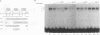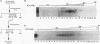Abstract
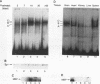
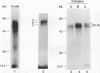
Transcription of the myelin basic protein (MBP) gene is regulated in a cell-type-specific and developmental stage-specific manner during myelin formation in the murine central nervous system. The 5'-flanking region of the MBP gene contains several regulatory elements that differentially contribute to the cell-type-specific transcription of MBP in cells derived from the central nervous system. The proximal element, termed MB1, which is located between nucleotides -14 and -50 with respect to the RNA start site, has previously been shown to have characteristics of a cell-type-specific enhancer element. In this study, we used band shift and UV cross-linking assays to identify DNA-binding proteins in mouse brain nuclear extract which interact with the MB1 element. Fractionation of these extracts has allowed the identification of a 38- to 41-kDa nuclear protein, derived from mouse brain tissue at the peak of myelination, which specifically binds the MB1 DNA sequence. Fractions enriched in the MB1-binding protein have been shown to stimulate transcription of the MBP promoter in extract derived from HeLa cells. MB1 binding protein activity is expressed in a tissue-specific and development stage-specific pattern which coincides with the pattern of MBP transcription, suggesting that this protein may be a biologically relevant transcription factor for the MBP gene in vivo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Rappaport J., Tada H., Kerr D., Khalili K. A nuclear protein derived from brain cells stimulates transcription of the human neurotropic virus promoter, JCVE, in vitro. J Biol Chem. 1990 Aug 15;265(23):13899–13905. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briggs M. R., Kadonaga J. T., Bell S. P., Tjian R. Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 1986 Oct 3;234(4772):47–52. doi: 10.1126/science.3529394. [DOI] [PubMed] [Google Scholar]
- Campagnoni A. T., Macklin W. B. Cellular and molecular aspects of myelin protein gene expression. Mol Neurobiol. 1988 Spring;2(1):41–89. doi: 10.1007/BF02935632. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davison B. L., Egly J. M., Mulvihill E. R., Chambon P. Formation of stable preinitiation complexes between eukaryotic class B transcription factors and promoter sequences. Nature. 1983 Feb 24;301(5902):680–686. doi: 10.1038/301680a0. [DOI] [PubMed] [Google Scholar]
- Deschamps J., Meijlink F. Mammalian homeobox genes in normal development and neoplasia. Crit Rev Oncog. 1992;3(1-2):117–173. [PubMed] [Google Scholar]
- Devine-Beach K., Haas S., Khalili K. Analysis of the proximal transcriptional element of the myelin basic protein gene. Nucleic Acids Res. 1992 Feb 11;20(3):545–550. doi: 10.1093/nar/20.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine-Beach K., Lashgari M. S., Khalili K. Myelin basic protein gene transcription. Identification of proximal and distal cis-acting regulatory elements. J Biol Chem. 1990 Aug 15;265(23):13830–13835. [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Gruss P., Walther C. Pax in development. Cell. 1992 May 29;69(5):719–722. doi: 10.1016/0092-8674(92)90281-g. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hoffman A., Sinn E., Yamamoto T., Wang J., Roy A., Horikoshi M., Roeder R. G. Highly conserved core domain and unique N terminus with presumptive regulatory motifs in a human TATA factor (TFIID). Nature. 1990 Jul 26;346(6282):387–390. doi: 10.1038/346387a0. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Inoue T., Tamura T., Furuichi T., Mikoshiba K. Isolation of complementary DNAs encoding a cerebellum-enriched nuclear factor I family that activates transcription from the mouse myelin basic protein promoter. J Biol Chem. 1990 Nov 5;265(31):19065–19070. [PubMed] [Google Scholar]
- Jackson S. P., Tjian R. Purification and analysis of RNA polymerase II transcription factors by using wheat germ agglutinin affinity chromatography. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1781–1785. doi: 10.1073/pnas.86.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. F., Pickett S. C., Barker D. L. Autoradiography using storage phosphor technology. Electrophoresis. 1990 May;11(5):355–360. doi: 10.1002/elps.1150110503. [DOI] [PubMed] [Google Scholar]
- Kamholz J., Toffenetti J., Lazzarini R. A. Organization and expression of the human myelin basic protein gene. J Neurosci Res. 1988 Sep;21(1):62–70. doi: 10.1002/jnr.490210110. [DOI] [PubMed] [Google Scholar]
- Kristensson K., Zeller N. K., Dubois-Dalcq M. E., Lazzarini R. A. Expression of myelin basic protein gene in the developing rat brain as revealed by in situ hybridization. J Histochem Cytochem. 1986 Apr;34(4):467–473. doi: 10.1177/34.4.2419396. [DOI] [PubMed] [Google Scholar]
- Lashgari M. S., Devine-Beach K., Haas S., Khalili K. Nuclear proteins in mouse brain cells bind specifically to the myelin basic protein regulatory region. J Clin Invest. 1990 Nov;86(5):1671–1677. doi: 10.1172/JCI114890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Olson E. N. Regulation of muscle cell growth and differentiation by the MyoD family of helix-loop-helix proteins. Adv Cancer Res. 1992;58:95–119. doi: 10.1016/s0065-230x(08)60292-4. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. J., Sutcliffe J. G. Gene expression in rat brain. Nucleic Acids Res. 1983 Aug 25;11(16):5497–5520. doi: 10.1093/nar/11.16.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Miura M., Tamura T., Aoyama A., Mikoshiba K. The promoter elements of the mouse myelin basic protein gene function efficiently in NG108-15 neuronal/glial cells. Gene. 1989 Jan 30;75(1):31–38. doi: 10.1016/0378-1119(89)90380-6. [DOI] [PubMed] [Google Scholar]
- Newman S., Kitamura K., Campagnoni A. T. Identification of a cDNA coding for a fifth form of myelin basic protein in mouse. Proc Natl Acad Sci U S A. 1987 Feb;84(3):886–890. doi: 10.1073/pnas.84.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach A., Takahashi N., Pravtcheva D., Ruddle F., Hood L. Chromosomal mapping of mouse myelin basic protein gene and structure and transcription of the partially deleted gene in shiverer mutant mice. Cell. 1985 Aug;42(1):149–155. doi: 10.1016/s0092-8674(85)80110-0. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Saffer J. D., Jackson S. P., Annarella M. B. Developmental expression of Sp1 in the mouse. Mol Cell Biol. 1991 Apr;11(4):2189–2199. doi: 10.1128/mcb.11.4.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp Z. D., Cao Z. Regulation of cell-type-specific transcription and differentiation of the pituitary. Bioessays. 1990 Feb;12(2):80–85. doi: 10.1002/bies.950120206. [DOI] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Sorg B. A., Smith M. M., Campagnoni A. T. Developmental expression of the myelin proteolipid protein and basic protein mRNAs in normal and dysmyelinating mutant mice. J Neurochem. 1987 Oct;49(4):1146–1154. doi: 10.1111/j.1471-4159.1987.tb10005.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Roach A., Teplow D. B., Prusiner S. B., Hood L. Cloning and characterization of the myelin basic protein gene from mouse: one gene can encode both 14 kd and 18.5 kd MBPs by alternate use of exons. Cell. 1985 Aug;42(1):139–148. doi: 10.1016/s0092-8674(85)80109-4. [DOI] [PubMed] [Google Scholar]
- Tamura T., Aoyama A., Inoue T., Miura M., Okano H., Mikoshiba K. Tissue-specific in vitro transcription from the mouse myelin basic protein promoter. Mol Cell Biol. 1989 Jul;9(7):3122–3126. doi: 10.1128/mcb.9.7.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Mikoshiba K. Demonstration of a transcription element in vitro between the capping site and translation initiation site of the mouse myelin basic protein gene. FEBS Lett. 1991 Mar 11;280(1):75–78. doi: 10.1016/0014-5793(91)80207-j. [DOI] [PubMed] [Google Scholar]
- Tamura T., Sumita K., Fujino I., Aoyama A., Horikoshi M., Hoffmann A., Roeder R. G., Muramatsu M., Mikoshiba K. Striking homology of the 'variable' N-terminal as well as the 'conserved core' domains of the mouse and human TATA-factors (TFIID). Nucleic Acids Res. 1991 Jul 25;19(14):3861–3865. doi: 10.1093/nar/19.14.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Sumita K., Hirose S., Mikoshiba K. Core promoter of the mouse myelin basic protein gene governs brain-specific transcription in vitro. EMBO J. 1990 Oct;9(10):3101–3108. doi: 10.1002/j.1460-2075.1990.tb07507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Sumita K., Mikoshiba K. Sequences involved in brain-specific in vitro transcription from the core promoter of the mouse myelin basic protein gene. Biochim Biophys Acta. 1991 Dec 2;1129(1):83–86. doi: 10.1016/0167-4781(91)90215-8. [DOI] [PubMed] [Google Scholar]
- Verity A. N., Campagnoni A. T. Regional expression of myelin protein genes in the developing mouse brain: in situ hybridization studies. J Neurosci Res. 1988 Oct-Dec;21(2-4):238–248. doi: 10.1002/jnr.490210216. [DOI] [PubMed] [Google Scholar]
- Wildeman A. G., Sassone-Corsi P., Grundström T., Zenke M., Chambon P. Stimulation of in vitro transcription from the SV40 early promoter by the enhancer involves a specific trans-acting factor. EMBO J. 1984 Dec 20;3(13):3129–3133. doi: 10.1002/j.1460-2075.1984.tb02269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman A. G., Zenke M., Schatz C., Wintzerith M., Grundström T., Matthes H., Takahashi K., Chambon P. Specific protein binding to the simian virus 40 enhancer in vitro. Mol Cell Biol. 1986 Jun;6(6):2098–2105. doi: 10.1128/mcb.6.6.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon L. I., Youssoufian H., Mather C., Lodish H. F., Orkin S. H. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferra F., Engh H., Hudson L., Kamholz J., Puckett C., Molineaux S., Lazzarini R. A. Alternative splicing accounts for the four forms of myelin basic protein. Cell. 1985 Dec;43(3 Pt 2):721–727. doi: 10.1016/0092-8674(85)90245-4. [DOI] [PubMed] [Google Scholar]