SUMMARY
In humans, skin is the largest organ and serves as a barrier between our body and the outside world. Skin protects our internal organs from external pathogens and other contaminants, and melanocytes within the skin protect the body from damage by ultraviolet light. These same pigment cells also determine our skin colour and complexion. Skin wounding triggers a repair response that includes a robust recruitment of inflammatory cells, which function to kill invading microbes and clear away cell and matrix debris. Once at the wound site, these innate immune cells release a barrage of cytokines that direct the activities of other cells during the repair process. Tissue damage and repair also frequently lead to alterations in skin pigmentation, in particular to wound hyperpigmentation. In this study, we describe a model of wound hyperpigmentation in the translucent zebrafish larva, where we can live-image the recruitment of melanocytes and their precursors, melanoblasts, to the wound site. We show that these pigment cells are drawn in after the initial recruitment of innate immune cells and that the inflammatory response is essential for wound hyperpigmentation. This new model will allow us to uncover the molecular link between immune and pigment cells during tissue repair and to screen for potential therapeutics to dampen wound hyperpigmentation.
INTRODUCTION
Although there are some significant differences in the way a wound heals in various organisms, and at different developmental stages, tissue repair generally takes place following a well-described series of overlapping steps (Martin, 1997; Gurtner et al., 2008). Haemostasis is the earliest event to occur after tissue damage and limits blood loss by forming a temporary plug to re-establish a barrier between internal tissues and the environment. Subsequently, the wound inflammatory response results in first neutrophils and then macrophages being drawn to sites of tissue damage, where they kill any infecting microbes and clear away cell and matrix debris. Furthermore, these innate immune cells release a battery of growth factors and cytokines that act on neighbouring cells and tissues in ways that can be both beneficial and detrimental, for example leading to formation of a collagenous scar at the healed wound site (Stramer et al., 2007).
Long known to be associated with tissue repair and scar formation is the process of wound hyperpigmentation. This is commonly observed in minor inflammatory conditions such as acne but can also be seen in fibrotic wounds and lesions (Halder and Nootheti, 2003; Cayce et al., 2004; King et al., 2005; Coley and Alexis, 2009). This wound-associated pigment disorder is most apparent in people with a dark complexion, but also occurs in people with pale skin (Halder and Nootheti, 2003; Coley and Alexis, 2009); currently, however, little is known about the cells and molecular signals that might drive wound hyperpigmentation.
We wondered whether wound hyperpigmentation might be a downstream consequence of the wound inflammatory response; here, we investigate this possibility in zebrafish, for which much is known about pigment cell development and migration (Parichy, 2006; Kelsh et al., 2009). In zebrafish, as in all other vertebrates, melanocytes develop from precursor melanoblasts, which are neural crest derivatives (Kelsh et al., 2009). As larval development proceeds, melanoblasts commence migration with their pathways influenced by guidance cues, including sdf-1, and eventually differentiate into melanocytes (Kelsh et al., 2000; Svetic et al., 2007). Stripes begin to form in the head region and subsequently extend in an anteroposterior manner. Once established, the larval pigment pattern will prevail until 14 days post-fertilisation (dpf) when the fish morphology changes from larval to juvenile during metamorphosis, and the adult stripe pattern is defined (Kelsh, 2004; Kelsh et al., 2009).
Zebrafish larvae have also become a well-established model in which to track the recruitment of immune cells to a wound or in response to early transformed cells (Cvejic et al., 2008; Mathias et al., 2009; Feng et al., 2010; Gray et al., 2011). In this study, we use live-imaging techniques to reveal the relationship between innate immune cell recruitment to wounds and the subsequent recruitment of melanoblasts and melanocytes. We show that relatively large or chronic wounds trigger recruitment of pigment cell lineages in both larvae and adults, which leads to hyperpigmentation, and that this process is associated with, and dependent on, the preceding inflammatory events.
TRANSLATIONAL IMPACT.
Clinical issue
Hyperpigmentation occurs when the skin affected by an inflammatory disorder (such as acne) or the scar left after a wound (for example, a deep cut or burn injury) remains more pigmented than the normal surrounding skin. Variations in skin pigmentation can be disfiguring if they occur on the face or forearms, and can cause psychological distress. There are no good medical treatments that prevent wound hyperpigmentation, or that reduce pigmentation after a wound has healed. Understanding the causes of wound hyperpigmentation could help in developing new therapies. In addition, some melanocytic lesions have been associated with melanoma, so increased understanding of wound hyperpigmentation might also have implications for skin cancer research.
Results
In this paper, the authors used zebrafish to establish a model in which wound hyperpigmentation could be visualised in real time in a live organism. They use several wounding strategies in larval and adult zebrafish to show that large (but not small) wounds result in hyperpigmentation owing to the recruitment of pigment cells (melanocytes). They found that both differentiated melanocytes and their undifferentiated precursors (melanoblasts) migrated to the wound. Live imaging revealed sequential recruitment of innate immune cells (neutrophils and, later, macrophages), followed by melanocytes. By depleting innate immune cells in zebrafish larvae, the authors showed that melanocyte recruitment and the overall wound hyperpigmentation response depends on the inflammatory response.
Implications and future directions
These results establish a model for examining cellular mechanisms of wound hyperpigmentation in vivo. The power of currently available imaging and genetic approaches in zebrafish means that this model can be applied to further dissect the wound healing response at the cellular and genetic level.
RESULTS
Larger or more persistent wounds in both larval and adult zebrafish can trigger wound hyperpigmentation
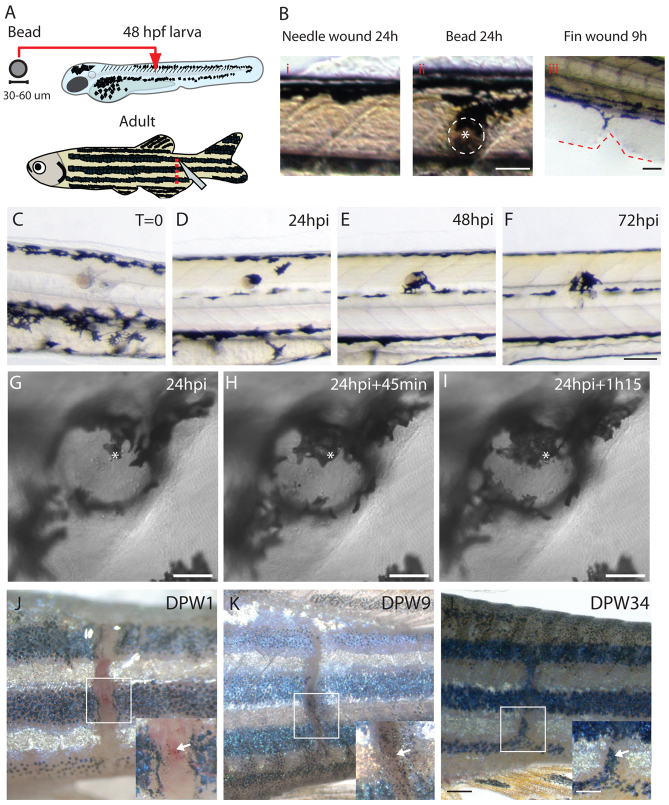
Our intention was to characterise the dynamic behaviour of melanocytes after tissue damage in order to capture the process of wound hyperpigmentation. We made several types of wound in larval and adult fish (Fig. 1A): larvae either received a puncture wound made with a tungsten needle or they received the same puncture wound prior to implantation of a bead beneath the flank skin. In adult fish, we made incisional wounds to their flanks using a scalpel. The small tungsten needle wounds in larval skin healed very rapidly, as previously described (Cvejic et al., 2008), and never resulted in recruitment of melanocytes to the wound (Fig. 1Bi) (0/30 wounds became pigmented, i.e. 0%). However, larger fin wounds made by nicking the tailfin with a tungsten needle (14/30 wounds became pigmented, i.e. 47%) or, more consistently, bead implants in 48-hpf zebrafish larvae resulted in a reproducible and time-dependent migration of melanocytes to the wound, resulting in long-lasting tissue hyperpigmentation (Fig. 1Bii,Biii,C-F) (36/40 wounds became pigmented, i.e. 90%). Usually, by 16-20 hours post-implantation (hpi) we observed one to three melanocytes drawn to, and occasionally in direct contact with, the implanted bead. By 24 hpi, the wound area was usually heavily pigmented (Fig. 1D,G-I; supplementary material Movie 1). Melanocytes remained in close contact with the bead for several days (longest time observed being 7 days; not shown) following implantation (Fig. 1E,F).
Fig. 1.
Large wounds induce hyperpigmentation in both zebrafish larvae and adults. (A) Schematics showing the wounding procedures used in this study. In zebrafish larvae, a small wound was made using a tungsten needle and then a bead was implanted through this wound into the muscle beneath the skin. In adults, a full thickness incisional wound through all skin layers was made on the fish flank using a scalpel. (B) A small needle wound to larval flank does not lead to melanocyte recruitment (Bi), whereas a wound resulting from the implantation of a bead (white asterisk) recruited melanocytes by 24 hpi (Bii). A large larval fin wound generally draws melanocytes to the site of tissue damage by 9 hours after wounding (Biii; red dashed line highlights the wound site). (C-F) Snapshot images showing time-course of progressive wound hyperpigmentation around an implanted bead in a zebrafish larva. (G-I) Still images, taken from supplementary material Movie 1, indicating the dynamic behaviour of melanocytes around the bead at 24 hpi after engraftment in a zebrafish larva. The same position is indicated by a white asterisk in sequential images to aid visualisation of a single melanocyte as it envelops the bead. (J-L) Time-course showing adult wound hyperpigmentation over a duration of 34 days post-wounding (DPW). Insets indicate how hyperpigmentation spreads into adult non-stripe domains. Scale bars: 50 μm (B); 100 μm (C-F); 25 μm (G-I); 500 μm (J-L); and 250 μm (J-L insets).
Incisional wounds in the flanks of adult zebrafish heal with a considerably longer time-course than larval wounds and they also become hyperpigmented (Fig. 1J-L). As with the larval bead wounds, melanocyte recruitment to adult wounds was not immediate; rather, their migration commenced about 12 hours after the initial tissue damage, which resulted in the wound being invaded by a significant number of melanocytes in the subsequent few days (Fig. 1J). Hyperpigmentation of these wounds persisted for at least several weeks after wounding and seemed not to diminish in those individuals that we kept on for longer times. Very clearly, the wound-recruited melanocytes did not ‘obey’ adult stripe territory boundaries: we saw many instances where melanocytes invaded the wound site between striped regions of skin (Fig. 1K). This inter-stripe hyperpigmentation persisted long after the wound had healed, frequently leaving the fish with a dark scar along the full length of the repaired wound (14/22 fish developed a pigmented scar, i.e. 64%) (Fig. 1L).
Dynamic studies reveal how both immature melanoblasts and differentiated, pigmented melanocytes migrate to the wound
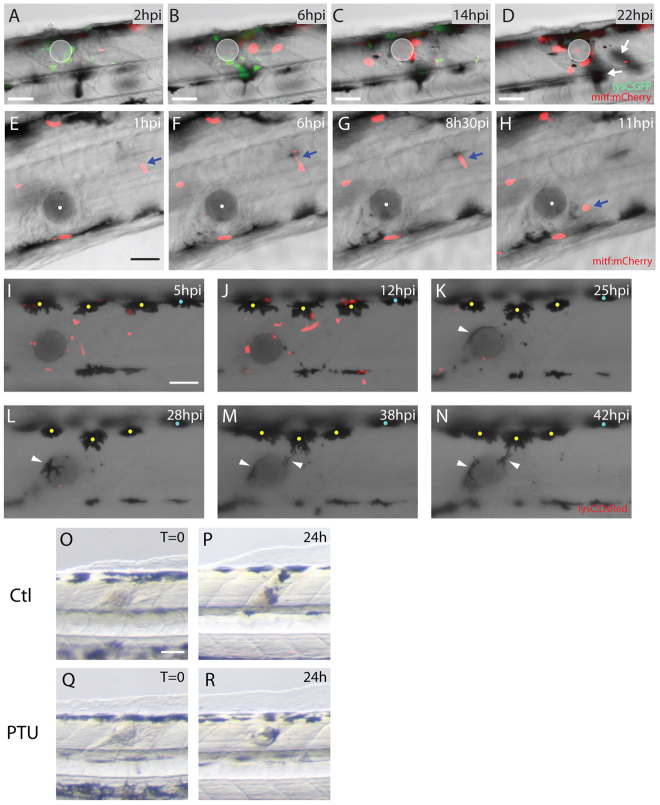
Two possible events could lead to hyperpigmented wounds: the local migration of melanocytes or the migration of melanoblasts, which subsequently differentiate into mature pigmented melanocytes. To analyse this further, we used live-imaging over a 48-hour period following wounding of zebrafish larvae expressing the mitf:Gal4-UAS:mCherry transgene (Santoriello et al., 2010), which recapitulates endogenous mitf expression (Lister et al., 1999), to reveal melanoblasts. Although recent lineage-tracing experiments revealed overlap of iridophore and melanoblast markers early in zebrafish development (Curran et al., 2010), the number of double-positive cells (for iridophore- and melanoblast-specific genes) was very low (between 3 and 8%) at 48 hours, when we perform our bead implant, encouraging us to believe that here we are looking largely at melanoblast migration. Migration of mature melanocytes can be imaged by virtue of their inherent pigmentation and we estimated their migration speed to be around 0.01 μm/minute. Our movies reveal the much faster melanoblasts as they migrate towards the wound at a speed of ∼1 μm/minute after an initial lag phase of 2-3 hours post-wounding (Fig. 2A-D; supplementary material Movie 2). Generally, by 3 hours the first cells have arrived at the wound and by 6 hours there are two or three melanoblasts clustered around the bead. Although some melanoblasts migrated from as far away as 200 μm distant from the wound (Fig. 2E-H; supplementary material Movie 2), other much closer melanoblasts failed to respond at all, suggesting a heterogeneity of responsiveness to wound signals amongst this population of cells that might be explained by a variable differentiation status of the melanoblasts (Kelsh et al., 2000). Migrating melanoblasts exhibited an amoeboid-type motility, as described for migrating Dictyostelium cells (Insall and Machesky, 2009) and for leukocytes as they migrate towards a wound (Herbomel et al., 1999; Cvejic et al., 2008) (Fig. 2E-H; supplementary material Movie 3). Just as for melanoblasts, not all nearby melanocytes are responsive to the wound. However, we did also observe some smaller, pigmented melanocytes, originating from the horizontal myoseptum pigment stripe, drawn towards the wound site (Fig. 2I-N). These smaller melanocytes, which might be recently differentiated (Kelsh et al., 2009), also attempted to wrap around the implanted bead (Fig. 1; Fig. 2K-N; supplementary material Movie 4).
Fig. 2.
Both melanoblasts and melanocytes are recruited to the wound. (A-D) Stills taken from supplementary material Movie 2 showing neutrophils and melanoblasts as they migrate to a bead implanted in a lysC:GFP/mitf:gal4-UAS:mCherryfish larva. (A) Neutrophils (lysCGFP, green) are rapidly recruited to the wound from 2 hours after implantation. (B) By 6 hours after wounding, melanoblasts (mitf:Gal4-UAS:mCherry, red) arrive close to the bead. (C) This continues even as the inflammatory response is resolving at 14 hpi. (D) By 22 hours, melanocytes have also migrated to the wound (out of focus, indicated by white arrows). (E-H) Stills taken from supplementary material Movie 3 showing activation and migration of an individual melanoblast (mitf:mCherry, red and highlighted by a blue arrow) towards the bead. (I-N) Stills taken from supplementary material Movie 4 showing the migration of both neutrophils (red) and melanocytes (black) to the wound (bead has been implanted in a lysC:DsRed fish larva). (I) Number of neutrophils (lysC:DsRed, red) peak at the wound several hours prior to any observable response by melanocytes (yellow and blue dots). (J) First indications of melanocyte response to the wound commence at about 12 hpi (only those with yellow dots), before the final resolution of neutrophils. (K) Several melanocytes make slow progress towards the bead and by 25 hpi a smaller melanocyte has also arrived and is in close contact with the bead (white arrowhead). (L-N) Movement of small and more mature dorsal melanocytes continues for many hours, with cells extending long protrusions to reach the bead. (OR) Comparison of fish treated with PTU at the time of bead implantation versus untreated controls. In both control (O,P) and PTU-treated fish (Q,R) the wound is pigmented by 24 hpi. Scale bars: 50 μm (A-R).
To experimentally test which of melanoblasts or melanocytes most significantly contribute to wound hyperpigmentation, we treated larvae with PTU (phenylthiourea), which blocks melanin synthesis, during the period post-wounding (Fig. 2O-R). If immature melanoblasts migrating into the wound make a significant contribution to the subsequent hyperpigmentation of wounds by rapidly differentiating on site into melanocytes, then PTU treatment to block melanin synthesis should largely prevent wound hyperpigmentation. After treating fish with PTU at the time of bead implantation and monitoring wound pigmentation for several days (only 24-hour results are shown), we saw no difference between untreated and PTU-treated fish (Fig. 2P,R) (18/21 PTU treated wounds become pigmented, i.e. 86%). This experiment suggests that, at least at the earliest wound stages, hyperpigmentation is a direct consequence of migration by differentiated melanocytes.
Innate immune cells precede pigment cell migration to the wound
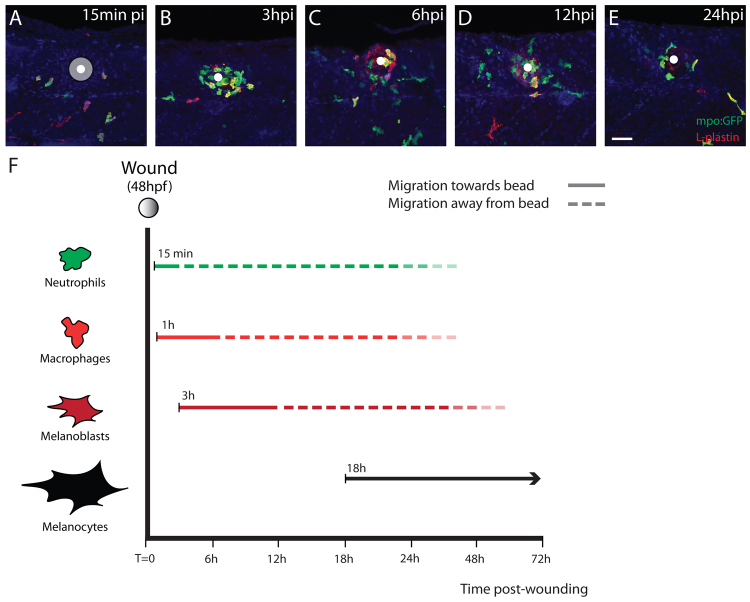
We observed that only a significant lesion in larvae or adult fish triggers wound hyperpigmentation (Fig. 1). Because inflammation plays a crucial role in wound healing and is significantly reduced in small, rapidly healing wounds, we wondered whether this might be the link between tissue damage and wound hyperpigmentation, with immune cells being responsible for drawing pigment cells to wounds. Previous experiments using lysC:GFP (Fig. 2A-D) and lysC:DsRed (Fig. 2I-N) transgenic fish to reveal neutrophil wound influx (Hall et al., 2007), indicated that neutrophils are recruited to the wound site a few hours before we see melanoblast and melanocyte migration. The earliest neutrophils were recruited from about 10-15 minutes post-wounding, with numbers peaking after about 1 hour, whereas the earliest melanoblasts observed in our movies arrived after 3 hours (Fig. 2A-D; supplementary material Movie 2). To further verify this, we implanted beads in mpo:GFP fish, again revealing neutrophils (Renshaw et al., 2006), and fixed and immunostained for the pan-leukocytic marker L-plastin (Redd et al., 2006) at different times following bead implantation (Fig. 3A-E). The time-course confirmed that neutrophils are the first of the innate immune cell lineages to arrive at the wound site, followed by macrophages, with these two lineages peaking at 3 and 6 hpi, respectively (Fig. 3B,C). As expected, considerable numbers of innate immune cells were still present at the wound site at 12 and 24 hours post-wounding, suggesting that our bead implant is indeed triggering an extended inflammatory response, much like a chronic wound (Fig. 3D,E) (Eming et al., 2007). We saw a much more transient inflammatory response when there was no bead and instead just a small puncture wound which, in turn, failed to trigger hyperpigmentation at the wound site (data not shown).
Fig. 3.
Inflammation precedes pigment cell migration to a wound. (A-E) Confocal images gathered at different time-points to reveal recruitment of innate immune cells to the implanted bead in a zebrafish larva. (A) By 15 minutes after implantation a small number of neutrophils (mpo:GFP, green and yellow cells) and occasional macrophages (L-plastin-positive, red) are already present in the wound vicinity. The bead is highlighted in grey with the centroid as a white dot. (B) The peak of neutrophil recruitment is at 3 hpi. (C) By 6 hpi, many innate immune cells are observed around the bead and this time is approximately the peak of macrophage recruitment. (D) Inflammation persists for some time and a large number of leukocytes can still be found close to the bead at 12 hpi. (E) Inflammation has partially resolved at 24 hpi, but a few leukocytes are still lingering around the bead. (F) Timeline highlighting the sequential recruitment of different cell lineages to the bead implant in zebrafish larvae. Full coloured lines represent the period during which cells are recruited to the bead. Dotted lines represent the period when cells are migrating away from the bead. The transition between the full and dotted line marks approximately the peak of recruitment for each cell lineage. Scale bar: 50 μm.
Wound hyperpigmentation is dependent on the inflammatory response
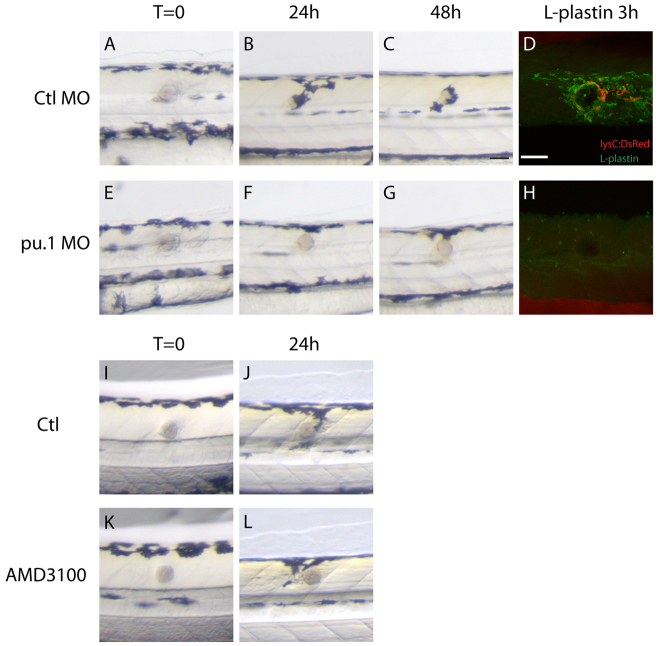
Because the inflammatory response to an implanted bead precedes the recruitment of pigment cells, it is possible that the same signals that draw immune cells to wounds might also be guiding the slower moving pigment cells, but it could also be that melanoblasts and melanocytes are secondarily responding to attractants released by the innate immune cells. To distinguish whether tissue damage signals are directly recruiting melanoblasts or melanocytes, or whether this is a secondary, inflammatory cell-mediated effect, we injected pu.1 morpholino (MO) or a combination of pu.1 and gcsfr morpholinos (same result, but data not shown) into one-cell-stage embryos to prevent the development of neutrophils and macrophages, and subsequently grafted beads into the flanks of these larvae (Rhodes et al., 2005; Liongue et al., 2009). Normally, the bead implant triggered wound pigmentation from 16-24 hours post-wounding, with one to four melanocytes around the bead (Fig. 1; Fig. 4B), and subsequently the wound became more pigmented as melanocytes got closer to the bead at 48 hpi (Fig. 4C). In innate immune cell-depleted larvae, we generally saw no recruitment of melanocytes towards the bead even at 24 hours post-wounding (Fig. 4F) (21/26 morphants showed no wound pigmentation, i.e. 81%). The fact that not all morphants showed a reduction in wound pigmentation could be attributed to the varying extent of gene knockdown in individual fish. After a further 24 hours, leukocyte-depleted larvae showed significantly delayed and reduced wound pigmentation compared with control fish (Fig. 4, compare C with G). Live imaging of mitf:GAL4-UAS:mCherry morphants (pu.1 MO) exhibited no recruitment of melanoblasts to the implanted bead (data not shown). Although these observations could be explained by our morpholino blocking a low level of pu.1 in pigment cells, pu.1 is generally considered to be uniquely expressed by haematopoietic tissues and so it is much more likely that the lack of wound pigmentation in our pu.1 morphants was due to the absence of innate immune cells (Tondravi et al., 1997; Martin et al., 2003; Rhodes et al., 2005). This transient genetic blocking experiment suggests that innate immune cells and the wound inflammatory response are a necessary trigger for recruitment of melanoblasts and melanocytes to sites of tissue damage. But what might the immune cell attractant to pigment cells be? One candidate regulator of pigment cell behaviour at wounds is stromal cell derived factor-1 (sdf-1), which is a guidance cue for pigment cells as they developmentally disperse; indeed, abnormal expression patterns of sdf-1a lead to an altered pigment pattern in the choker zebrafish mutant (Svetic et al., 2007). However, our preliminary experiments using the sdf-1 signalling inhibitor drug AMD3100 did not block wound hyperpigmentation (21 of 27 wounds in treated fish became hyperpigmented, i.e. 78%; Fig. 4, compare J with L). Thus, sdf-1 does not seem to be a major player in melanocyte recruitment following wounding.
Fig. 4.
Wound recruitment of melanocytes requires innate immune cells. (A-H) Time-course of melanocyte recruitment to the bead in control and pu.1 MO-injected fish. (A-C) As shown in Fig. 1, there is a time-dependent migration of melanocytes to the wound over the first 48 hours following bead implantation in control fish. (E-G) Depletion of innate immune cells in pu.1 MO-injected fish delays and reduces the recruitment of melanocytes to the wound. (D) Immunostaining of innate immune cells with anti-L-plastin antibody shows the presence of leukocytes around the wound in control fish; neutrophils (lysC:DsRed) are red and macrophages (L-plastin-positive cells) are green. (H) No leukocytes are evident in pu.1 MO-injected fish. (I-L) Compared with control fish (I,J), AMD3100-treated fish do not show a reduction in wound hyperpigmentation at 24 hpi (K,L). Scale bar: 50 μm (A-L).
DISCUSSION
In this paper, we describe a new model for live-imaging and genetic dissection of the wound hyperpigmentation response in zebrafish larvae. A similar response is seen in adult fish and might also be widely conserved across phyla because insects develop a melanised clot following wounding (Galko and Krasnow, 2004), and hyperpigmentation is commonly seen following various skin damage and healing responses in humans (Ruiz-Maldonado and Orozco-Covarrubias, 1997; Galko and Krasnow, 2004). This response might have been evolutionary conserved because of the protective role that melanin and its derivatives play against UV in damaged tissues, but also because pigment cells might play an antimicrobial role at the wound. In Drosophila, a specialist haemocyte, the crystal cell, releases a cocktail of enzymes at the site of a wound, which trigger tissue melanisation and encapsulation of invading microbes (Galko and Krasnow, 2004; Bidla et al., 2007). The by-products generated during melanin synthesis in human skin are known to be highly oxidative and toxic for bacteria in ways that are related to the defence system used by plants (Mackintosh, 2001; Chisholm et al., 2006). These potential roles for pigment cells at the wound site are amenable to testing in our zebrafish model.
In some human inflammatory conditions, the signals released by skin keratinocytes and leukocytes have been shown to stimulate melanocytes to produce more melanin and become more dendritic (Hara et al., 1995; Scott et al., 2004). However, recently a clinical study has hinted that the wound environment triggers directed migration of melanocytes to the wound site (Sugata et al., 2008). Our own studies in zebrafish provide clear evidence for migration of melanocytes and their precursors, melanoblasts, to the wound and, moreover, indicate that this is a direct consequence of signals from the earlier recruited innate immune cells. The molecular nature of these signals is not yet clear but we have ruled out one potential candidate, sdf-1a, which plays a clear role in guidance of pigment cells during development to define a stripe pattern. Our results also highlight a threshold inflammatory influence, whereby only if the wound is relatively large or long lasting (chronic) will there be a hyperpigmentation response; a deeper understanding of what defines this threshold will be of major clinical relevance. Our findings that innate immune cells are instructive to pigment cells at the wound site adds to the list of cell lineages that inflammatory cells regulate during the repair process (Eming et al., 2007; Stramer et al., 2007). Wound pigmentation is a major clinical problem that can greatly affect the life of a person by altering his or her appearance, and often causes considerable psychological distress (Brown et al., 2008). Aside from aesthetic considerations, it is known that melanocytic lesions can also lead to melanoma (Sina and Goldner, 1990). Our new model of wound hyperpigmentation in zebrafish will allow live-imaging and further genetic dissection of this process and is amenable to genetic and/or small molecule screens that could lead the way to therapeutics for altering pigment responses to tissue damage.
MATERIALS AND METHODS
Zebrafish strains and maintenance
Adult zebrafish (Danio rerio) were maintained and crossed as described (Westerfield, 1993). Strains used included LonAB and SAT (Sanger AB Tübingen, obtained from the Sanger Institute, Hinxton, UK), Tg(mpx:eGFP)i113 (also called mpo:GFP; originally obtained from Stephen Renshaw, University of Sheffield, UK), and Tg (mitf:Gal4VP16-UAS:mCherry) (obtained from Adam Hurlstone, University of Manchester, UK). We crossed the Tg (mitf:Gal4VP16-UAS:mCherry) line with Tg(lyz:eGFP)nz117 line (originally obtained from Phil Crosier, University of Auckland, New Zealand). Adult fish were wounded with a sterile scalpel blade (WPI, Hitchin, UK) after being anaesthetised in MS-222 (ethyl 3-aminobenzoate; Sigma-Aldrich) and were monitored daily.
Bead implantation assay
We used Heparin-Ceramic HyperD M Hydrogel Composite beads (H0532, Sigma-Aldrich) for implantation in zebrafish larvae. The beads come in various sizes; the smaller ones (around 40 μm) were selected to implant in fish. The beads were rinsed for 5-10 minutes in 0.3% Danieau's solution five times before implantation. Larvae (48 hpf) were anaesthetised in MS-222 and laid on an agarose dish. A puncture was made through the skin and muscle using a fine tungsten needle (World Precision Instruments). A bead was then picked with fine tweezers (World Precision Instruments) and gently pushed into the wound. More than 95% of larvae survived the anaesthetic and implantation. Migration of various cell lineages (leukocytes and pigment cells) to the wound was monitored in the hours or days following implantation. Treated larvae were either live-imaged or fixed in 4% paraformaldehyde overnight at 4°C. We also tested other types of beads to verify that the migration of pigment cells was not due to the composition of the bead itself; AG1-X beads (made of resin, from Bio-Rad) and Sephadex G-25 beads (Sigma-Aldrich) gave identical results.
Microscopy and live imaging
Still images of fish were taken using a Leica DFC320 camera attached to a Leica MZFLIII stereomicroscope. Confocal images were taken using a Leica SP5 confocal imaging system attached to a Leica DMI 6000 inverted microscope with a motorised XYZ stage for multiple-site imaging. Some other images were taken using a Hamamatsu CCD camera attached to a wide-field microscope system (Leica DMIRB inverted microscope). Live imaging was performed using the wide-field microscope and Volocity 5.0.2 Acquisition (Perkin-Elmer, UK) with image capture every 1, 6 or 10 minutes during the time-lapse experiments. Images series were exported as QuickTime movies using Sorenson 3 compression and Volocity 5.0.2 visualisation.
Drug treatments
PTU (phenylthiourea) was prepared as a 100× stock (0.3%) and diluted to a final concentration (0.003%) in 0.3× Danieau's solution for use in our melanisation blocking experiments. The Sdf1-α inhibitor, AMD3100 (A5602, Sigma-Aldrich) was also diluted in Danieau's solution to a final concentration of 10 μM. Fish larvae were treated with PTU or AMD3100 at the time of bead implantation and pictures were taken the following days.
Morpholino injections and immunohistochemistry
All morpholinos were obtained from GeneTools (Philomath, OR). Translation-blocking morpholino against pu.1 (5′-GATATACT-GATACTCCATTGGTGGT-3′) (Rhodes et al., 2005) and splice-block morpholino against gcsfr (5′-TTTGTCTTTACAGATCCGCC-AGTTC-3′) (Liongue et al., 2009) or standard control morpholino were injected at 0.4 mM into one-cell-stage embryos. Whole-mount immunostaining was performed using rabbit anti-L-plastin antibody diluted 1:500 in PBS containing 0.1% Triton X-100 and 5% goat serum. Anti-rabbit secondary antibodies (conjugated to Cy3 or DyLight 488) were used at 1:500 and purchased from Jackson ImmunoResearch Europe (Newmarket, UK).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr Elizabeth Patton (MRC Human Genetics Unit, Edinburgh, UK) for advice, insightful discussions and reagents. We would also like to thank Dr Chris Hall and Prof. Phil Crosier (University of Auckland, Auckland, New Zealand), and Dr Adam Hurlstone (Manchester University, Manchester, UK) for providing zebrafish lines, and all members of the Martin and Nobes' labs for support and discussions. All experiments requiring animal use were approved by the University of Bristol animal care committee and the UK Home Office.
FUNDING: M.L. is funded by a postdoctoral fellowship awarded by the Fonds de Recherche en Santé du Québec and this work was also partially funded by a Wellcome Trust grant to P.M. Y.F. was supported by an ISSF grant from the Wellcome Trust allocated by the University of Bristol.
Footnotes
AUTHOR CONTRIBUTIONS M.L. and P.M. conceived and designed the experiments. M.L., Y.F. and R.J. performed the experiments. M.L., Y.F. and P.M. analysed the data. M.L., Y.F. and P.M. wrote the paper.
COMPETING INTERESTS: The authors declare that they do not have any competing or financial interests.
SUPPLEMENTARY MATERIAL: Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.010371/-/DC1
REFERENCES
- Bidla G., Dushay M. S., Theopold U. (2007). Crystal cell rupture after injury in Drosophila requires the JNK pathway, small GTPases and the TNF homolog Eiger. J. Cell Sci. 120, 1209-1215 [DOI] [PubMed] [Google Scholar]
- Brown B. C., McKenna S. P., Siddhi K., McGrouther D. A., Bayat A. (2008). The hidden cost of skin scars: quality of life after skin scarring. J. Plast. Reconstr. Aesthet. Surg. 61, 1049-1058 [DOI] [PubMed] [Google Scholar]
- Cayce K. A., McMichael A. J., Feldman S. R. (2004). Hyperpigmentation: an overview of the common afflictions. Dermatol. Nurs. 16, 401-406 [PubMed] [Google Scholar]
- Chisholm S. T., Coaker G., Day B., Staskawicz B. J. (2006). Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803-814 [DOI] [PubMed] [Google Scholar]
- Coley M. K., Alexis A. F. (2009). Managing common dermatoses in skin of color. Semin. Cutan. Med. Surg. 28, 63-70 [DOI] [PubMed] [Google Scholar]
- Curran K., Lister J. A., Kunkel G. R., Prendergast A., Parichy D. M., Raible D. W. (2010). Interplay between Foxd3 and Mitf regulates cell fate plasticity in the zebrafish neural crest. Dev. Biol. 344, 107-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvejic A., Hall C., Bak-Maier M., Flores M. V., Crosier P., Redd M. J., Martin P. (2008). Analysis of WASp function during the wound inflammatory response – live-imaging studies in zebrafish larvae. J. Cell Sci. 121, 3196-3206 [DOI] [PubMed] [Google Scholar]
- Eming S. A., Krieg T., Davidson J. M. (2007). Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514-525 [DOI] [PubMed] [Google Scholar]
- Feng Y., Santoriello C., Mione M., Hurlstone A., Martin P. (2010). Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 8, e1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko M. J., Krasnow M. A. (2004). Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2, e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C., Loynes C. A., Whyte M. K., Crossman D. C., Renshaw S. A., Chico T. J. (2011). Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb. Haemost. 105, 811-819 [DOI] [PubMed] [Google Scholar]
- Gurtner G. C., Werner S., Barrandon Y., Longaker M. T. (2008). Wound repair and regeneration. Nature 453, 314-321 [DOI] [PubMed] [Google Scholar]
- Halder R. M., Nootheti P. K. (2003). Ethnic skin disorders overview. J. Am. Acad. Dermatol. 48, S143-S148 [DOI] [PubMed] [Google Scholar]
- Hall C., Flores M. V., Storm T., Crosier K., Crosier P. (2007). The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol. 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M., Yaar M., Gilchrest B. A. (1995). Endothelin-1 of keratinocyte origin is a mediator of melanocyte dendricity. J. Invest. Dermatol. 105, 744-748 [DOI] [PubMed] [Google Scholar]
- Herbomel P., Thisse B., Thisse C. (1999). Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 126, 3735-3745 [DOI] [PubMed] [Google Scholar]
- Insall R. H., Machesky L. M. (2009). Actin dynamics at the leading edge: from simple machinery to complex networks. Dev. Cell 17, 310-322 [DOI] [PubMed] [Google Scholar]
- Kelsh R. N. (2004). Genetics and evolution of pigment patterns in fish. Pigment Cell Res. 17, 326-336 [DOI] [PubMed] [Google Scholar]
- Kelsh R. N., Schmid B., Eisen J. S. (2000). Genetic analysis of melanophore development in zebrafish embryos. Dev. Biol. 225, 277-293 [DOI] [PubMed] [Google Scholar]
- Kelsh R. N., Harris M. L., Colanesi S., Erickson C. A. (2009). Stripes and belly-spots – a review of pigment cell morphogenesis in vertebrates. Semin. Cell Dev. Biol. 20, 90-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R., Googe P. B., Page R. N., Mihm M. C., Jr (2005). Melanocytic lesions associated with dermatofibromas: a spectrum of lesions ranging from junctional nevus to malignant melanoma in situ. Mod. Pathol. 18, 1043-1047 [DOI] [PubMed] [Google Scholar]
- Liongue C., Hall C. J., O'Connell B. A., Crosier P., Ward A. C. (2009). Zebrafish granulocyte colony-stimulating factor receptor signaling promotes myelopoiesis and myeloid cell migration. Blood 113, 2535-2546 [DOI] [PubMed] [Google Scholar]
- Lister J. A., Robertson C. P., Lepage T., Johnson S. L., Raible D. W. (1999). nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development 126, 3757-3767 [DOI] [PubMed] [Google Scholar]
- Mackintosh J. A. (2001). The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J. Theor. Biol. 211, 101-113 [DOI] [PubMed] [Google Scholar]
- Martin P. (1997). Wound healing – aiming for perfect skin regeneration. Science 276, 75-81 [DOI] [PubMed] [Google Scholar]
- Martin P., D'Souza D., Martin J., Grose R., Cooper L., Maki R., McKercher S. R. (2003). Wound healing in the PU.1 null mouse – tissue repair is not dependent on inflammatory cells. Curr. Biol. 13, 1122-1128 [DOI] [PubMed] [Google Scholar]
- Mathias J. R., Dodd M. E., Walters K. B., Yoo S. K., Ranheim E. A., Huttenlocher A. (2009). Characterization of zebrafish larval inflammatory macrophages. Dev. Comp. Immunol. 33, 1212-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parichy D. M. (2006). Evolution of danio pigment pattern development. Heredity 97, 200-210 [DOI] [PubMed] [Google Scholar]
- Redd M. J., Kelly G., Dunn G., Way M., Martin P. (2006). Imaging macrophage chemotaxis in vivo: studies of microtubule function in zebrafish wound inflammation. Cell Motil. Cytoskeleton 63, 415-422 [DOI] [PubMed] [Google Scholar]
- Renshaw S. A., Loynes C. A., Trushell D. M., Elworthy S., Ingham P. W., Whyte M. K. (2006). A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976-3978 [DOI] [PubMed] [Google Scholar]
- Rhodes J., Hagen A., Hsu K., Deng M., Liu T. X., Look A. T., Kanki J. P. (2005). Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev. Cell 8, 97-108 [DOI] [PubMed] [Google Scholar]
- Ruiz-Maldonado R., Orozco-Covarrubias M. L. (1997). Postinflammatory hypopigmentation and hyperpigmentation. Semin. Cutan. Med. Surg. 16, 36-43 [DOI] [PubMed] [Google Scholar]
- Santoriello C., Gennaro E., Anelli V., Distel M., Kelly A., Köster R. W., Hurlstone A., Mione M. (2010). Kita driven expression of oncogenic HRAS leads to early onset and highly penetrant melanoma in zebrafish. PLoS ONE 5, e15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G., Leopardi S., Printup S., Malhi N., Seiberg M., Lapoint R. (2004). Proteinase-activated receptor-2 stimulates prostaglandin production in keratinocytes: analysis of prostaglandin receptors on human melanocytes and effects of PGE2 and PGF2alpha on melanocyte dendricity. J. Invest. Dermatol. 122, 1214-1224 [DOI] [PubMed] [Google Scholar]
- Sina B., Goldner R. (1990). Malignant melanoma and pigmented lesions: a diagnostic and management dilemma. South. Med. J. 83, 1218-1223 [DOI] [PubMed] [Google Scholar]
- Stramer B. M., Mori R., Martin P. (2007). The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Invest. Dermatol. 127, 1009-1017 [DOI] [PubMed] [Google Scholar]
- Sugata K., Kitahara T., Takema Y. (2008). Changes of human skin in subepidermal wound healing process. Skin Res. Technol. 14, 436-439 [DOI] [PubMed] [Google Scholar]
- Svetic V., Hollway G. E., Elworthy S., Chipperfield T. R., Davison C., Adams R. J., Eisen J. S., Ingham P. W., Currie P. D., Kelsh R. N. (2007). Sdf1a patterns zebrafish melanophores and links the somite and melanophore pattern defects in choker mutants. Development 134, 1011-1022 [DOI] [PubMed] [Google Scholar]
- Tondravi M. M., McKercher S. R., Anderson K., Erdmann J. M., Quiroz M., Maki R., Teitelbaum S. L. (1997). Osteopetrosis in mice lacking haematopoietic transcription factor PU.1. Nature 386, 81-84 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (1993). The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio Rerio). Eugene, OR: M. Westerfield [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.