic and environmental influences that govern the expression of this behaviour. Tests used on rodent models of impulsivity share strong parallels with tasks used to assess this trait in humans, and studies in both suggest a crucial role of monoaminergic corticostriatal systems in the expression of this behavioural trait. Furthermore, rodent models have enabled investigation of the causal relationship between drug abuse and impulsivity. Here, we review the use of rodent models of impulsivity for investigating the mechanisms involved in this trait, and how these mechanisms could contribute to the pathogenesis of addiction.
Defining impulsivity: a human trait
Impulsivity is typically classified as a predisposition for premature, poorly planned, unduly risky or inappropriate actions (Daruma and Barnes, 1993). This behavioural construct consists of a heterogeneous repertoire of factors, each with independent but potentially overlapping neurobiological substrates (Evenden, 1999). Impulsivity in its simplest form consists of at least two major components: motor disinhibition (impulsive action) and impulsive decision-making (impulsive choice). Theorists, however, suggest that impulsivity encompasses a more complex array of behavioural processes, including urgency, risk-taking, sensation-seeking, nonplanning, lack of premeditation, a disregard for future consequences and insensitivity to punishment (Barratt, 1985; Evenden, 1999; Moeller et al., 2001; Monterosso and Ainslie, 1999; Whiteside and Lynam, 2003).
Although still a matter of considerable debate, impulsivity has been postulated to originate from complex, dynamic ecological variables. In this sense, impulsivity might offer a biological advantage in certain social or environmental settings (Williams and Taylor, 2006) and, as such, ‘functional’ impulsivity favours advantageous outcomes and indeed is an important aspect of human behaviour without which individuals would fail to take acceptable risks or pursue unexpected opportunities. In contrast,‘maladaptive’ impulsivity is akin to the largely accepted definition of impulsivity as excessively risky, premature and inappropriate actions associated with negative outcomes. Maladaptive impulsivity has been shown to predict antisocial behaviour, unlike functional indices of impulsivity, which facilitate extraversion and sociability (Cale and Lilienfeld, 2006). Indeed, in its extreme form, maladaptive impulsivity has been associated with a wide range of neuropsychiatric morbidities, including personality (Perry and Körner, 2011) and mood (Lombardo et al., 2012) disorder, drug abuse and addiction (Ersche et al., 2010), suicide (Dougherty et al., 2004), and attention deficit hyperactivity disorder (ADHD) (Avila et al., 2004).
The diverse taxonomies of impulsivity have led to the development of a variety of self-report questionnaires evaluating these factors [e.g. Eysenck personality questionnaire (Eysenck and Eysenck, 1984), Barratt (Barratt, 1985), UPPS (Whiteside and Lynam, 2003) and Dickman Impulsiveness Scales (Monterosso and Ainslie, 1999)]. There are, however, a number of inherent limitations associated with self-report measures (Wilson and Dunn, 2004), which can be exacerbated in impulsive individuals, who might lack introspection and the ability to appropriately perceive their behaviour. Indeed, it has been suggested that questionnaire-based methods have contributed to the heterogeneity of findings relating to the biological basis of impulsivity (Eisenberg et al., 2007). Computer-based clinical psychometric behavioural tests, which subjectively assess aspects of impulsive choice or impulsive action, provide a more objective measure of impulsivity (reviewed by Chamberlain and Sahakian, 2007; Kertzman et al., 2006). Among these tests, delay-discounting is the most commonly employed measure of impulsive choice and involves individuals making a series of choices between small, immediate rewards versus larger rewards received after a longer delay. Impulsive individuals prefer immediate rewards even if they are smaller than those offered at a later period of time (Richards et al., 1999). The go/no-go task is used to assess deficits in response initiation/inhibition (impulsive action). This test requires subjects to perform a binary-choice reaction time task where they respond to one stimulus (e.g. a plain square) and must inhibit a response to a second stimulus (e.g. a patterned square). Impulsive individuals tend to make more rapid, but incorrect, responses during this task (Riccio et al., 2002). In the stop-signal reaction time task, which is also used to assess impulsive action, subjects perform a primary visual binary-choice reaction time task and, on a portion of trials, are instructed to inhibit that response upon the presentation of a stop signal (e.g. an auditory or visual stimulus), which can occur at any time at or after the onset of the primary stimulus. Impulsive individuals show a higher rate of failure to inhibit responses following presentation of the stop signal (Logan et al., 1997).
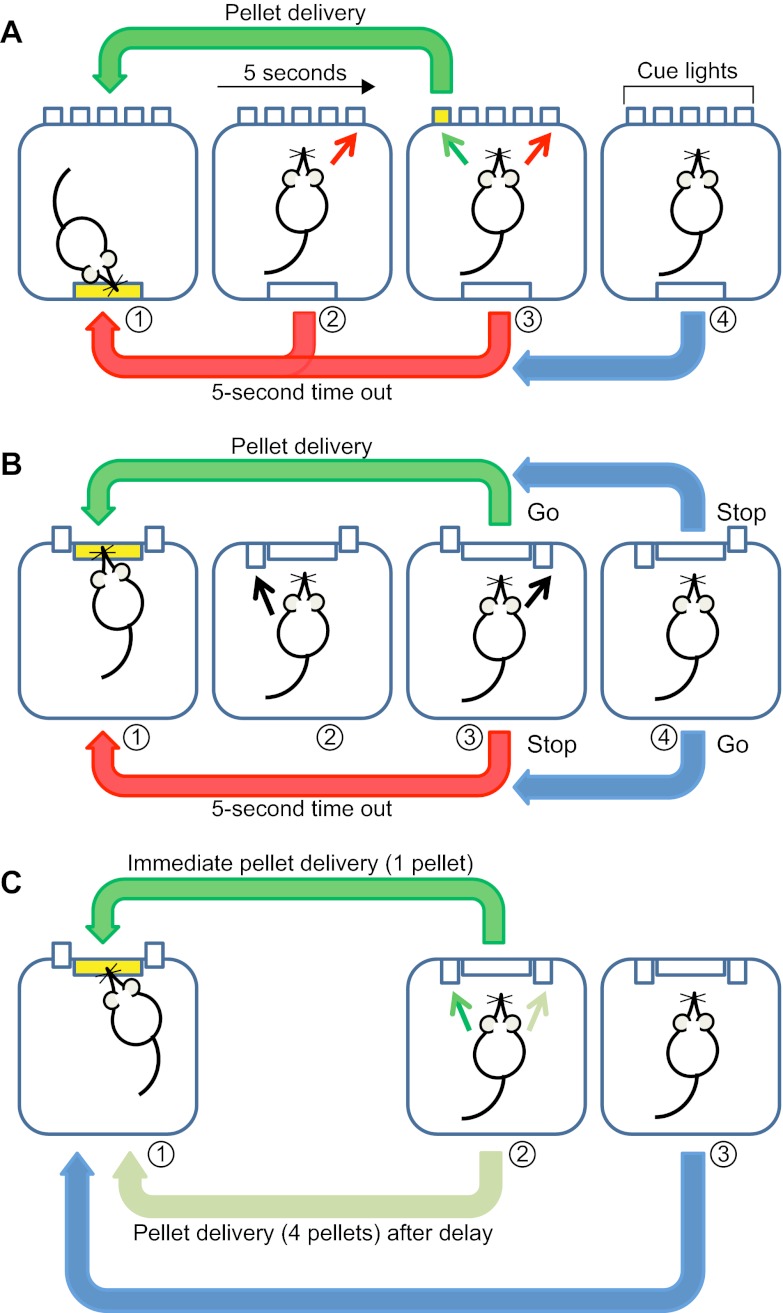
Importantly for the investigation of the biological basis of impulsivity, indices of impulsive behaviour in humans have analogues in animal behaviour. Delay-discounting, go/no-go and stop-signal tasks have all been translated to operant-based paradigms and have been used to assess impulsivity in rodents (reviewed by Winstanley, 2011). Other operant-based methods, particularly the five-choice serial reaction time task (5CSRTT) (Fig.1), which assays impulsive action based on premature responses for a food-predictive, brief light stimulus (Robbins, 2002), are also widely used. In this Review, we discuss the use of rodent models of impulsivity, particularly in relati;on to the endogenous expression of impulsive behaviour (referred to here as trait or trait-like impulsivity), for investigating the underlying mechanisms of this behaviour and its potential aetiological relationship as an endophenotype of the pathogenesis of addiction.
Fig. 1.
Schematic representation of operant-based tests of impulsivity in rodents. Green arrows represent correct responses and outcomes, whereas red arrows denote incorrect responses and associated outcomes. Blue arrows denote outcomes associated following an omitted response. Adapted with permission from Dalley and Roiser (Dalley and Roiser, 2012). (A) The five-choice serial reaction time task (5CSRTT) requires animals to wait for a food-predictive light cue before carrying out the response. The trial is initiated when the animal enters the illuminated food magazine (panel 1) and, following a delay (5 seconds), one of five cue lights is briefly illuminated (panel 3). If subjects nose-poke in the corresponding aperture, they receive a food reward (green arrow, panel 3). Responses that are made prior to the presentation of the cue light (impulsive responses; panel 2), are incorrect (red arrow, panel 3) or are withheld (panel 4) are punished by a 5-second time out during which the house light is extinguished. (B) The stop-signal reaction time task requires subjects to withhold reinforced responding following presentation of a tone cue (stop signal). The task begins following entry into the food magazine (panel 1), after which the left lever is introduced into the arena (panel 2). Responding on this lever introduces the right ‘reward’ lever, which, if depressed during a go trial (no tone), results in delivery of a food reward (panel 3). During a stop trial (during which a tone is presented), responding on the right lever is punished by a time out. If no responses are made during a stop trial, a food reward is delivered (panel 4). Conversely, if no response is made during a go trial, this is punished by a time-out period. Impulsive individuals have difficulty inhibiting responses during stop trials. (C) The delay-discounting task requires subjects to choose between a small immediate food reward or a larger reward delivered following a delay. The tasks begins following entry into the food magazine (panel 1), after which animals are presented with two levers (panel 2): one provides a small immediate food reward (left lever) and the other a larger reward following a delay (right lever). Omitted responses (panel 3) are unrewarded. Impulsive individuals prefer the immediate over the delayed reward.
Neurobiology of impulsivity
Clinical studies investigating the neuroanatomical and psychopharmacological substrates of impulsivity have largely implicated monoaminergic corticostriatal systems. Brain imaging studies in humans have identified structural and functional alterations in distinct regions of the prefrontal cortex (PFC) (Wilbertz et al, 2012) and associated corticostriatal circuitry (Carmona et al, 2009) in impulsive individuals. It has been suggested that dysfunctional monoaminergic signalling, particularly related to dopaminergic and noradrenergic function, contribute to impulsivity based on the therapeutic efficacy of drugs used to treat ADHD, such as the mixed dopamine (DA)/noradrenaline reuptake inhibitor methylphenidate and the noradrenaline reuptake inhibitor atomoxetine (Del Campo et al, 2011). Indeed, positron emission tomography studies have identified alterations in DA release (Buckholtz et al, 2010; Oswald et al, 2007) and the availability of DA receptors D2 and/or D3 (referred to here as D2-like receptors) in the striatum (Ghahremani et al, 2012; Lee et al, 2009) of impulsive individuals. Serotonergic (5HT) dysfunction has also been implicated, with enhanced levels of impulsivity predictive for reduced 5HT2A receptor and 5HT transporter binding in the PFC (Lindström et al., 2004; Meyer et al., 2008).
Investigations of the heritability of impulsivity have also identified polymorphisms in genes involved in monoaminergic function, including those encoding the DA transporter (Congdon et al., 2008), the 5HT2A (Reist et al., 2004) and 5HT2B (Bevilacqua et al., 2010) receptors, and the monoamine oxidase A enzyme (Liu et al., 2011). However, there is also increasing evidence for environmental risk factors in impulsivity, particularly relating to the expression of genetic vulnerability for impulsivity (Bezdjian et al., 2011). For example, a polymorphism in the DA D4 receptor was only found to be associated with impulsivity in individuals who were exposed to low socioeconomic status during childhood (Sweitzer et al., 2012).
Clinical investigations into the biological basis of impulsive behaviour are often confounded by co-expression of diseasespecific symptoms. Although limited by anthropomorphism, animal models enable the relatively selective investigation of behavioural endophenotypes. Experimental approaches that evoke impulsive behaviour in animals, effected either pharmacologically or by selective brain lesions, have dissected the contribution of individual brain regions and transmitter systems to impulsive behaviour (Dalley and Roiser, 2012; Eagle and Baunez, 2010; Winstanley, 2011) (Tables 1, 2). These studies accord with findings in humans implicating the monoaminergic corticostriatal systems in impulsivity; however, there is additional evidence for an involvement of cannabinoid, opioid, glutamatergic and cholinergic signalling mechanisms in impulsive behaviour (Pattij and Vanderschuren, 2008). Furthermore, the contribution of each of these anatomical regions and transmitter systems varies between different impulsivity subtypes (Pattij and Vanderschuren, 2008).
Table 1.
Selected references for effects of anatomically localised brain lesions on impulsive responding in rats
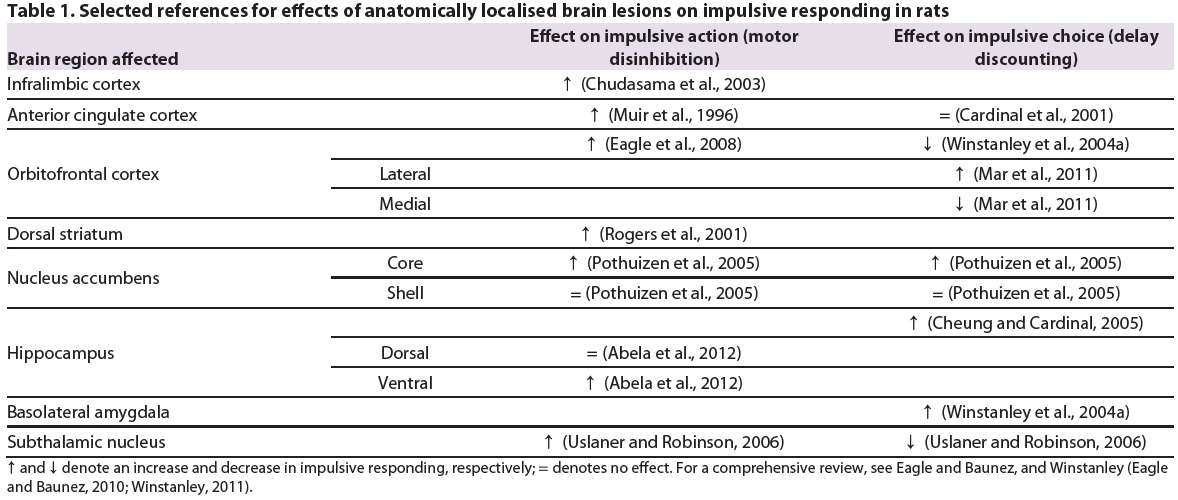
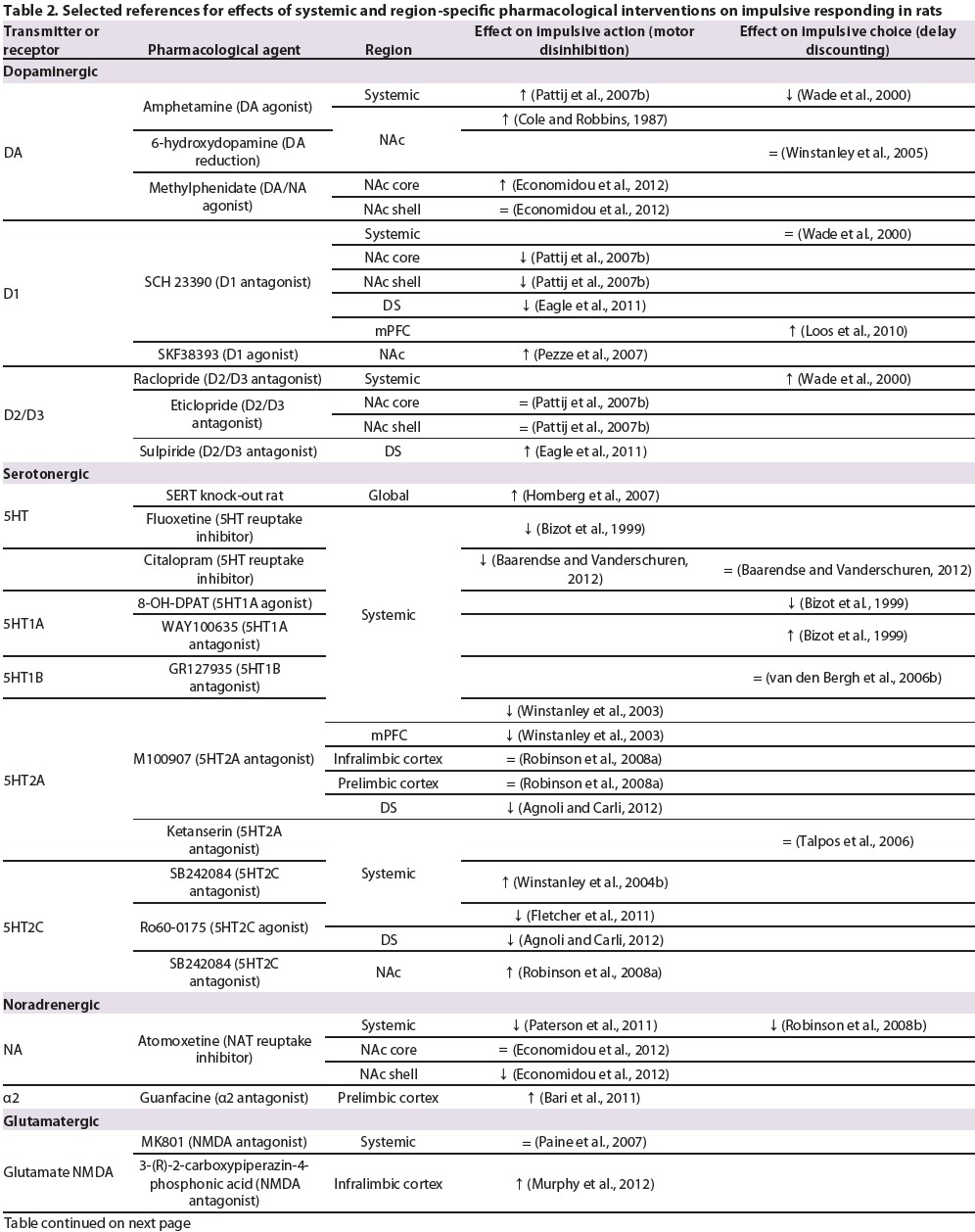
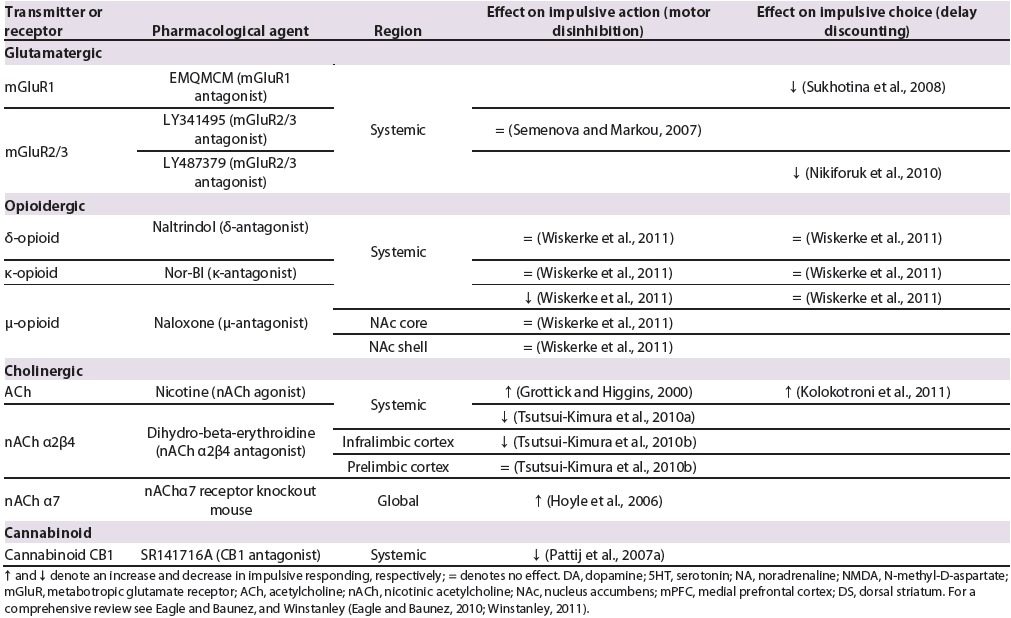
Table 2.
Selected references for effects of systemic and region-specifc pharmacological interventions on impulsive responding in rats
Animal models of trait-like impulsivity
Ecologically valid animal models that capture the intrinsic qualities of impulsive behaviour arguably have greater face and construct validity than those generated using invasive approaches (e.g. brain lesions, administration of pharmacological agents). Studies that modulate impulsivity using invasive approaches can be confounded by inadvertently investigating the effects associated with the particular manipulation, rather than the underlying neurobiology that is responsible for maladaptive impulsivity. Thus, animals that exhibit trait impulsivity have provided a useful experimental approach to the investigation of impulsivity. To date, a number of studies have identified particular strains of rodents that demonstrate increased levels of impulsivity (trait or trait-like impulsivity), including Roman high avoidance (RHA) rats (Moreno et al., 2010), spontaneously hypertensive rats (SHRs) (Adriani et al., 2003), and rats exhibiting naturally high impulsive behaviour on the 5CSRTT (Dalley et al., 2007) and delay-discounting task (Broos et al., 2012; Perry et al., 2005).
A limited number of studies have investigated the neurobiology of trait impulsivity in rodents; these have predominantly been carried out using animals displaying enhanced impulsive responding on the 5CSRTT. A diminished availability of D2-like receptors in the ventral striatum is observed in rats impulsive for the 5CSRTT (Dalley et al., 2007). Enhanced D1-receptor-mediated neurotransmission in the nucleus accumbens core (Ohno et al., 2012), and increased mRNA expression of this receptor in the PFC, has been shown to be predictive of delay-discounting impulsivity in SHRs (Loos et al., 2010). Pharmacological enhancement of DA signalling has been shown to increase impulsive responding, whereas administration of D2-like receptor agonists reduces impulsivity in rats exhibiting impulsive behaviour on the 5CSRTT (Fernando et al., 2012). These findings suggest that dopaminergic modulation of impulsivity can be receptor-subtype- and brainregion-dependent. For example, administration of the D2-like receptor antagonist nafadotride into to the nucleus accumbens shell was found to enhance premature responding, whereas infusions into the core reduced premature responding in trait impulsive rats (Besson et al., 2010). There is also evidence for dysfunction in noradrenergic systems in trait impulsive animals: systemic administration of a noradrenaline reuptake inhibitor was found to reduce impulsivity, an effect potentially mediated by alpha 2A receptors (Fernando et al., 2012).
It is notable that, similar to ADHD (Sullivan et al., 2012), there is growing evidence for genetic influences in trait impulsivity in rodents. For example, there are reports demonstrating the stability of impulsive behaviour within, and variability between, inbred strains of mice (Gubner et al., 2010; Isles et al., 2004; Logue et al., 1998; Loos et al., 2009; Peña-Oliver et al., 2012), and a genomewide association study in our own laboratory has found significant evidence of heritability of impulsivity in a multi-generational pedigree of outbred rats (S. Pitzoi, A. Mar, T. Robbins, E. Petretto, T. Aitman and J.W.D., unpublished findings). Moreover, studies using transgenic animals have provided persuasive evidence that genetic influences contribute to impulsivity (e.g. Peña-Oliver et al., 2012). Many of these studies aim to elucidate the contribution of genes and polymorphisms already identified as risk factors for impulsive behaviour in human studies [e.g. the serotonin transporter knockout rat (Homberg et al., 2007)]; however, previously unknown genetic mechanisms have also been identified, for example relating to genomic imprinting (Davies et al., 2005; Doe et al., 2009), that suggest a potential role for epigenetic mechanisms and gene-environment interactions in the expression of impulsivity.
Genetic studies in inbred mice have confirmed an environmental component and role for gene-environment interactions in the expression of impulsive behaviour (Isles et al., 2004; Loos et al., 2009). Factors such as rearing conditions [e.g. maternal separation, (Lovic et al., 2011)], prenatal or adolescent exposure to certain drugs such as alcohol (Bañuelos et al., 2012) and nicotine (Schneider et al., 2011), and environmental conditions [e.g. enrichment (Perry et al., 2008)], reportedly alter levels of impulsivity in rodents. The mechanisms through which environmental manipulations affect levels of impulsivity are still under investigation; however, plasticity mechanisms in dopaminergic function in corticostriatal circuitry are implicated in these phenomena. For example, both environmental enrichment and maternal separation alter levels and/or functioning of DA transporters in the corticostriatal system (Womersley et al., 2011; Zhu et al., 2005).
Trait-like impulsivity in rodents: a vulnerability marker of addiction
Animal models of trait impulsivity provide the opportunity to investigate the role of impulsivity in neuropsychiatric disorders such as addiction. Although the link between addiction and relapse vulnerability and impulsivity is well established in the clinical literature (reviewed by Verdejo-García et al., 2008), it has been difficult to dissect the causal effect of this relationship. Studies in rodents indicate that trait impulsivity is predictive of addiction-related behaviours, although this relationship is dependent on impulsivity sub-type and drug class (Belin et al., 2008; Dalley et al., 2007; Perry et al., 2005; Poulos et al., 1995). Both RHA (Moreno et al., 2010) and SHR (Harvey et al., 2011) lines display susceptibility to psychostimulants, whereas rats selected for trait action impulsivity show enhanced self-administration of cocaine (Dalley et al., 2007), nicotine (Diergaarde et al., 2008), alcohol (Radwanska and Kaczmarek, 2012) and methylphenidate (Marusich and Bardo, 2009), but not heroin (McNamara et al., 2010). Trait-like impulsive rats further show enhanced conditioned place preference to amphetamine (Yates et al., 2012), have a higher propensity to develop compulsive cocaine self-administration (Belin et al., 2008) and show increased rates of 3,4-methylenedioxymethamphetamine (MDMA)-primed drug-seeking (Bird and Schenk, 2012) and cueinduced relapse for cocaine-seeking (Economidou et al., 2009). Furthermore, impulsive choice has been shown to predict increased alcohol (Oberlin and Grahame, 2009; Poulos et al., 1995) and nicotine (Diergaarde et al., 2008) consumption, as well as resistance to extinction and enhanced relapse propensity to both nicotine (Diergaarde et al., 2008) and cocaine (Broos et al., 2012). There is conflicting evidence, however, regarding the relationship of impulsive choice to cocaine and opiate consumption, with studies both supporting (Anker et al., 2009; García-Lecumberri et al., 2011) and refuting (Broos et al., 2012; Schippers et al., 2012) an association. Although these findings are perhaps surprising given that heroin and cocaine addicts are also impulsive (Kirby and Petry, 2004; Moreno-López et al., 2012), it is possible that these clinical findings reflect the influence of chronic drug use and/or repeated cycles of drug bingeing and withdrawal on impulsivity levels. Consistent with this notion, both heroin (Schippers et al., 2012) and cocaine (Mendez et al., 2010; Paine et al., 2003; Roesch et al., 2007; Winstanley et al., 2009) exposure increases impulsivity in nonimpulsive animals. However, in striking contrast, cocaine exposure in trait impulsive rats has the dramatic effect of decreasing impulsivity (Dalley et al., 2007), an effect that might be mediated by a restoration of dopaminergic function in the ventral striatum of this group of animals (D.C., Y. Hong, B.J., B. Everitt, T. Robbins, T. Fryer and J.W.D., unpublished findings).
The disparity between the contribution of impulsive action and impulsive choice to addiction susceptibility – and, as discussed previously, the apparent differences in neural correlates underlying these behaviours – brings to question whether impulsivity in these models reflects a unitary construct. To this point, studies have found that RHA rats demonstrate both enhanced impulsive action and choice (Moreno et al., 2010). Similarly, rats selected for high impulsivity on 5CSRTT were also impulsive on a delay-discounting task (Robinson et al., 2009). However, there is also evidence to suggest that impulsive action and impulsive choice reflect independent constructs. Thus, SHRs were found to be impulsive on a delay-discounting task (Adriani et al., 2003) but not a 5CSRTT (van den Bergh et al., 2006a), and no correlation was observed between the performance of Wistar rats on a stop-signal or 5CSRTT and delay discounting (Broos et al., 2012), a dissociation that also extends to humans (Broos et al., 2012). It is possible that impulsive action and impulsive choice represent distinct but related constructs with overlapping mechanisms, making it possible for the two types of impulsivity to co-exist without being directly correlated. To this effect, although these two forms of impulsivity reflect deficits in ‘failing to wait’, tasks assessing impulsive choice also incorporate a value assessment. Therefore, deficits in performance can also be related to mechanisms involved in interpreting reward value that are distinct from those involved in ‘waiting’ per se. Interestingly, the neural substrates involved in coding reward value are similar to those involved in impulsivity (reviewed by Levy and Glimcher, 2012). Thus, it might be possible for an individual to be impulsive on delay-discounting choice tasks without being impulsive on motor tasks (e.g. stop-signal), but not vice versa.
Impulsivity seems to be one of several behavioural endophenotypes associated with addiction-like behaviour. Others include novelty reactivity (Piazza et al., 1990), novelty preference (Belin et al., 2011), anxiety (Dilleen et al., 2012) and sign-tracking (Flagel et al., 2010). Although novelty-reactive rats demonstrate enhanced measures of impulsive action, and acquire a conditioned approach response to an appetitive stimulus [i.e. sign-trackers (Flagel et al., 2010)], trait impulsive animals show no obvious heightened sensitivity to novelty (Dalley et al., 2007; Molander et al., 2011), nor do they differentially acquire appetitive conditioned approach compared with low-impulsive rats (Robinson et al., 2009). However, both RHA and rats impulsive on the 5CSRTT show a preference for novel objects and contexts (Giorgi et al., 2007; Molander et al., 2011), which might reflect a reduction in noveltyinduced anxiety in these animals (Duclot et al., 2011). Indeed, it has been suggested that anxiety is inversely related to impulsivity (Schneider et al., 2012), an intriguing relationship considering that both impulsivity and anxiety predict addiction vulnerability. Although there is some evidence to suggest that anxiolytics enhance impulsivity (Bizot et al., 1988; Evenden and Ryan, 1996), it is unclear whether this is due to an effect to reduce anxiety, given that doses shown to be anxiolytic had no effect on premature responding in these animals (Molander et al., 2011). Furthermore, there is little evidence to suggest that trait impulsive animals are anxious (Loos et al., 2009); thus, their preference for novelty might simply reflect an underlying deficit in behavioural inhibition.
However, trait-like impulsivity does predict the emergence of compulsive cocaine taking in rodents (Belin et al., 2008), defined by the persistence of drug intake despite punishing feedback (Everitt et al., 2008). Furthermore, impulsive SHR and RHA lines show compulsive drinking on a schedule-induced polydipsia (SIP) task (Ibias and Pellón, 2011; Moreno et al., 2010), whereas individual variation in SIP predicts impulsivity in delay-discounting (Cardona et al., 2011) and 5CSRTT (Moreno et al., 2012). Collectively, these findings demonstrate that impulsivity, in its various forms, is a vulnerability marker for the development of compulsive drug taking. However, it remains unclear whether impulsivity and compulsivity subtypes co-exist in the same individual, or whether they are separable entities that are serially expressed in a manner dependent on drug-induced plasticity in the PFC and striatum (Dalley et al., 2011).
Implications for addiction
Findings in naturally impulsive rodents suggest that impulsivity represents a susceptibility factor for addiction rather than occurring directly as a consequence of chronic drug use. Clinical studies demonstrating enhanced impulsivity in the non-drug-using siblings of chronic stimulant abusers support this assertion (Ersche et al., 2012; Ersche et al., 2010). Given this, it is possible that treating impulsivity might prevent the development of compulsive drug use and/or the occurrence of relapse in susceptible individuals, although clinical and preclinical studies confirming the efficacy of this therapeutic intervention are still required. However, atomoxetine, a selective noradrenaline reuptake inhibitor, reduces impulsive responding in highly impulsive rats (Fernando et al., 2012), as well as decreasing cocaine-seeking in these animals (Economidou et al., 2009). Moreover, clinical trials have provided some evidence for enhanced rates of abstinence following treatment with the related drug reboxetine in cocaine-dependent patients (Szerman et al., 2005).
Psychostimulant drugs have been shown to reduce some forms of impulsivity in animals (Sagvolden and Xu, 2008; Wooters and Bardo, 2011). Furthermore, cocaine self-administration has been shown to normalise excessive impulsive responding in rats (Dalley et al., 2007). A plausible hypothesis, given that impulsive animals are susceptible to compulsive cocaine self-administration, follows that drug intake potentially represents a form of ‘self-medication’ in impulsive subjects. Indeed, this might correct an apparent hypodopaminergic state in these animals, which itself predicts increased cocaine self-administration (Michaelides et al., 2012; Nader et al., 2006). It should be noted, however, that cocaine reduces striatal binding of the D2-like PET ligand [18F]fluoroclebopride (Nader and Czoty, 2005), an effect that might in fact drive continued cocaine use, reflecting the cycle of chronic cocaine abuse observed in addicts. The observed ventral-to-dorsal shift in dopaminergic dysfunction that results from prolonged cocaine self-administration (Porrino et al., 2004) has been suggested to underlie the development of maladaptive habit learning in addiction (Everitt and Robbins, 2005). There is also evidence implicating D2/D3 function in the dorsal striatum in behavioural inhibition (e.g. Ghahremani et al., 2012), providing another avenue through which D2/D3 receptor dysfunction might contribute to the link between impulsivity and addiction. At least in rats, impulsivity on the 5CSRTT does not seem to be related to D2/D3 receptor function in the dorsal striatum (Dalley et al., 2007); however, D2/D3 receptor availability in this region has been shown to predict irrational, biased decision making in rats (Cocker et al., 2012), a finding of possible relevance to behavioural addictions such as pathological gambling.
Conclusions
Animal models of impulsivity have provided significant insight into the underlying neurobiological, genetic and environmental contributions to impulsivity as a behavioural endophenotype, and as a susceptibility marker for addiction. Such findings have implicated dysfunction in monoaminergic corticostriatal systems in the expression of impulsivity and have specifically identified a putative involvement of striatal D2/D3 receptors. Further refinement of such models are likely to reveal currently unknown mechanisms underlying the apparent causal relationship between impulsivity and addiction, thereby informing the development of new therapeutic strategies for disorders of behavioural control.
Acknowledgments
FUNDING: This work was supported by the Medical Research Council (G0701500) and by a joint award from the Medical Research Council and Wellcome Trust in support of the Behavioural and Clinical Neuroscience Institute at Cambridge University. B.J. is supported by a postdoctoral fellowship from the National Health and Medical Research Council (Australia) (1016313).
Footnotes
COMPETING INTERESTS: None to declare.
References
- Abela A. R., Dougherty S. D., Fagen E. D., Hill C. J., Chudasama Y. (2012). Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb. Cortex. [Epub ahead of print] 10.1093/cercor/bhs121 [DOI] [PubMed] [Google Scholar]
- Adriani W., Caprioli A., Granstrem O., Carli M., Laviola G. (2003). The spontaneously hypertensive-rat as an animal model of ADHD: evidence for impulsive and non-impulsive subpopulations. Neurosci. Biobehav. Rev. 27, 639-651 [DOI] [PubMed] [Google Scholar]
- Agnoli L., Carli M. (2012). Dorsal-striatal 5-HT2A and 5-HT2C receptors control impulsivity and perseverative responding in the 5-choice serial reaction time task. Psychopharmacology (Berl.) 219, 633-645 [DOI] [PubMed] [Google Scholar]
- Anker J. J., Perry J. L., Gliddon L. A., Carroll M. E. (2009). Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol. Biochem. Behav. 93, 343-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila C., Cuenca I., Félix V., Parcet M. A., Miranda A. (2004). Measuring impulsivity in school-aged boys and examining its relationship with ADHD and ODD ratings. J. Abnorm. Child Psychol. 32, 295-304 [DOI] [PubMed] [Google Scholar]
- Baarendse P. J., Vanderschuren L. J. (2012). Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl.) 219, 313-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañuelos C., Gilbert R. J., Montgomery K. S., Fincher A. S., Wang H., Frye G. D., Setlow B., Bizon J. L. (2012). Altered spatial learning and delay discounting in a rat model of human third trimester binge ethanol exposure. Behav. Pharmacol. 23,54-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A., Mar A. C., Theobald D. E., Elands S. A., Oganya K. C., Eagle D. M., Robbins T. W. (2011). Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J. Neurosci. 31, 9254-9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt E. S. (1985). Impulsiveness subtraits, arousal and information processing In Motivation, Emotion and Personality (ed. Spence J. T., Itard C. T.), pp. 137-146 New York, NY: Elsevier Science [Google Scholar]
- Belin D., Mar A. C., Dalley J. W., Robbins T. W., Everitt B. J. (2008). High impulsivity predicts the switch to compulsive cocaine-taking. Science 320, 1352-1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D., Berson N., Balado E., Piazza P. V., Deroche-Gamonet V. (2011). High-novelty-preference rats are predisposed to compulsive cocaine selfadministration. Neuropsychopharmacology 36, 569-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M., Belin D., McNamara R., Theobald D. E., Castel A., Beckett V. L., Crittenden B. M., Newman A. H., Everitt B. J., Robbins T. W., et al. (2010). Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology 35, 560-569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L., Doly S., Kaprio J., Yuan Q., Tikkanen R., Paunio T., Zhou Z., Wedenoja J., Maroteaux L., Diaz S., et al. (2010). A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature 468, 1061-1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdjian S., Baker L. A., Tuvblad C. (2011). Genetic and environmental influences on impulsivity: a meta-analysis of twin, family and adoption studies. Clin. Psychol. Rev. 31, 1209-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J., Schenk S. (2012). Contribution of impulsivity and novelty-seeking to the acquisition and maintenance of MDMA self-administration. Addict. Biol. [Epub ahead of print] 10.1111/j.1369-1600.2012.00477.x [DOI] [PubMed] [Google Scholar]
- Bizot J. C., Thiébot M. H., Le Bihan C., Soubrié P., Simon P. (1988). Effects of imipramine-like drugs and serotonin uptake blockers on delay of reward in rats. Possible implication in the behavioral mechanism of action of antidepressants. J. Pharmacol. Exp. Ther. 246, 1144-1151 [PubMed] [Google Scholar]
- Bizot J., Le Bihan C., Puech A. J., Hamon M., Thiébot M. (1999). Serotonin and tolerance to delay of reward in rats. Psychopharmacology (Berl.) 146, 400-412 [DOI] [PubMed] [Google Scholar]
- Broos N., Diergaarde L., Schoffelmeer A. N., Pattij T., De Vries T. J. (2012). Trait impulsive choice predicts resistance to extinction and propensity to relapse to cocaine seeking: a bidirectional investigation. Neuropsychopharmacology 37, 1377-1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz J. W., Treadway M. T., Cowan R. L., Woodward N. D., Li R., Ansari M. S., Baldwin R. M., Schwartzman A. N., Shelby E. S., Smith C. E., et al. (2010). Dopaminergic network differences in human impulsivity. Science 329, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cale E. M., Lilienfeld S. O. (2006). Psychopathy factors and risk for aggressive behavior: a test of the “threatened egotism” hypothesis. Law Hum. Behav. 30, 51-74 [DOI] [PubMed] [Google Scholar]
- Cardinal R. N., Pennicott D. R., Sugathapala C. L., Robbins T. W., Everitt B. J. (2001). Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science 292, 2499-2501 [DOI] [PubMed] [Google Scholar]
- Cardona D., López-Crespo G., Sánchez-Amate M. C., Flores P., Sánchez-Santed F. (2011). Impulsivity as long-term sequelae after chlorpyrifos intoxication: time course and individual differences. Neurotox. Res. 19, 128-137 [DOI] [PubMed] [Google Scholar]
- Carmona S., Proal E., Hoekzema E. A., Gispert J. D., Picado M., Moreno I., Soliva J. C., Bielsa A., Rovira M., Hilferty J., et al. (2009). Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attentiondeficit/hyperactivity disorder. Biol. Psychiatry 66, 972-977 [DOI] [PubMed] [Google Scholar]
- Chamberlain S. R., Sahakian B. J. (2007). The neuropsychiatry of impulsivity. Curr. Opin. Psychiatry 20, 255-261 [DOI] [PubMed] [Google Scholar]
- Cheung T. H., Cardinal R. N. (2005). Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neurosci. 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y., Passetti F., Rhodes S. E., Lopian D., Desai A., Robbins T. W. (2003). Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav. Brain Res. 146, 105-119 [DOI] [PubMed] [Google Scholar]
- Cocker P. J., Dinelle K., Kornelson R., Sossi V., Winstanley C. A. (2012). Irrational choice under uncertainty correlates with lower striatal D2/3 receptor binding in rats. J. Neurosci. 32, 15450-15457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. J., Robbins T. W. (1987). Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl.) 91, 458-466 [DOI] [PubMed] [Google Scholar]
- Congdon E., Lesch K. P., Canli T. (2008). Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am. J. Med. Genet. 147B,27-32 [DOI] [PubMed] [Google Scholar]
- Dalley J. W., Roiser J. P. (2012). Dopamine, serotonin and impulsivity. Neuroscience 215, 42-58 [DOI] [PubMed] [Google Scholar]
- Dalley J. W., Fryer T. D., Brichard L., Robinson E. S., Theobald D. E., Lääne K., Pena Y., Murphy E. R., Shah Y., Probst K., et al. (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315, 1267-1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley J. W., Everitt B. J., Robbins T. W. (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron 69, 680-694 [DOI] [PubMed] [Google Scholar]
- Daruma J., Barnes P. (1993). A neurodevelopmental view of impulsivity and its relationship to the superfactors of personality. In The Impulsive Client: Theory, Research and Treatment (ed. McCown W., Johnson J., Shure M.). Washington, DC: American Psychological Association [Google Scholar]
- Davies W., Isles A., Smith R., Karunadasa D., Burrmann D., Humby T., Ojarikre O., Biggin C., Skuse D., Burgoyne P., et al. (2005). Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat. Genet. 37, 625-629 [DOI] [PubMed] [Google Scholar]
- Del Campo N., Chamberlain S. R., Sahakian B. J., Robbins T. W. (2011). The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol. Psychiatry 69, e145-e157 [DOI] [PubMed] [Google Scholar]
- Diergaarde L., Pattij T., Poortvliet I., Hogenboom F., de Vries W., Schoffelmeer A. N., De Vries T. J. (2008). Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol. Psychiatry 63, 301-308 [DOI] [PubMed] [Google Scholar]
- Dilleen R., Pelloux Y., Mar A. C., Molander A., Robbins T. W., Everitt B. J., Dalley J. W., Belin D. (2012). High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology (Berl.) 222, 89-97 [DOI] [PubMed] [Google Scholar]
- Doe C. M., Relkovic D., Garfield A. S., Dalley J. W., Theobald D. E., Humby T., Wilkinson L. S., Isles A. R. (2009). Loss of the imprinted snoRNA mbii-52 leads to increased 5htr2c pre-RNA editing and altered 5HT2CR-mediated behaviour. Hum. Mol. Genet. 18, 2140-2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D. M., Mathias C. W., Marsh D. M., Papageorgiou T. D., Swann A. C., Moeller F. G. (2004). Laboratory measured behavioral impulsivity relates to suicide attempt history. Suicide Life Threat. Behav. 34, 374-385 [DOI] [PubMed] [Google Scholar]
- Duclot F., Hollis F., Darcy M. J., Kabbaj M. (2011). Individual differences in novelty-seeking behavior in rats as a model for psychosocial stress-related mood disorders. Physiol. Behav. 104, 296-305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle D. M., Baunez C. (2010). Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci. Biobehav. Rev. 34, 50-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle D. M., Baunez C., Hutcheson D. M., Lehmann O., Shah A. P., Robbins T. W. (2008). Stop-signal reaction-time task performance: role of prefrontal cortex and subthalamic nucleus. Cereb. Cortex 18, 178-188 [DOI] [PubMed] [Google Scholar]
- Eagle D. M., Wong J. C., Allan M. E., Mar A. C., Theobald D. E., Robbins T. W. (2011). Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J. Neurosci. 31, 7349-7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D., Pelloux Y., Robbins T. W., Dalley J. W., Everitt B. J. (2009). High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol. Psychiatry 65, 851-856 [DOI] [PubMed] [Google Scholar]
- Economidou D., Theobald D. E., Robbins T. W., Everitt B. J., Dalley J. W. (2012). Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology 37, 2057-2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. T., Mackillop J., Modi M., Beauchemin J., Dang D., Lisman S. A., Lum J. K., Wilson D. S. (2007). Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav. Brain Funct. 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche K. D., Turton A. J., Pradhan S., Bullmore E. T., Robbins T. W. (2010). Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol. Psychiatry 68, 770-773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche K. D., Jones P. S., Williams G. B., Turton A. J., Robbins T. W., Bullmore E. T. (2012). Abnormal brain structure implicated in stimulant drug addiction. Science 335, 601-604 [DOI] [PubMed] [Google Scholar]
- Evenden J. L. (1999). Varieties of impulsivity. Psychopharmacology (Berl.) 146, 348-361 [DOI] [PubMed] [Google Scholar]
- Evenden J. L., Ryan C. N. (1996). The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl.) 128, 161-170 [DOI] [PubMed] [Google Scholar]
- Everitt B. J., Robbins T. W. (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481-1489 [DOI] [PubMed] [Google Scholar]
- Everitt B. J., Belin D., Economidou D., Pelloux Y., Dalley J. W., Robbins T. W. (2008). Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3125-3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck H. J., Eysenck S. B. G. (1984). The Manual of the Eysenck Personality Inventory.London, UK: University of London Press [Google Scholar]
- Fernando A. B., Economidou D., Theobald D. E., Zou M. F., Newman A. H., Spoelder M., Caprioli D., Moreno M., Hipólito L., Aspinall A. T., et al. (2012). Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology (Berl.) 219, 341-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel S. B., Robinson T. E., Clark J. J., Clinton S. M., Watson S. J., Seeman P., Phillips P. E., Akil H. (2010). An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology 35, 388-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P. J., Rizos Z., Noble K., Higgins G. A. (2011). Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology 61, 468-477 [DOI] [PubMed] [Google Scholar]
- García-Lecumberri C., Torres I., Martín S., Crespo J. A., Miguéns M., Nicanor C., Higuera-Matas A., Ambrosio E. (2011). Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J. Psychopharmacol. 25, 783-791 [DOI] [PubMed] [Google Scholar]
- Ghahremani D. G., Lee B., Robertson C. L., Tabibnia G., Morgan A. T., De Shetler N., Brown A. K., Monterosso J. R., Aron A. R., Mandelkern M. A., et al. (2012). Striatal dopamine D2/D3 receptors mediate response inhibition and relatedactivity in frontostriatal neural circuitry in humans. J. Neurosci. 32, 7316-7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi O., Piras G., Corda M. G. (2007). The psychogenetically selected Roman high- and low-avoidance rat lines: a model to study the individual vulnerability to drug addiction. Neurosci. Biobehav. Rev. 31, 148-163 [DOI] [PubMed] [Google Scholar]
- Grottick A. J., Higgins G. A. (2000). Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav. Brain Res. 117, 197-208 [DOI] [PubMed] [Google Scholar]
- Gubner N. R., Wilhelm C. J., Phillips T. J., Mitchell S. H. (2010). Strain differences in behavioral inhibition in a Go/No-go task demonstrated using 15 inbred mouse strains. Alcohol. Clin. Exp. Res. 34, 1353-1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. C., Sen S., Deaciuc A., Dwoskin L. P., Kantak K. M. (2011). Methylphenidate treatment in adolescent rats with an attention deficit/hyperactivity disorder phenotype: cocaine addiction vulnerability and dopamine transporter function. Neuropsychopharmacology 36, 837-847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg J. R., Pattij T., Janssen M. C., Ronken E., De Boer S. F., Schoffelmeer A. N., Cuppen E. (2007). Serotonin transporter deficiency in rats improves inhibitory control but not behavioural flexibility. Eur. J. Neurosci. 26, 2066-2073 [DOI] [PubMed] [Google Scholar]
- Hoyle E., Genn R. F., Fernandes C., Stolerman I. P. (2006). Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl.) 189, 211-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibias J., Pellón R. (2011). Schedule-induced polydipsia in the spontaneously hypertensive rat and its relation to impulsive behaviour. Behav. Brain Res. 223, 58-69 [DOI] [PubMed] [Google Scholar]
- Isles A. R., Humby T., Walters E., Wilkinson L. S. (2004). Common genetic effects on variation in impulsivity and activity in mice. J. Neurosci. 24, 6733-6740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertzman S., Grinspan H., Birger M., Kotler M. (2006). Computerized neuropsychological examination of impulsiveness: A selective review. Isr. J. Psychiatry Relat. Sci. 43, 74-80 [PubMed] [Google Scholar]
- Kirby K. N., Petry N. M. (2004). Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction 99, 461-471 [DOI] [PubMed] [Google Scholar]
- Kolokotroni K. Z., Rodgers R. J., Harrison A. A. (2011). Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology (Berl.) 217, 455-473 [DOI] [PubMed] [Google Scholar]
- Lee B., London E. D., Poldrack R. A., Farahi J., Nacca A., Monterosso J. R., Mumford J. A., Bokarius A. V., Dahlbom M., Mukherjee J., et al. (2009). Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependenceand is linked to impulsivity. J. Neurosci. 29, 14734-14740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. J., Glimcher P. W. (2012). The root of all value: a neural common currency for choice. Curr. Opin. Neurobiol. [Epub ahead of print] 10.1016/j.conb.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström M. B., Ryding E., Bosson P., Ahnlide J. A., Rosén I., Träskman-Bendz L. (2004). Impulsivity related to brain serotonin transporter binding capacity in suicide attempters. Eur. Neuropsychopharmacol. 14, 295-300 [DOI] [PubMed] [Google Scholar]
- Liu L., Guan L. L., Chen Y., Ji N., Li H. M., Li Z. H., Qian Q. J., Yang L., Glatt S. J., Faraone S. V., et al. (2011). Association analyses of MAOA in Chinese Han subjects with attention-deficit/hyperactivity disorder: family-based association test, case-control study, and quantitative traits of impulsivity. Am. J. Med. Genet. 156B,737-748 [DOI] [PubMed] [Google Scholar]
- Logan G. D., Schachar R. J., Tannock R. (1997). Impulsivity and inhibitory control. Psychol. Sci. 8, 60-64 [Google Scholar]
- Logue S. F., Swartz R. J., Wehner J. M. (1998). Genetic correlation between performance on an appetitive-signaled nosepoke task and voluntary ethanol consumption. Alcohol. Clin. Exp. Res. 22, 1912-1920 [PubMed] [Google Scholar]
- Lombardo L. E., Bearden C. E., Barrett J., Brumbaugh M. S., Pittman B., Frangou S., Glahn D. C. (2012). Trait impulsivity as an endophenotype for bipolar I disorder. Bipolar Disord. 14, 565-570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loos M., van der Sluis S., Bochdanovits Z., van Zutphen I. J., Pattij T., Stiedl O., Neuro-BSIK Mouse Phenomics consortium. Smit A. B., Spijker S. (2009). Activity and impulsive action are controlled by different genetic and environmental factors. Genes Brain Behav. 8, 817-828 [DOI] [PubMed] [Google Scholar]
- Loos M., Pattij T., Janssen M. C., Counotte D. S., Schoffelmeer A. N., Smit A. B., Spijker S., van Gaalen M. M. (2010). Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb. Cortex 20, 1064-1070 [DOI] [PubMed] [Google Scholar]
- Lovic V., Keen D., Fletcher P. J., Fleming A. S. (2011). Early-life maternal separation and social isolation produce an increase in impulsive action but not impulsive choice. Behav. Neurosci. 125, 481-491 [DOI] [PubMed] [Google Scholar]
- Mar A. C., Walker A. L., Theobald D. E., Eagle D. M., Robbins T. W. (2011). Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J. Neurosci. 31, 6398-6404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich J. A., Bardo M. T. (2009). Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav. Pharmacol. 20, 447-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara R., Dalley J. W., Robbins T. W., Everitt B. J., Belin D. (2010). Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacology (Berl.) 212, 453-464 [DOI] [PubMed] [Google Scholar]
- Mendez I. A., Simon N. W., Hart N., Mitchell M. R., Nation J. R., Wellman P. J., Setlow B. (2010). Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav. Neurosci. 124, 470-477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. H., Wilson A. A., Rusjan P., Clark M., Houle S., Woodside S., Arrowood J., Martin K., Colleton M. (2008). Serotonin2A receptor binding potential in people with aggressive and violent behaviour. J. Psychiatry Neurosci. 33, 499-508 [PMC free article] [PubMed] [Google Scholar]
- Michaelides M., Thanos P. K., Kim R., Cho J., Ananth M., Wang G. J., Volkow N. D. (2012). PET imaging predicts future body weight and cocaine preference. Neuroimage 59, 1508-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F. G., Barratt E. S., Dougherty D. M., Schmitz J. M., Swann A. C. (2001). Psychiatric aspects of impulsivity. Am. J. Psychiatry 158, 1783-1793 [DOI] [PubMed] [Google Scholar]
- Molander A. C., Mar A., Norbury A., Steventon S., Moreno M., Caprioli D., Theobald D. E., Belin D., Everitt B. J., Robbins T. W., et al. (2011). High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology (Berl.) 215, 721-731 [DOI] [PubMed] [Google Scholar]
- Monterosso J., Ainslie G. (1999). Beyond discounting: possible experimental models of impulse control. Psychopharmacology (Berl.) 146, 339-347 [DOI] [PubMed] [Google Scholar]
- Moreno M., Cardona D., Gómez M. J., Sánchez-Santed F., Tobeña A., Fernández-Teruel A., Campa L., Sunol C., Escarabajal M. D., Torres C., et al. (2010). Impulsivity characterization in the Roman high- and low-avoidance rat strains: behavioral and neurochemical differences. Neuropsychopharmacology 35, 1198-1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M., Gutiérrez-Ferre V. E., Ruedas L., Campa L., Suñol C., Flores P. (2012). Poor inhibitory control and neurochemical differences in high compulsive drinker rats selected by schedule-induced polydipsia. Psychopharmacology (Berl.) 219, 661-672 [DOI] [PubMed] [Google Scholar]
- Moreno-López L., Catena A., Fernández-Serrano M. J., Delgado-Rico E., Stamatakis E. A., Pérez-García M., Verdejo-García A. (2012). Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 125, 208-214 [DOI] [PubMed] [Google Scholar]
- Muir J. L., Everitt B. J., Robbins T. W. (1996). The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb. Cortex 6, 470-481 [DOI] [PubMed] [Google Scholar]
- Murphy E. R., Fernando A. B., Urcelay G. P., Robinson E. S., Mar A. C., Theobald D. E., Dalley J. W., Robbins T. W. (2012). Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology (Berl.) 219, 401-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader M. A., Czoty P. W. (2005). PET imaging of dopamine D2 receptors in monkey models of cocaine abuse: genetic predisposition versus environmental modulation. Am. J. Psychiatry 162, 1473-1482 [DOI] [PubMed] [Google Scholar]
- Nader M. A., Morgan D., Gage H. D., Nader S. H., Calhoun T. L., Buchheimer N., Ehrenkaufer R., Mach R. H. (2006). PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat. Neurosci. 9, 1050-1056 [DOI] [PubMed] [Google Scholar]
- Nikiforuk A., Popik P., Drescher K. U., van Gaalen M., Relo A. L., Mezler M., Marek G., Schoemaker H., Gross G., Bespalov A. (2010). Effects of a positive allosteric modulator of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats. J. Pharmacol. Exp. Ther. 335, 665-673 [DOI] [PubMed] [Google Scholar]
- Oberlin B. G., Grahame N. J. (2009). High-alcohol preferring mice are more impulsive than low-alcohol preferring mice as measured in the delay discounting task. Alcohol. Clin. Exp. Res. 33, 1294-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y., Okano M., Masui A., Imaki J., Egawa M., Yoshihara C., Tatara A., Mizuguchi Y., Sasa M., Shimizu S. (2012). Region-specific elevation of D1 receptor-mediated neurotransmission in the nucleus accumbens of SHR, a rat model of attention deficit/hyperactivity disorder. Neuropharmacology 63, 547-554 [DOI] [PubMed] [Google Scholar]
- Oswald L. M., Wong D. F., Zhou Y., Kumar A., Brasic J., Alexander M., Ye W., Kuwabara H., Hilton J., Wand G. S. (2007). Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. Neuroimage 36, 153-166 [DOI] [PubMed] [Google Scholar]
- Paine T. A., Dringenberg H. C., Olmstead M. C. (2003). Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav. Brain Res. 147, 135-147 [DOI] [PubMed] [Google Scholar]
- Paine T. A., Tomasiewicz H. C., Zhang K., Carlezon W. A., Jr(2007). Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol. Psychiatry 62, 687-693 [DOI] [PubMed] [Google Scholar]
- Paterson N. E., Ricciardi J., Wetzler C., Hanania T. (2011). Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and d-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci. Res. 69, 41-50 [DOI] [PubMed] [Google Scholar]
- Pattij T., Vanderschuren L. J. (2008). The neuropharmacology of impulsive behaviour. Trends Pharmacol. Sci. 29, 192-199 [DOI] [PubMed] [Google Scholar]
- Pattij T., Janssen M. C., Schepers I., González-Cuevas G., de Vries T. J., Schoffelmeer A. N. (2007a). Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology (Berl.) 193, 85-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T., Janssen M. C., Vanderschuren L. J., Schoffelmeer A. N., van Gaalen M. M. (2007b). Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shell in inhibitory response control. Psychopharmacology (Berl.) 191, 587-598 [DOI] [PubMed] [Google Scholar]
- Peña-Oliver Y., Buchman V. L., Dalley J. W., Robbins T. W., Schumann G., Ripley T. L., King S. L., Stephens D. N. (2012). Deletion of alpha-synuclein decreases impulsivity in mice. Genes Brain Behav. 11, 137-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. C., Körner A. C. (2011). Impulsive phenomena, the impulsive character (der Triebhafte Charakter) and DSM personality disorders. J. Pers. Disord. 25, 586-606 [DOI] [PubMed] [Google Scholar]
- Perry J. L., Larson E. B., German J. P., Madden G. J., Carroll M. E. (2005). Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl.) 178, 193-201 [DOI] [PubMed] [Google Scholar]
- Perry J. L., Stairs D. J., Bardo M. T. (2008). Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav. Brain Res. 193, 48-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze M. A., Dalley J. W., Robbins T. W. (2007). Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology 32, 273-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza P. V., Deminière J. M., Maccari S., Mormède P., Le Moal M., Simon H. (1990). Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav. Pharmacol. 1, 339-345 [DOI] [PubMed] [Google Scholar]
- Porrino L. J., Daunais J. B., Smith H. R., Nader M. A. (2004). The expanding effects of cocaine: studies in a nonhuman primate model of cocaine self-administration. Neurosci. Biobehav. Rev. 27, 813-820 [DOI] [PubMed] [Google Scholar]
- Pothuizen H. H., Jongen-Rêlo A. L., Feldon J., Yee B. K. (2005). Double dissociation of the effects of selective nucleus accumbens core and shell lesions on impulsive-choice behaviour and salience learning in rats. Eur. J. Neurosci. 22, 2605-2616 [DOI] [PubMed] [Google Scholar]
- Poulos C. X., Le A. D., Parker J. L. (1995). Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav. Pharmacol. 6, 810-814 [PubMed] [Google Scholar]
- Radwanska K., Kaczmarek L. (2012). Characterization of an alcohol addiction-prone phenotype in mice. Addict. Biol. 17, 601-612 [DOI] [PubMed] [Google Scholar]
- Reist C., Mazzanti C., Vu R., Fujimoto K., Goldman D. (2004). Inter-relationships of intermediate phenotypes for serotonin function, impulsivity, and a 5-HT2A candidate allele: His452Tyr. Mol. Psychiatry 9, 871-878 [DOI] [PubMed] [Google Scholar]
- Riccio C. A., Reynolds C. R., Lowe P., Moore J. J. (2002). The continuous performance test: a window on the neural substrates for attention? Arch. Clin. Neuropsychol. 17, 235-272 [PubMed] [Google Scholar]
- Richards J. B., Zhang L., Mitchell S. H., de Wit H. (1999). Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J. Exp. Anal. Behav. 71, 121-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T. W. (2002). The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl.) 163, 362-380 [DOI] [PubMed] [Google Scholar]
- Robinson E. S., Dalley J. W., Theobald D. E., Glennon J. C., Pezze M. A., Murphy E. R., Robbins T. W. (2008a). Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology 33, 2398-2406 [DOI] [PubMed] [Google Scholar]
- Robinson E. S., Eagle D. M., Mar A. C., Bari A., Banerjee G., Jiang X., Dalley J. W., Robbins T. W. (2008b). Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33, 1028-1037 [DOI] [PubMed] [Google Scholar]
- Robinson E. S., Eagle D. M., Economidou D., Theobald D. E., Mar A. C., Murphy E. R., Robbins T. W., Dalley J. W. (2009). Behavioural characterisation of high impulsivity on the 5-choice serial reaction time task: specific deficits in ‘waiting’ versus ‘stopping’. Behav. Brain Res. 196, 310-316 [DOI] [PubMed] [Google Scholar]
- Roesch M. R., Takahashi Y., Gugsa N., Bissonette G. B., Schoenbaum G. (2007). Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J. Neurosci. 27, 245-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R. D., Baunez C., Everitt B. J., Robbins T. W. (2001). Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav. Neurosci. 115, 799-811 [DOI] [PubMed] [Google Scholar]
- Sagvolden T., Xu T. (2008). l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of Attention-Deficit/Hyperactivity Disorder (ADHD). Behav. Brain Funct. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers M. C., Binnekade R., Schoffelmeer A. N., Pattij T., De Vries T. J. (2012). Unidirectional relationship between heroin self-administration and impulsive decision-making in rats. Psychopharmacology (Berl.) 219, 443-452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T., Ilott N., Brolese G., Bizarro L., Asherson P. J., Stolerman I. P. (2011). Prenatal exposure to nicotine impairs performance of the 5-choice serial reaction time task in adult rats. Neuropsychopharmacology 36, 1114-1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T., Bizarro L., Asherson P. J., Stolerman I. P. (2012). Hyperactivity, increased nicotine consumption and impaired performance in the five-choice serial reaction time task in adolescent rats prenatally exposed to nicotine. Psychopharmacology (Berl.) 223, 401-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S., Markou A. (2007). The effects of the mGluR5 antagonist MPEP and the mGluR2/3 antagonist LY341495 on rats' performance in the 5-choice serial reaction time task. Neuropharmacology 52, 863-872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhotina I. A., Dravolina O. A., Novitskaya Y., Zvartau E. E., Danysz W., Bespalov A. Y. (2008). Effects of mGlu1 receptor blockade on working memory, time estimation, and impulsivity in rats. Psychopharmacology (Berl.) 196, 211-220 [DOI] [PubMed] [Google Scholar]
- Sullivan P. F., Daly M. J., O'Donovan M. (2012). Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat. Rev. Genet. 13, 537-551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer M. M., Halder I., Flory J. D., Craig A. E., Gianaros P. J., Ferrell R. E., Manuck S. B. (2012). Polymorphic variation in the dopamine D4 receptor predicts delay discounting as a function of childhood socioeconomic status: evidence for differential susceptibility. Soc. Cogn. Affect. Neurosci. [Epub ahead of print] 10.1093/scan/nss020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szerman N., Peris L., Mesías B., Colis P., Rosa J., Prieto A. (2005). Reboxetine for the treatment of patients with Cocaine Dependence Disorder. Hum. Psychopharmacol. 20, 189-192 [DOI] [PubMed] [Google Scholar]
- Talpos J. C., Wilkinson L. S., Robbins T. W. (2006). A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J. Psychopharmacol. 20, 47-58 [DOI] [PubMed] [Google Scholar]
- Tsutsui-Kimura I., Ohmura Y., Izumi T., Yamaguchi T., Yoshida T., Yoshioka M. (2010a). Endogenous acetylcholine modulates impulsive action via alpha4beta2 nicotinic acetylcholine receptors in rats. Eur. J. Pharmacol. 641, 148-153 [DOI] [PubMed] [Google Scholar]
- Tsutsui-Kimura I., Ohmura Y., Izumi T., Yamaguchi T., Yoshida T., Yoshioka M. (2010b). Nicotine provokes impulsive-like action by stimulating alpha4beta2 nicotinic acetylcholine receptors in the infralimbic, but not in the prelimbic cortex. Psychopharmacology (Berl.) 209, 351-359 [DOI] [PubMed] [Google Scholar]
- Uslaner J. M., Robinson T. E. (2006). Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice – mediation by enhanced incentive motivation? Eur. J. Neurosci. 24, 2345-2354 [DOI] [PubMed] [Google Scholar]
- van den Bergh F. S., Bloemarts E., Chan J. S., Groenink L., Olivier B., Oosting R. S. (2006a). Spontaneously hypertensive rats do not predict symptoms of attention-deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 83, 380-390 [DOI] [PubMed] [Google Scholar]
- van den Bergh F. S., Bloemarts E., Groenink L., Olivier B., Oosting R. S. (2006b). Delay aversion: effects of 7-OH-DPAT, 5-HT1A/1B-receptor stimulation and D-cycloserine. Pharmacol. Biochem. Behav. 85, 736-743 [DOI] [PubMed] [Google Scholar]
- Verdejo-García A., Lawrence A. J., Clark L. (2008). Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 32, 777-810 [DOI] [PubMed] [Google Scholar]
- Wade T. R., de Wit H., Richards J. B. (2000). Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl.) 150, 90-101 [DOI] [PubMed] [Google Scholar]
- Whiteside S. P., Lynam D. R. (2003). Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp. Clin. Psychopharmacol. 11, 210-217 [DOI] [PubMed] [Google Scholar]
- Wilbertz G., van Elst L. T., Delgado M. R., Maier S., Feige B., Philipsen A., Blechert J. (2012). Orbitofrontal reward sensitivity and impulsivity in adult attention deficit hyperactivity disorder. Neuroimage 60, 353-361 [DOI] [PubMed] [Google Scholar]
- Williams J., Taylor E. (2006). The evolution of hyperactivity, impulsivity and cognitive diversity. J. R. Soc. Interface 3, 399-413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T. D., Dunn E. W. (2004). Self-knowledge: its limits, value, and potential for improvement. Annu. Rev. Psychol. 55, 493-518 [DOI] [PubMed] [Google Scholar]
- Winstanley C. A. (2011). The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br. J. Pharmacol. 164, 1301-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C. A., Chudasama Y., Dalley J. W., Theobald D. E., Glennon J. C., Robbins T. W. (2003). Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl.) 167, 304-314 [DOI] [PubMed] [Google Scholar]
- Winstanley C. A., Theobald D. E., Cardinal R. N., Robbins T. W. (2004a). Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J. Neurosci. 24, 4718-4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C. A., Theobald D. E., Dalley J. W., Glennon J. C., Robbins T. W. (2004b). 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl.) 176, 376-385 [DOI] [PubMed] [Google Scholar]
- Winstanley C. A., Theobald D. E., Dalley J. W., Robbins T. W. (2005). Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology 30, 669-682 [DOI] [PubMed] [Google Scholar]
- Winstanley C. A., Bachtell R. K., Theobald D. E., Laali S., Green T. A., Kumar A., Chakravarty S., Self D. W., Nestler E. J. (2009). Increased impulsivity during withdrawal from cocaine self-administration: role for DeltaFosB in the orbitofrontalcortex. Cereb. Cortex 19, 435-444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J., Schetters D., van Es I. E., van Mourik Y., den Hollander B. R., Schoffelmeer A. N., Pattij T. (2011). μ-Opioid receptors in the nucleus accumbens shell region mediate the effects of amphetamine on inhibitory control but not impulsive choice. J. Neurosci. 31, 262-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womersley J. S., Hsieh J. H., Kellaway L. A., Gerhardt G. A., Russell V. A. (2011). Maternal separation affects dopamine transporter function in the spontaneously hypertensive rat: an in vivo electrochemical study. Behav. Brain Funct. 7, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters T. E., Bardo M. T. (2011). Methylphenidate and fluphenazine, but not amphetamine, differentially affect impulsive choice in spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rats. Brain Res. 1396, 45-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. R., Marusich J. A., Gipson C. D., Beckmann J. S., Bardo M. T. (2012). High impulsivity in rats predicts amphetamine conditioned place preference. Pharmacol. Biochem. Behav. 100, 370-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Apparsundaram S., Bardo M. T., Dwoskin L. P. (2005). Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J. Neurochem. 93, 1434-1443 [DOI] [PubMed] [Google Scholar]