SUMMARY
The pathology of sudden infant death syndrome (SIDS) is poorly understood. Many risk factors, including hypoxia, have been identified. Prolongation of the ECG QTc interval is associated with elevated risk of SIDS but its aetiology in most cases remains unknown. We have characterised ECG changes in the newborn mouse in the hours and days following birth. There was a steady increase in heart rate alongside significant decreases in QTc interval, QRS duration and QTc dispersion over the first 10 postnatal days. Birth into hypoxia (10% FiO2) prevented electrocardiac maturation, downregulated cardiac ion-channel expression and led to neonatal death. We found that risk of death decreased with increasing age of exposure to hypoxia. Genetic elevation of cardiac hypoxia-signalling after birth in αMHC-Cre::VHLfl/fl mice also prevented electrocardiographic maturation, leading to arrhythmia and death before weaning. Immunohistochemistry and western blotting revealed internalisation and dephosphorylation of Connexin43. We conclude that increased ambient oxygen concentration after birth drives maturation of the cardiac electrical conduction system, failure of which leads to aberrant ion channel and Connexin43 expression and predisposes to arrhythmia and sudden death. This is consistent with known risk factors of SIDS and provides a link between neonatal hypoxia, ECG abnormalities and sudden death.
INTRODUCTION
Sudden infant death syndrome (SIDS) is death of an infant that is neither attributable to medical history nor explained after autopsy or by death scene investigation. SIDS is the leading cause of death in the first year of life after the neonatal period and is currently responsible for 0.53 deaths per 1000 infants. High incidence, catastrophic impact on affected families and absence of mechanistic insight means that SIDS represents a major medical challenge. Since the ‘back to sleep’ campaign in 1994, there have been no further reductions in SIDS incidence. A number of causative mechanisms have been proposed to lead to SIDS, but without any unifying theory or correlation with pathological findings (Goldwater, 2011).
A study of 33,034 infants found that 50% of infants who died of SIDS had a prolonged QTc interval in the first week of life (Schwartz et al., 1998). Approximately 10% of SIDS cases carry functionally significant genetic variants in sodium and potassium channels causing long QT (Arnestad et al., 2007), or variants in the gap junction protein Connexin43 (Cx43) (Van Norstrand et al., 2012). This circumstantial evidence suggests a role for abnormal electrical conduction in SIDS, but the underlying cause(s) in the vast majority of cases remains unexplained.
Most risk factors for SIDS, including prone sleeping position, respiratory disorders and high altitude, are associated with a reduced oxygen environment. Furthermore, hypoxia is associated with a prolonged QT interval in the adult (Roche et al., 2003; Tirlapur and Mir, 1982). We therefore hypothesised that neonatal hypoxia leading to abnormal electrical conduction is a potential cause of sudden death.
We used non-invasive electrocardiography to characterize the postnatal maturation of the cardiac electrical conduction system in neonatal mice (Chu et al., 2001). We investigated whether reduced ambient oxygen environment or genetically manipulated hypoxic signalling affected maturation of the cardiac electrical conduction system and the subsequent risk of sudden death.
RESULTS
Maturation of ECG morphology in wild-type mice
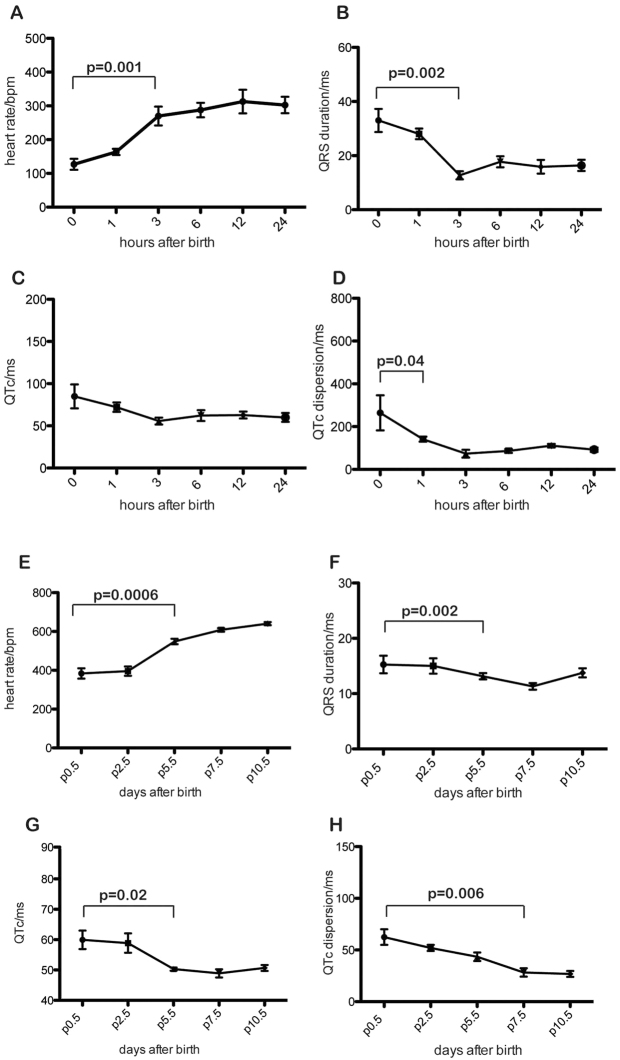
To assess electrocardiographic changes immediately following birth, unborn pups were removed from pregnant females at embryonic day (E)18.5 and placed with a foster mother. We performed electrocardiography in the same pups sequentially at 0, 1, 3, 6, 12 and 24 hours after birth. ECG morphology changes were detectable at 1 hour after birth, becoming significant at 3 hours. Heart rate increased whereas QRS, QTc and QTc dispersion (where ‘c’ denotes correction for heart rate) declined rapidly and then plateaued over the 24 hours (Fig. 1). We recorded resting ECGs from postnatal day (P) 0.5 to P10 (Fig. 1). The trends of increased heart rate and declining QRS, QTc and QTc dispersion continued over this timescale (Fig. 1).
Fig. 1.
Postnatal maturation of cardiac electrical conduction. (A-H) Sequential ECG in a cohort of neonatal mice revealed increase in heart rate (A), decrease in QRS duration (B), QTc (C) and QT dispersion (D) in the hours after birth. P values in A, B and D indicate the first point at which parameters become significantly different from immediate postnatal values (two-tailed t-test; n=8). Error bars show s.e.m. The trends noted in the first 24 hours continued over the 10 days following birth (E,F,G,H).
Hypoxia prevents maturation of the ECG
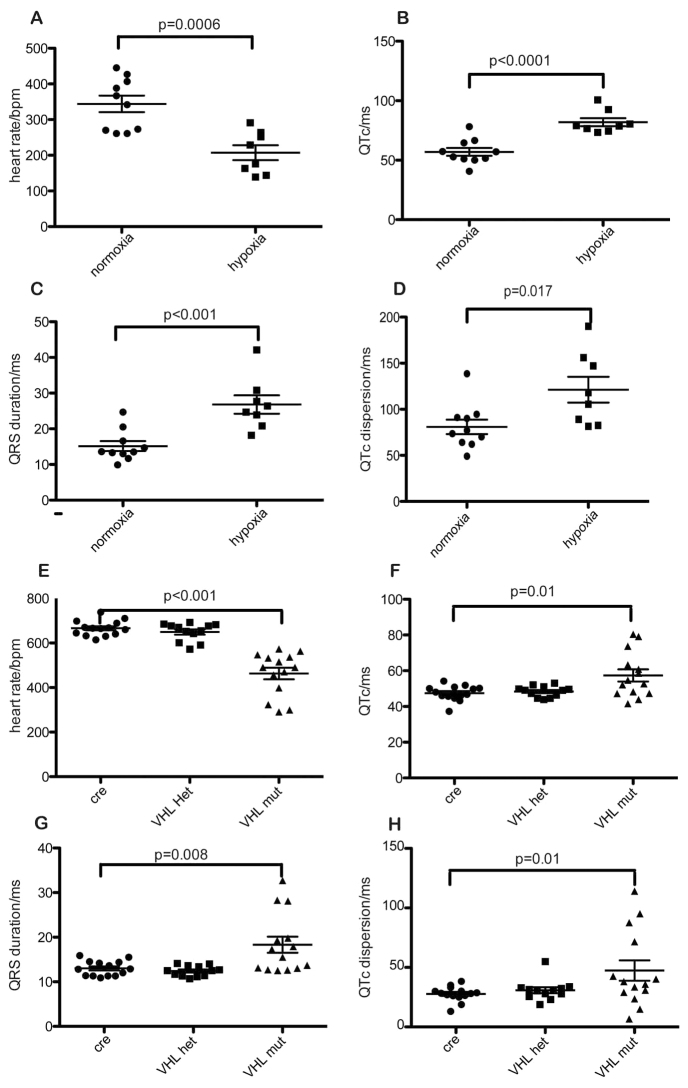
Neonates reared in 10% oxygen for 24 hours showed reduced heart rate and increased QTc and QTc dispersion compared with normoxic controls (Fig. 2). These parameters were similar to those in newborn neonates.
Fig. 2.
Rearing in hypoxia or activation of cardiac HIF signalling retards postnatal maturation of cardiac conduction. (A-H) Wild-type neonates reared in 10% oxygen for the first 24 hours after birth show decreased heart rate (A) and increased QTc (B), QRS duration (C) and QTc dispersion (D) compared with normoxic controls (n=9). A similar trend was noted in αMHC-Cre::VHLfl/fl mice (VHL-mut), which upregulate cardiac HIF signalling constitutively, but not in heterozygote αMHC-Cre::VHLfl/+ (VHL-het) or αMHC-Cre littermates (cre) (E,F,G,H). Means ± s.e.m. are shown.
αMHC-Cre::VHLfl/fl mice exhibit immature ECG morphology and sudden death
αMHC-Cre::VHLfl/fl mice have cardiac-specific deletion of von Hippel-Lindau protein (VHL), causing constitutive upregulation of cardiac hypoxia inducible factor (HIF) signalling. Their neonates showed decreased heart rate with increased QRS, QTc and QTc dispersion compared with control αMHC-Cre::VHL+/+or αMHC-Cre::VHLfl/+littermates at 10 days after birth (Fig. 2). αMHC-Cre::VHLfl/fl mice died between P16 and P18. Before death, there were no observable differences between mutant and control littermates in behaviour or weight. αMHC-Cre::VHLfl/fl mice exhibited frequent cardiac arrhythmia, consistent with sudden cardiac death, as did hypoxic wild-type mice (Fig. 3).
Fig. 3.
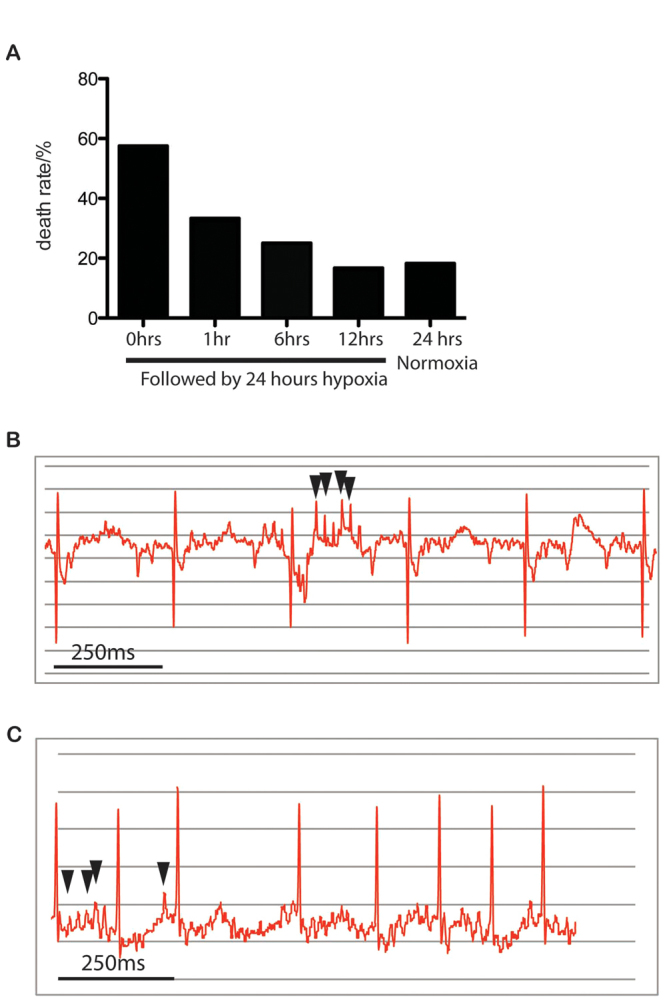
Postnatal exposure to hypoxia leads to cardiac arrhythmia and sudden death in neonatal mice. (A-C) 24-hour incubation in 10% oxygen with a foster mother caused sudden death in wild-type F1(CBA/Ca × C57BL/10) mice. Death rates were reduced by longer times in normoxia before hypoxia (A), implying decreased sensitivity to hypoxia with time. Cardiac arrhythmia is apparent on the ECG of P1 mice reared in hypoxia for 24 hours (B) and P10.5 αMHC-Cre::VHLfl/fl mice (C). Arrows indicate ectopic beats.
TRANSLATIONAL IMPACT.
Clinical issue
Sudden infant death syndrome (SIDS) remains one of the major enigmas in modern medicine. The ‘back to sleep’ campaign in 1994, which encouraged parents to place infants on their backs to sleep, promoted a reduction in SIDS incidence from 2 to 0.53 infants per 1000 births. Since then, there has been no reduction in this figure. Thus, advice for parents remains limited, and tragedies that might be preventable continue to occur. It has been previously documented that ∼50% of infants that die from SIDS display a prolonged QTc interval in the first few weeks of life, but the mechanisms underlying this observation have been elusive (except in cases where rare channelopathies and other genetic abnormalities are present).
Results
In this study, the authors addressed this issue using a recent innovation in electrocardiography that allows non-invasive recording of the electrocardiogram (ECG) in mice. This enabled the first reported catalogue of ECG changes from birth to 10 days postnatally, measuring changes in heart rate, QTc interval and QRS duration. By altering ambient oxygen concentration or genetically manipulating cellular hypoxic signalling in neonatal mice, the authors show that an increase in ambient oxygen concentration after birth is important for driving maturation of cardiac electrical conduction. Reduced oxygen predisposed mice to arrhythmia and sudden death, which was associated with ECG abnormalities. At the cellular level, reduced oxygen caused aberrant gap junction phosphorylation and distribution, and misexpression of ion channels, in the heart. These findings are consistent with known risk factors of SIDS – such as head covering, high altitude, respiratory infections, central nervous system abnormalities and the prone sleeping position – all of which are directly or indirectly associated with a hypoxic environment.
Implications and future directions
This study provides a link between neonatal hypoxia, ECG abnormalities and sudden death, which might provide an explanation for many SIDS cases. The results support the use of regular ECG screening of infants, and subsequent close monitoring of infants displaying long QTc interval, as well as ensuring a well-ventilated environment in cots and the use of other hypoxia-prevention strategies. The mouse models used in this study will facilitate further investigation into SIDS, and the non-invasive ECG approach used here can be applied by other researchers investigating cardiac conduction defects in mice.
Risk of sudden death on exposure to hypoxia decreases with age in neonates
We hypothesised that postnatal electrocardiac maturation is oxygen dependent and that exposure of neonatal mice to hypoxia at later points after birth would result in lower rates of sudden death. When neonates were raised from birth in a hypoxic environment for 24 hours, mortality was 58%. When neonates were raised from birth in normoxia for 1, 6 and 12 hours, then placed into 10% hypoxia for 24 hours, mortality was reduced to 33, 25 and 17%, respectively (Fig. 3).
Connexin43 distribution and quantification
Cx43 is essential for normal electrical conduction in the heart; cardiac-restricted inactivation of Cx43 leads to slower ventricular conduction and lethal arrhythmias in mice (Gutstein et al., 2001). We therefore performed immunohistochemistry to investigate left ventricular distribution of Cx43 in neonatal mice reared in 10% oxygen, in αMHC-Cre::VHLfl/fl mice and in normoxic controls. We found no difference between hypoxic mice and controls in Cx43 distribution and quantification (data not shown). In αMHC-Cre::VHLfl/fl mice, Cx43 was observed in intracellular aggregates rather than at the cell membrane (Fig. 4). Western blots using antibodies to phosphorylated and non-phosphorylated Cx43 revealed that total Cx43 was unaltered, whereas the presence of phosphorylated Cx43, thought to be targeted to the plasma membrane (Solan and Lampe, 2007), was nearly undetectable (Fig. 4).
Fig. 4.
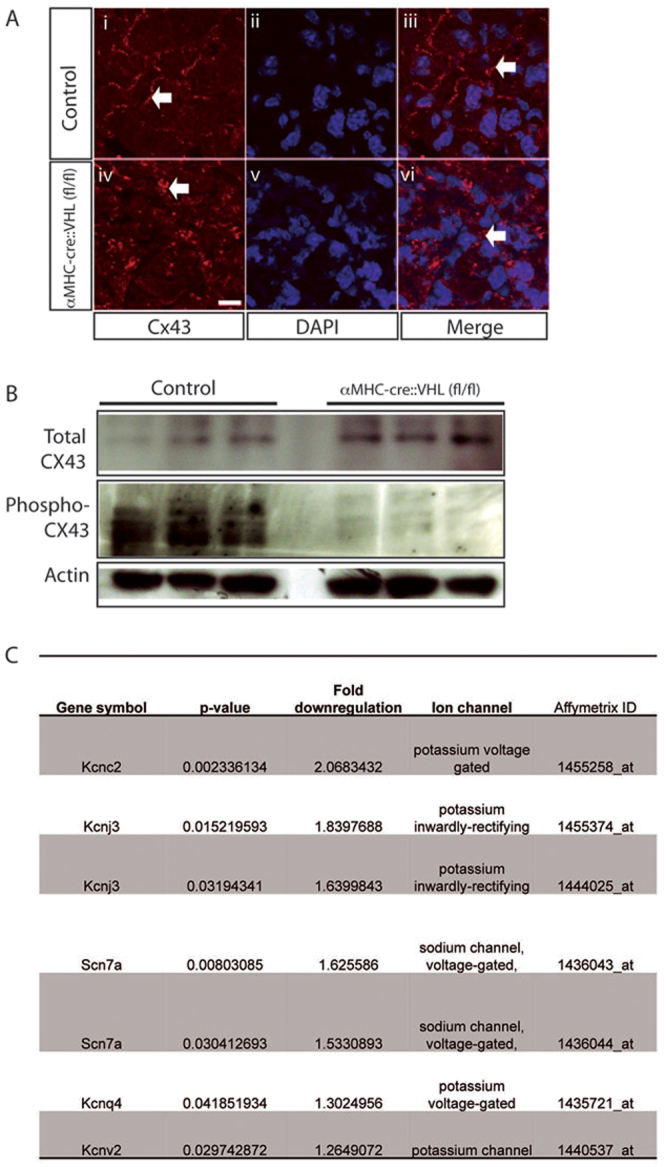
Connexin43 misregulation in αMHC-Cre::VHLfl/fl mice. (A) Immunohistochemical analysis of total Cx43 revealed predominantly cell membrane Cx43 (arrows) in αMHC-Cre control mice (Ai-Aiii). In αMHC-Cre::VHLfl/fl mice, Cx43 is predominantly internal (arrows) (Aiv-Avi). Scale bar: 10 mm. (B) Western blotting of protein extract revealed no overall decrease in total Cx43 protein but almost total Cx43 dephosphorylation in αMHC-Cre::VHLfl/fl hearts. (C) Affymetrix analysis of RNA expression revealed downregulation of several genes encoding ion channels in 24-hour-old mice reared in 10% oxygen compared with controls.
Ion channel expression
Microarray gene expression analysis showed reduced expression of several cardiac ion channels (potassium channels, potassium inwardly rectifying channels and sodium channels) in neonates reared in 10% oxygen for 24 hours compared with normoxic controls (Fig. 4).
DISCUSSION
We describe, for the first time, oxygen-dependent maturation of cardiac conduction in the mouse over the hours and days following birth. Increased postnatal heart rate and decreased QRS duration, QTc interval and QT dispersion during the first postnatal week are dependent on downregulation of hypoxia signalling in the heart. Elevation of neonatal cardiac hypoxia signalling leads to arrhythmia and sudden death. This in turn suggests a previously unknown mechanism for SIDS pathogenesis. Our results link hypoxia, a major risk factor for SIDS, with several genetic mutations found in SIDS victims (Arnestad et al., 2007; Van Norstrand et al., 2012).
The link between prolonged QT interval and risk of SIDS has been firmly established (Schwartz et al., 1998); however, with the exception of genetic variations in ion channels (Arnestad et al., 2007), the root of prolonged QT remains unknown in most SIDS cases. In some studies of human SIDS, no correlation has been made with QT prolongation. This might reflect the relatively poor sensitivity of surface ECGs to detect changes in QT interval, the age of testing or the use of small cohorts. Our mouse models suggest that hypoxia could be an important precipitant of prolonged QT, and thus sudden death, by hypoxia-induced downregulation of ion channels and Cx43 dephosphorylation. Indeed, hypoxia, prolonged QT interval and risk of lethal cardiac arrhythmias are causally linked in adults (Roche et al., 2003; Tirlapur and Mir, 1982). It is unclear whether hypoxia alone can be causal in human SIDS cases, as in our models, or whether it exacerbates underlying genetic variations and is additive with other SIDS risk factors.
We found overall levels of Cx43 to be unaltered in mice with constitutively elevated cardiac HIF signalling, but a significant reduction in membrane targeting consistent with Cx43 dephosphorylation (Fig. 4), which has been reported in adult hypoxic myocardium (Beardslee et al., 2000). We also found significant downregulation of potassium, sodium and calcium channels when neonates were raised in hypoxia (Fig. 4). In our current study, dead pups were rapidly eaten by the dam, so the quality of tissue available for autopsy was poor. It will be important to analyse the cause of death by ECG telemetry and immediate autopsy, to compare with human pathological findings in SIDS (Goldwater, 2011).
Sensitivity to myocardial hypoxia decreases with time after birth, with risk of death declining with age of exposure to hypoxia (Fig. 3). It is not definitively known how the timescale of postnatal development in mice relates to that of humans, but it is well documented that sensitivity to SIDS in humans decreases 4 months after birth. Interestingly, this is when the QTc interval is known to peak in humans (Schaffer et al., 1991), whereas mice display unidirectional change (Fig. 1). We propose a ‘ratchet’ effect whereby oxygen causes maturation of the electrical conduction system, with declining ability to revert to immature phenotype with increasing age. These pathological cardiac changes could represent a predisposition to cardiac death and might themselves be serious enough to lead to death (as in our model), or be lethal in combination with other risk factors such as brain-stem malfunction.
The discrepancies between hypoxia-reared neonates and αMHC-Cre::VHLfl/+ mice might be due to a dosage effect of HIF signalling. It could be that, at 10% FiO2, neonatal cardiac HIF signalling is not maximally upregulated, whereas it is in VHL deletion. Systemic effects of generalised hypoxia, such as increased sympathetic activation, might also contribute to the slightly differing phenotypes.
In summary, we propose a model that links neonatal hypoxia with sudden death by cardiac arrhythmia by misregulation of cardiac Cx43 and ion channels. Our model is consistent with existing theories of SIDS pathogenesis and links hypoxia, the major known risk factor for SIDS, with many of the candidate genes for pathogenesis. Our electrocardiographic characterisation in the developing neonatal mouse serves as a benchmark for future studies and we believe that the neonatal hypoxic model and the αMHC-Cre::VHLfl/flmouse will facilitate further investigation into SIDS. The lack of validated animal models of SIDS is puzzling given that this is a large clinical problem with little mechanistic insight at the moment. We feel that our study adds further evidence to prompt the use of regular ECG screening of infants and subsequent close-monitoring of those infants displaying long QTc interval to ensure a well-ventilated environment in the infant's cot and the use of other strategies to prevent hypoxia.
MATERIALS AND METHODS
Animal husbandry
All studies were performed in accordance with the Home Office Animal Procedures Act (1986) and guidelines established by the European Convention for the Protection of Laboratory Animals. In studies characterising ECG maturation in the hours after birth and in hypoxic studies, embryonic F1(CBA/Ca × C57BL/10) mice were removed and fostered at E18.5 onto a Parkes mouse who had littered the previous day. We were able to distinguish the fostered mice by the black colouration of the eyes in the F1(CBA/Ca × C57BL/10) pups compared with unpigmented eyes of the Parkes strain pups. The transgenic mouse strain, αMHC Cre+::VHLfl/fl,was created by crossing transgenic mice with a floxed VHL allele (Haase, 2005) with mice containing Cre driven by the α-myosin heavy chain promoter (αMHC Cre), resulting in cardiac specificity (Agah et al., 1997). PCR amplification was performed on tail-derived genomic DNA to determine genotype.
Electrocardiography
ECGs were recorded non-invasively in conscious mice using the ECGenie system (Mouse Specifics). Data acquisition was carried out using the program LabChart 6 (ADInstruments). Analysis of individual ECG signals was then performed using e-MOUSE physiologic waveform analysis software (Mouse Specifics) as described (Chu et al., 2001). In this system, ECG recordings are assessed by the user before being analysed by automated algorithms; signals which contain too much noise or incorrectly called waveforms are removed. All data were obtained during daylight hours, when the mouse heart rate is more stable than during the more active nocturnal hours. In evaluating waveforms and intervals, the end of the T wave was determined as the return of the signal to the isoelectric line as previously described (Chu et al., 2001). QTc was calculated according to Bazett's formula modified specifically for mice (Mitchell et al., 1998), i.e. QTc=QT0/[(RR0/100)1/2].
Connexin43 studies
Hearts from embryos and neonatal mice were dissected in cold PBS and immediately snap-frozen in liquid nitrogen. Immunohistochemistry and western blotting for Cx43 was performed as described (Breckenridge et al., 2009). Anti-Connexin43 (Zymed) and anti-phosphorylated-Connexin43 (Invitrogen) were diluted 1:200 for histology. An immunoblot for actin protein (anti-actin antibody; Sigma-Aldrich) was used as a control for equal protein loading in western blotting. All antibodies including secondary antibodies (Sigma-Aldrich) were diluted 1:5000. Full details of immunochemistry and western blotting procedures are available on request.
Ion channel gene expression
RNA extraction and microarray analysis was performed on hearts as previously described (Bozdech et al., 2003). Briefly, the specimen was placed in 1 ml of TRIzol reagent and homogenised using glass homogenisers and plungers (Uniform, Jencoms, England). Chloroform (200 μl)was added, samples mixed by vortex and left at room temperature for 5 minutes. The tubes were then centrifuged at 13,000 r.p.m. for 15 minutes at 4°C. The aqueous phase was then transferred to a new centrifuge tube and 500 μl of chilled isopropanol added and mixed by vortex. After incubation at room temperature for 20 minutes, tubes were centrifuged at 13,000 r.p.m. for 30 minutes at 4°C. The supernatant was discarded and 500 μl of ice cold 70% ethanol (v/v) added to the residual pellet. Tubes were vortexed before centrifuging at 8000 r.p.m. for 5 minutes at 4°C. The supernatant was discarded and the pellets air-dried. The resulting RNA was resuspended in 50 μl of nuclease-free water (Ambion, Huntingdon, UK) and frozen at −80°C until use. RNA cleanup was carried out on samples prior to microarray analysis, followed by assessment of RNA yield and purity (full details available on request).
Labelled RNA was hybridised to the mouse 430Plus 2.0 chip (Affymetrix) (full details available on request) and the raw data analysed using GeneSpring software version 11.0 (Silicon Genetics/Agilent Technologies). Gene lists were quality filtered to remove genes with expression levels below background and limited to report genes that changed by 1.5-fold or greater with a significance of P<0.05 according to an unpaired t-test.
Acknowledgments
FUNDING: This work was funded by the Medical Research Council to T.M. [grant number U117562103].
Footnotes
COMPETING INTERESTS:The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS:M.T.N., R.A.B. and T.J.M. conceived and designed the experiments. M.T.N. performed the experiments. M.T.N. analysed the data. M.T.N. and R.A.B. wrote the paper. T.J.M. edited the paper.
References
- Agah R., Frenkel P. A., French B. A., Michael L. H., Overbeek P. A., Schneider M. D. (1997). Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J. Clin. Invest. 100, 169-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnestad M., Crotti L., Rognum T. O., Insolia R., Pedrazzini M., Ferrandi C., Vege A., Wang D. W., Rhodes T. E., George A. L., Jr, et al. (2007). Prevalence of long-QT syndrome gene variants in sudden infant death syndrome. Circulation 115, 361-367 [DOI] [PubMed] [Google Scholar]
- Beardslee M. A., Lerner D. L., Tadros P. N., Laing J. G., Beyer E. C., Yamada K. A., Kléber A. G., Schuessler R. B., Saffitz J. E. (2000). Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ. Res. 87, 656-662 [DOI] [PubMed] [Google Scholar]
- Bozdech Z., Llinás M., Pulliam B. L., Wong E. D., Zhu J., DeRisi J. L. (2003). The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge R. A., Zuberi Z., Gomes J., Orford R., Dupays L., Felkin L. E., Clark J. E., Magee A. I., Ehler E., Birks E. J., et al. (2009). Overexpression of the transcription factor Hand1 causes predisposition towards arrhythmia in mice. J. Mol. Cell. Cardiol. 47, 133-141 [DOI] [PubMed] [Google Scholar]
- Chu V., Otero J. M., Lopez O., Morgan J. P., Amende I., Hampton T. G. (2001). Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater P. N. (2011). A perspective on SIDS pathogenesis. the hypotheses: plausibility and evidence. BMC Med. 9, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein D. E., Morley G. E., Tamaddon H., Vaidya D., Schneider M. D., Chen J., Chien K. R., Stuhlmann H., Fishman G. I. (2001). Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ. Res. 88, 333-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase V. H. (2005). The VHL tumor suppressor in development and disease: functional studies in mice by conditional gene targeting. Semin. Cell Dev. Biol. 16, 564-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Jeron A., Koren G. (1998). Measurement of heart rate and Q-T interval in the conscious mouse. Am. J. Physiol. 274, H747-H751 [DOI] [PubMed] [Google Scholar]
- Roche F., Reynaud C., Pichot V., Duverney D., Costes F., Garet M., Gaspoz J. M., Barthélémy J. C. (2003). Effect of acute hypoxia on QT rate dependence and corrected QT interval in healthy subjects. Am. J. Cardiol. 91, 916-919 [DOI] [PubMed] [Google Scholar]
- Schaffer M. S., Trippel D. L., Buckles D. S., Young R. H., Dolan P. L., Gillette P. C. (1991). The longitudinal time course of QTc in early infancy. Preliminary results of a prospective sudden infant death syndrome surveillance program. J. Perinatol. 11, 57-62 [PubMed] [Google Scholar]
- Schwartz P. J., Stramba-Badiale M., Segantini A., Austoni P., Bosi G., Giorgetti R., Grancini F., Marni E. D., Perticone F., Rosti D., et al. (1998). Prolongation of the QT interval and the sudden infant death syndrome. N. Engl. J. Med. 338, 1709-1714 [DOI] [PubMed] [Google Scholar]
- Solan J. L., Lampe P. D. (2007). Key connexin 43 phosphorylation events regulate the gap junction life cycle. J. Membr. Biol. 217, 35-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirlapur V. G., Mir M. A. (1982). Nocturnal hypoxemia and associated electrocardiographic changes in patients with chronic obstructive airways disease. N. Engl. J. Med. 306, 125-130 [DOI] [PubMed] [Google Scholar]
- Van Norstrand D. W., Asimaki A., Rubinos C., Dolmatova E., Srinivas M., Tester D. J., Saffitz J. E., Duffy H. S., Ackerman M. J. (2012). Connexin43 mutation causes heterogeneous gap junction loss and sudden infant death. Circulation 125, 474-481 [DOI] [PMC free article] [PubMed] [Google Scholar]