SUMMARY
Human prostatic cancer-associated fibroblasts (CAFs) can elicit malignant changes in initiated but non-tumorigenic human prostate epithelium, demonstrating that they possess pro-tumorigenic properties. We set out to reduce the pro-tumorigenic activity of patient CAFs using the Dlk1 and SCUBE1 molecules that we had previously identified in prostate development. Our hypothesis was that mesenchymally expressed molecules might reduce CAF pro-tumorigenic activity, either directly or indirectly. We isolated primary prostatic CAFs and characterised their expression of CAF markers, expression of Notch2, Dlk1 and SCUBE1 transcripts, and confirmed their ability to stimulate BPH1 epithelial cell proliferation. Next, we expressed Dlk1 or SCUBE1 in CAFs and determined their effects upon tumorigenesis in vivo following recombination with BPH1 epithelia and xenografting in SCID mice. Tumour size was reduced by about 75% and BPH1 proliferation was reduced by about 50% after expression of Dlk1 or SCUBE1 in CAFs, and there was also a reduction in invasion of BPH1 epithelia into the host kidney. Inhibition of Notch signalling, using inhibitor XIX, led to a reduction in BPH1 cell proliferation in CAF-BPH1 co-cultures, whereas inhibition of Dlk1 in NIH3T3-conditioned media led to an increase in BPH1 growth. Our results suggest that pro-tumorigenic CAF activity can be reduced by the expression of developmental pathways.
INTRODUCTION
The stromal microenvironment plays an important role in prostate development and prostate cancer progression. Stromal changes during tumorigenesis have been documented in breast, colon, lung and prostate tumours (Bhowmick et al., 2004). Tumour stroma contains activated or carcinoma-associated fibroblasts (CAFs) and stimulates prostate carcinogenesis (Franco et al., 2011; He et al., 2007; Kiskowski et al., 2011; Olumi et al., 1999; Orimo et al., 2005; Tuxhorn et al., 2002). Using tissue recombination and renal capsule xenografting, human prostate CAFs have been shown to induce tumour formation from initiated but non-tumorigenic human prostate epithelial cells (the SV40 immortalised BPH1 cell line), whereas normal prostate fibroblasts (NPFs) did not (Barclay et al., 2005; Olumi et al., 1999).
Prostate cancer shows some similarities to embryonic prostate development, notably the importance of stromal-epithelial signalling and of paracrine regulation of stromal and epithelial compartments. Similarities in gene expression between prostate cancer and development have been documented (Joesting et al., 2005; Orr et al., 2011). Our gene profiling studies of embryonic (inductive) prostate mesenchyme identified pathways that are expressed or dysregulated in prostate cancer, including the deltalike 1 (Dlk1)/Notch2 and SCUBE1 molecules (Vanpoucke et al., 2007). WFDC1, which was identified as a growth inhibitor expressed in fetal urogenital mesenchyme, has been shown to be downregulated in reactive prostatic stroma (Ressler and Rowley, 2011). Several independent studies have demonstrated the potency of developmental mesenchyme and microenvironments in normalising the growth and differentiation of tumour epithelia (Abbott et al., 2008; Hayashi and Cunha, 1991). Although these studies have shown how potent the developmental microenvironment can be in controlling malignant epithelial growth, there is a poor understanding of the molecular mediators of this activity.
Dlk1 encodes a transmembrane protein that belongs to the Notch family, which regulates cell fate decisions and might potentiate or inhibit cell differentiation depending on cell context (Nueda et al., 2007). Previously, we showed Notch/Dlk1 signalling plays an important role in prostate development, regulating stromal survival, and stromal and epithelial differentiation (Orr et al., 2009).
SCUBE1 is a secreted glycoprotein with epidermal growth factor repeats and a CUB domain (Grimmond et al., 2000). Studies in zebrafish suggested that Scube family members are involved in sonic hedgehog (Shh) signalling (Woods and Talbot, 2005) and other extracellular signalling pathways (Kawakami et al., 2005). Previously, we demonstrated SCUBE1 transcript expression is decreased in patient-matched pairs of CAFs in comparison with normal prostate fibroblasts (Vanpoucke et al., 2007).
The present study was designed to determine whether we could use molecules identified in prostate development as the basis for manipulation of CAF pro-tumorigenicity, and whether these might be effective in regulating tumour growth. CAFs were modified to express Dlk1 or to increase expression of SCUBE1. Manipulation of these pathways led to reduced tumorigenicity in an in vivo model of prostate cancer.
TRANSLATIONAL IMPACT.
Clinical issue
The tumour microenvironment, and particularly cancer-associated fibroblasts (CAFs) within it, are increasingly recognised as playing an important role in the growth of tumour epithelia and cancer progression. One experimental strategy for manipulating the tumour microenvironment is to promote programmes from the embryonic mesenchyme or microenvironment. This approach aims to normalise the growth and differentiation of tumour epithelia by inducing redifferentiation.
Results
The authors previously identified several molecules in developing prostate mesenchyme. In this study, they set out to determine the effects of these molecules on prostate CAFs in an in vivo model system of tumour reconstitution. Their hypothesis was that mesenchymally expressed developmental regulators might inhibit the pro-tumorigenic activities of CAFs – either directly or indirectly. Indeed, their data show that expression of Deltalike 1 (Dlk1, a transmembrane protein of the Notch family) or SCUBE1 (a secreted glycoprotein) in CAFs reduced tumour size, epithelial growth and invasion of reconstituted tumours in vivo.
Implications and future directions
These data suggest that certain mesenchymal secreted regulators of development could be applied to alter the tumour microenvironment, and thereby to reduce tumour growth and progression, offering a new avenue to therapeutic intervention.
RESULTS
Isolation and characterisation of cancer-associated fibroblasts
We isolated and characterised CAFs from patients with a prostate cancer diagnosis undergoing transurethral resection of the prostate. Tissue sections were analysed using haematoxylin and eosin (H&E) and Microseminoprotein B (MSMB) staining to confirm the presence of cancer (supplementary material Fig. S1). RT-PCR analysis of CAFs confirmed transcript expression of mesenchymal markers vimentin and fibroblast-specific protein 1 (VIM, FSP1), smooth muscle markers (CNN1, MYH11), pro-tumorigenic chemokine CXCL12 (Orimo et al., 2005), reactive stroma protease fibroblast activated protein (FAP) and androgen receptor (supplementary material Fig. S2 and Table S1). CAF isolates were analysed by immunocytochemistry; all showed VIM expression and CAF subsets showed FSP1, CNN1, SMACT and FAP expression (Fig. 1A). Fibroblast heterogeneity in cancer stroma in vivo is documented and consistent with observations by ourselves and others (Franco et al., 2011; Kiskowski et al., 2011; Orr et al., 2011; Sugimoto et al., 2006). Similarly, weak or absent E-cadherin transcript expression and few pan-cytokeratin-positive cells indicated a low epithelial contamination rate in CAFs at P4, consistent with other published studies.
Fig. 1.
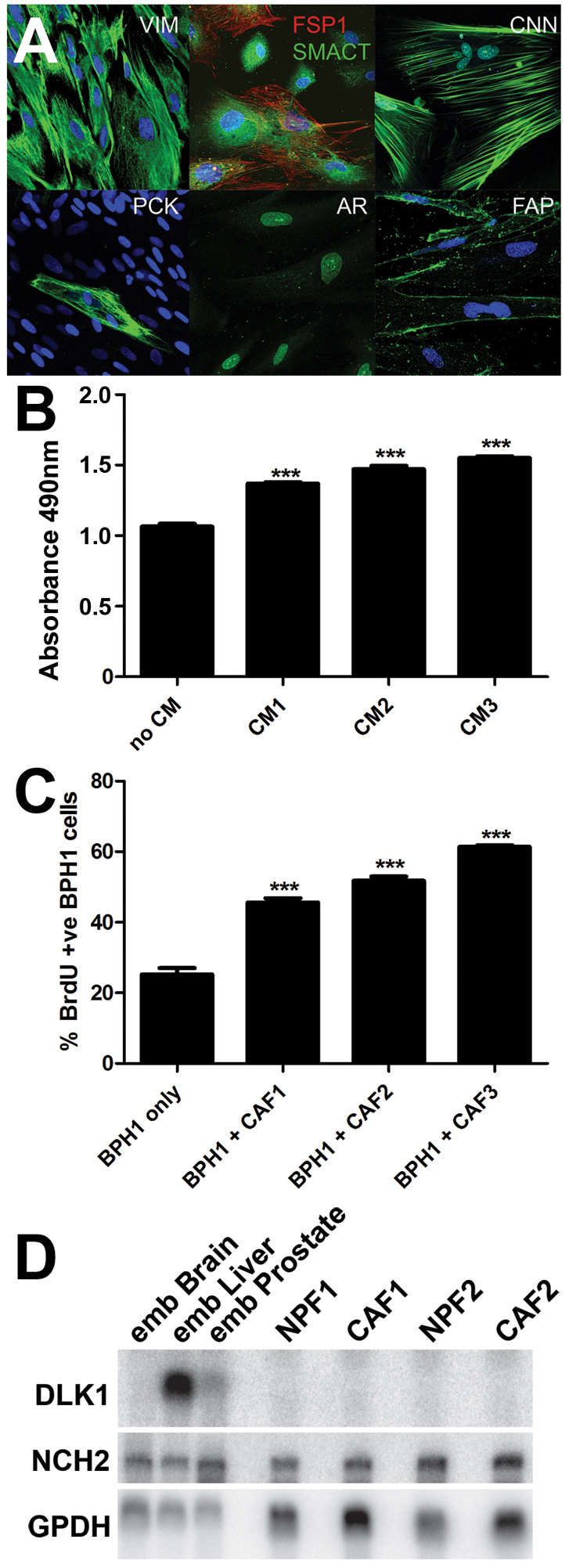
Expression of CAF markers, pro-proliferative CAF activity and Dlk1 and Notch2 mRNAs in CAFs. (A) Immunocytochemical detection of VIM, FSP1, CNN1, PCK, AR, FAP (green) and SMACT (red) in CAFs. VIM, CNN1 and AR were expressed in most or all cells in the cultures, whereas SMACT and FAP were co-expressed in CAF subsets. PCK identified rare epithelial cells in the CAFs. (B) CAF-conditioned medium (CM) from three CAF lines was collected and added to BPH1 cells; this elicited a small but statistically significant increase in BPH1 growth. (C) Co-culture of BPH1 cells with three CAF isolates produced a significant stimulation of BPH1 growth. (D) Northern blotting was used to establish the expression of transcripts for DLK1, NOTCH2 and GAPDH in two pairs of matched CAFs and NPFs. Transcripts for DLK1 were absent, whereas NOTCH2 was expressed. ***P<0.001, using the Student's t-test.
To verify that our CAF isolates stimulated epithelial growth, we confirmed that BPH1 cells proliferated faster in conditioned medium from CAFs, the effect being modest but significant (Fig. 1B). Similarly, BPH1 cells proliferated faster in co-culture with CAFs than did BPH1 cells grown in isolation (Fig. 1C). Co-culture of CAFs with BPH1 cells resulted in a stronger effect on BPH1 proliferation, suggesting that cell-cell contact might be important.
Our previous studies identified the expression of Dlk1 and SCUBE1 in embryonic mesenchyme and observed that SCUBE1 was decreased in CAFs (Vanpoucke et al., 2007). We showed that Dlk1 and Notch signalling was important for mesenchymal survival and differentiation and important in prostate development (Orr et al., 2009). By northern blotting analysis we established NOTCH2 expression in CAFs and NPFs, but observed no significant difference in transcript levels between normal or cancer samples. DLK1 expression was absent from both CAFs and NPFs (Fig. 1D), consistent with its predominantly embryonic expression pattern (Yevtodiyenko and Schmidt, 2006).
Expression of Dlk1 or SCUBE1 reduces pro-tumorigenic activity of prostate CAFs
We selected four CAF isolates and used lentiviral constructs to upregulate SCUBE1 or express DLK1 in our CAF isolates. Patient-matched CAFs not transduced with a lentivirus (no virus), and CAFs transfected with an LV-emGFP control vector (expressing green fluorescent protein, GFP), EV, were included as controls. Successful cell transfection with EV was confirmed by using GFP; we estimated that about 80-90% of CAFs expressed GFP protein, 72 hours after transfection in culture. Western blot analysis confirmed successful transduction of CAFs with LV-Dlk1 and LV-SCUBE1-myc (supplementary material Fig. S3). To study the effects of DLK1 or SCUBE1 on CAF activity we used a xenograft model of prostate cancer (Ao et al., 2007; Olumi et al., 1999). Four CAF isolates were modified with lentivirus to express Dlk1 or to increase SCUBE1 levels. Un-infected or EV-, Dlk1-LV- or SCUBE1-LV-infected CAFs were recombined with BPH1 cells in collagen gels and grafted under the kidney capsule of adult male SCID mice (Fig. 2A). CAFs from three additional patients were infected with Dlk1-LV (supplementary material Fig. S4).
Fig. 2.
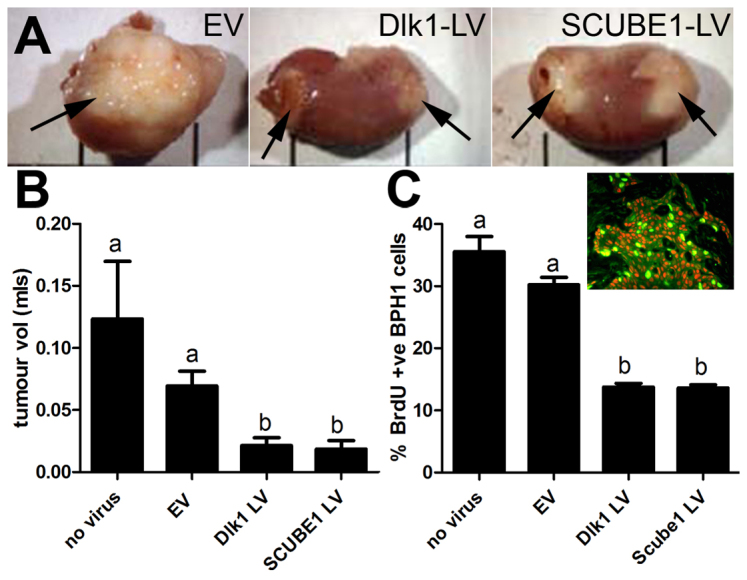
Effect of expression of Dlk1 or SCUBE1 in CAFs on tumour size and BPH1 cell proliferation after growth in vivo. (A) Gross morphology of reconstituted CAF-BPH1 tumours (arrows) on host kidney. Patient-matched CAF isolates (n=4) were modified with lentivirus to express Dlk1 or increase SCUBE1. EV-, Dlk1-LV- or SCUBE1-LV-infected CAFs were recombined with BPH1 cells to form tumours. EV-CAF tumours were large, whereas those CAFs infected with Dlk1-LV or SCUBE1-LV appeared smaller. (B) Tumour volume was measured; infection of CAFs with Dlk1-LV or SCUBE1-LV resulted in a statistically significant reduction in tumour volume (one-way ANOVA with post-hoc Dunnett's test, P<0.001). (C)The proliferation rate of SV40T-BPH1 cells was measured by BrdU incorporation. Inset, immunofluorescent colocalisation was performed using SV40T and BrdU antibodies. SV40T-expressing cells (BPH1) were red, BrdU-expressing cells were green and cells co-expressing SV40T and BrdU were yellow. CAFs infected with Dlk1-LV or SCUBE1-LV resulted in tumours with lower epithelial (SV40T-BPH1) proliferation rates and showed a statistically significant difference from uninfected or EV-infected CAFs (one-way ANOVA with post-hoc Tukey multiple comparison, b, P<0.001).
The gross morphology of the grafts showed that tumours made using CAFs overexpressing SCUBE1 or expressing Dlk1 showed a significant decrease (P<0.0001) in volume in comparison with the control tumours (no virus) and EV-infected CAFs (Fig. 2A,B).
Next, we measured the effect on BPH1 cell proliferation in our reconstituted tumours, because CAF modification might reduce BPH1 cell proliferation and account for the decrease in tumour volume. The xenograft host mice were treated with BrdU prior to harvesting the tumours, and BPH1 cell proliferation was quantified by counting BrdU incorporation in SV40T-positive cells (Fig. 2C, inset). This excluded non-tumour-, host- or patient-derived epithelia because only BPH1 cells express SV40T. We observed that tumour volume was relative to BPH1 cell proliferation: larger tumours demonstrated higher proliferative indices of %BrdU-positive BPH1-SV40T cells in comparison with smaller tumours. Also, tumours prepared with CAFs expressing Dlk1 or SCUBE1 were smaller and demonstrated significantly lower (P<0.001) values of %BrdU-positive BPH1-SV40T cells than control tumours (Fig. 2C).
Modifying CAFs had an effect on reconstituted tumour size and BPH1 cell proliferation. To further investigate the mechanism of action, we tested whether altering Dlk1 or SCUBE1 in CAFs or BPH1 cells cultured in vitro would affect cell growth. An MTS assay identified no difference in cell proliferation of BPH1 cells or CAFs transfected with LV-SCUBE1, LV-Dlk1, EV or no virus (supplementary material Fig. S5). It has been demonstrated that expression of cyclin D1 in normal fibroblasts leads to increased proliferation and acquisition of pro-tumorigenic activity (He et al., 2007). Because neither Dlk1 or SCUBE1 reduced CAF proliferation, we conclude that their inhibition of pro-tumorigenic activity was not mediated by reducing CAF proliferation. Similarly, neither Dlk1 nor SCUBE1 directly inhibited BPH1 proliferation in vitro.
Reduction of invasion in reconstituted tumours containing modified CAFs
In all CAF-BPH1 tumours, SV40T-positive BPH1 cells were distributed at the periphery of the tumour and some formed large nests of cells that pushed against the margin between the tumour and kidney, consistent with previous studies (Ao et al., 2007; Franco et al., 2011; Kiskowski et al., 2011) (Fig. 3A). Control tumours (EV-infected or without virus) recapitulated some of the features of prostate cancer. E-cadherin expression in SV40T-expressing BPH1 cells had a characteristic cell-surface and cytoplasmic ‘patchy’ distribution pattern (supplementary material Fig. S6A). Also, infiltrating BPH1 cells at the kidney tumour margin exhibited markers of epithelial-mesenchymal transition characterised by SV40T-positive BPH1 cells mis-expressing the mesenchymal marker vimentin (supplementary material Fig. S6B). Some tumours demonstrated SV40T-BPH1 cells crossing the kidney tumour margin and intermingling with kidney tubules, and these were classified as invasive (Fig. 3A). In grafts prepared from CAFs from three patients (infected with empty virus, EV), we determined that 77% (17 of 22 grafts) showed BPH1 cells migrating into the kidney, forming invasive tumours. In comparison, tumours containing CAFs modified with LV-Dlk1 or LV-SCUBE1 (Fig. 3B-D) showed a modest reduction in the proportion of tumours with an invasive phenotype: LV-Dlk1, 33% (3 out of 9 grafts); and LV-SCUBE1, 59% (10 out of 17 grafts).
Fig. 3.
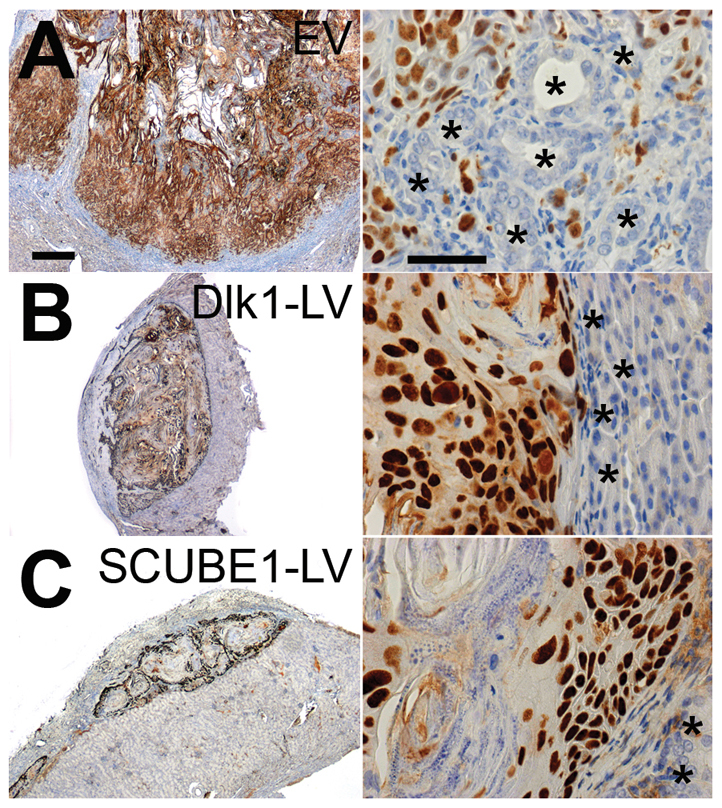
Histology of CAF-BPH1 tumours and evidence of BPH1 invasion into host kidney. (A-C) Images of a reconstituted tumour prepared from (A) EV- (empty vector), (B) Dlk1-LV- or (C) SCUBE1-LV-infected CAFs (n=4 patients), plus BPH1 cells. Immunolocalisation of SV40T (brown) shows BPH1 cell distribution in representative tumour sections at low power (on the left) and high power (on the right). BPH1 cell invasion into the host kidney was found in the majority of tumours prepared with CAFs infected with EV. There was a modest reduction in the proportion of grafts with BPH1 cells (SV40-positive) invading the kidney in tumours prepared with CAFs infected with EV (77%) compared with Dlk1-LV (33%) or SCUBE1-LV (59%). Kidney tubules are marked with asterisks. Scale bars: 500 μm (left) and 50 μm (right).
The Notch pathway plays a role in CAF pro-tumorigenic activity
Dlk1 has been shown to function as an inhibitor of the Notch pathway in Drosophila and mammals (Baladrón et al., 2005 Bray et al., 2008; Nueda et al., 2007), and expression of Dlk1 in CAFs led to a decrease in the size of reconstituted tumours. The Notch pathway has been shown to play a role in prostate development (Orr et al., 2009) and cancer (Santagata et al., 2004), and we have used small molecule inhibitors of presenilin and γ-secretase to inhibit Notch signalling (although there might be off-target effects). CAFs, BPH1 cells and co-cultures of CAFs and BPH1 cells were grown in the presence of a γ-secretase inhibitor (Notch inhibitor XIX). Loss of Notch activity had no effect on cell proliferation of CAF or BPH1 cells when cells were cultured alone (Fig. 4A). Inhibition of Notch signalling in co-cultures of CAF and BPH1 cells caused a significant and dose-dependent decrease in BPH1 cell proliferation but not CAF proliferation (Fig. 4B). Taken together, the results suggest that loss of Notch signalling in CAFs has an indirect effect on BPH1 cell proliferation.
Fig. 4.
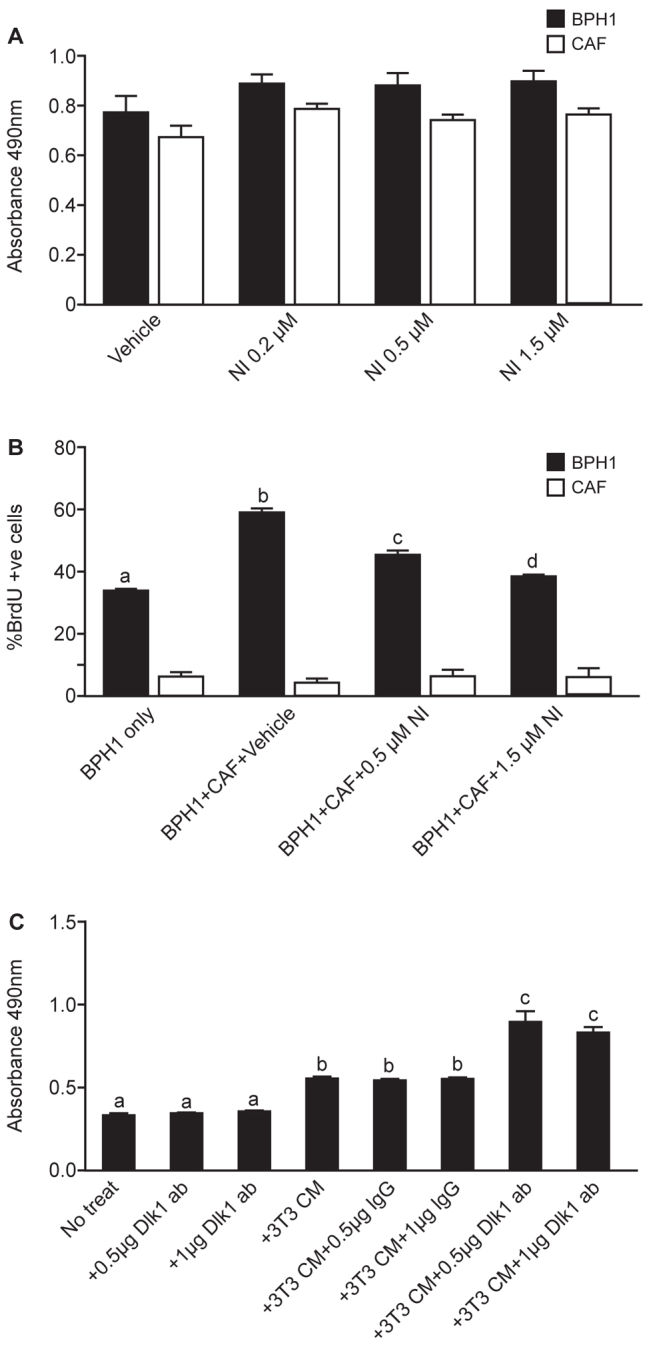
Effect of inhibition of Notch signalling on CAF-BPH1 interactions in vitro, and inhibition of Dlk1 signalling in NIH3T3-conditioned medium upon BPH1 proliferation. (A) MTS cell number assay of BPH1 cells or CAFs cultured individually with different concentrations of Notch inhibitor XIX (NI). Addition of Notch inhibitor XIX had no effect on either BPH1 or CAF growth when cultured individually. (B) BrdU incorporation into BPH1 cells cultured in the presence or absence of CAFs and with or without Notch inhibitor XIX. Addition of Notch inhibitor led to a statistically significant decrease in BPH1 proliferation (black bars) (one-way ANOVA with Tukey multiple comparison, b c, d, P<0.001) but no effect on CAF proliferation (white bars). (C) MTS assay of BPH1 cells cultured in NIH-3T3-conditioned medium, with addition of goat IgG or anti-Dlk1 antibody. BPH1 cell proliferation increased significantly in the presence of NIH-3T3-conditioned medium and cell proliferation increased further with addition of anti-Dlk1 antibody (one-way ANOVA with Tukey multiple comparison, b, c, P<0.001).
To examine the importance of Dlk1 in regulating BPH1 proliferation, we cultured BPH1 cells in the presence of conditioned medium prepared from NIH3T3 fibroblasts, which express Dlk1. CAFs do not express Dlk1 (Fig. 2, and unpublished observations). We observed increased proliferation of BPH1 cells when cultured in 3T3-conditioned medium. Inhibition of Dlk1 by addition of an anti-Dlk1 antibody to 3T3-conditioned medium further increased BPH1 cell proliferation (Fig. 4C). We conclude that Dlk1 can inhibit BPH1 cell proliferation in the presence of fibroblast-secreted factors. The cellular microenvironment is important in determining Notch and Dlk1 activity (Baladrón et al., 2005; Nueda et al., 2007). In the absence of fibroblast-conditioned medium, Dlk1-LV had no effect on proliferation of BPH1 cells or CAFs in vitro (supplementary material Fig. S5).
DISCUSSION
We set out to examine the potential utility of developmental mesenchymally expressed molecules as inhibitors of tumorigenesis, via indirect action in CAFs. Our hypothesis was that DLK1 or SCUBE1 might directly or indirectly reduce pro-tumorigenic CAF activity by altering CAF homeostasis or differentiation. This was based upon their proposed role in embryonic mesenchyme. Embryonic mesenchyme has been shown to affect the differentiation of rat Dunning tumour (a prostate tumour model) (Hayashi and Cunha, 1991), whereas malignant melanoma cells can be normalised by insertion into an embryonic environment (Abbott et al., 2008). These studies demonstrate the potency and applicability of re-differentiating tumour cells using embryonic mesenchyme and developmental signals. However, the precise identity of the re-differentiating signals is unknown, as is the importance of their cellular context, ECM availability and the presence of extracellular inhibitors. We suggest that our studies have added further support by demonstrating the ability of the microenvironment and CAFs to regulate tumorigenesis.
We showed that expression of Dlk1 and SCUBE1 in CAFs reduced their pro-tumorigenic activity, resulting in smaller tumours with lower epithelial proliferation rates. These molecules are extracellular and might be used as novel therapeutic inhibitors of tumorigenesis. We are not aware of other studies using such signalling pathways to inhibit CAF pro-tumorigenic signalling in a similar way and suggest the term ‘deCAF’ to denote the partial or complete inhibition of pro-tumorigenic signalling from CAFs. We suggest that other molecules expressed within the mesenchymal compartment during development might have similar or greater anti-tumorigenic properties, and hope that this paradigm is further investigated. It will also be important to determine whether our findings can be confirmed in other tumour types such as breast, colon, pancreatic and lung – or wherever there is evidence of significant microenvironmental effects.
Recently, it has emerged that heterogeneity within CAFs is a key element required for their pro-tumorigenicity (Franco et al., 2011; Kiskowski et al., 2011). The CAFs used in our studies were heterogeneous with regard to their expression of FSP1 and SMACT (Fig. 1), which is a feature of most CAFs (Sugimoto et al., 2006). Few markers of CAF heterogeneity exist and thus it is not yet possible to define the extent of CAF heterogeneity. However, it is likely that molecules that control the heterogeneity of the CAF population could alter CAF pro-tumorigenic signalling indirectly. For example, any molecule that reduces CAF heterogeneity but promotes homogeneity would be predicted to inhibit CAF pro-tumorigenic activity. Therefore, Dlk1 or SCUBE1 might reduce CAF pro-tumorigenicity via effects upon CAF differentiation or heterogeneity, but currently we have no markers with which to test this possibility.
We suggest that Dlk1 might be acting as a Notch inhibitor in our in vitro and in vivo studies via direct and/or indirect pathways. A reduction in Notch signalling or increase in Dlk1 expression in CAFs had similar effects on BPH1 cell proliferation, both causing a decrease. Other studies have shown that downregulation of Notch signalling can inhibit prostate cancer cell growth, migration and invasion, though these are probably direct effects within the epithelial compartment (Santagata et al., 2004). Also, decreasing Notch signalling by blocking Notch receptor activation with Dlk1 has been proposed as an anti-cancer therapy (Dikic and Schmidt, 2010).
We have shown that manipulation of Dlk1 or SCUBE1 expression in CAFs can be used to reduce CAF-mediated tumour growth and invasion. The results of studies on patient-derived primary CAFs in xenografts in vivo support the idea that our approach might work in patients, because our studies are based upon primary non-immortalised cells. However, the epithelial cells in our model system are SV40T immortalised and it will be important to extend this work into a model system using both patient tumour epithelia as well as CAFs. This will be a substantial undertaking, given the difficulties associated with culture and propagation of primary tumour epithelia. This notwithstanding, we hope that the use of developmental mesenchymal pathways might become a general therapeutic target for novel anti-tumour molecules.
MATERIALS AND METHODS
CAF isolation and cell line culture
CAFs were isolated from human transurethral resection of the prostate samples from patients with a cancer diagnosis undergoing surgery at the Western General Hospital, Edinburgh (ethical approval MREC 02/5/63) as previously described (Olumi et al., 1999; Orr et al., 2011). Informed consent was obtained from all CAF donors. Cells were seeded at 2×103 cells per well in 96-well plates (for growth assays) or at 75×104 cells in a 75-cm2 flask. All cell culture experiments were performed in triplicate wells on at least two replicate experiments.
Preparation and use of conditioned medium
CAFs or NIH3T3s were plated in 75-cm2 flasks and grown overnight. Medium was replaced with serum-free DMEM and incubated for 24 hours. The medium was then collected, centrifuged to remove cell debris, passed through a 0.45 μm filter and stored at −80°C. BPH1 cells were incubated in serum-free medium for 24 hours prior to addition of CAF-conditioned medium. Cell growth was measured by MTS assay after 72 hours.
Co-culture of CAF and BPH1 cells
CAF cells were seeded 5×104 in six-well plates and BPH1 cells were added at 2×104 cells/well with CAFs or at 7×104 cells/well without. Cells were washed in PBS and co-cultured in serum-free medium for 72 hours. To examine loss of Notch signalling, cells were incubated in serum-free medium for 24 hours prior to addition of 0.5 or 1.5 μM Notch inhibitor XIX (Calbiochem, Nottingham, UK) in 1% serum medium or equal volume of vehicle (20% DMSO in ethanol). Cells were cultured for 72 hours and then incubated with BrdU (100 μg/ml) for 2 hours prior to fixation. CAFs and BPH1 cells were treated with 0.2, 0.5 or 1.5 μM Notch inhibitor XIX and growth was measured by MTS assay after 72 hours.
Cell culture with 3T3-conditioned medium and Dlk1 antibody
BPH1 cells were cultured in serum-free medium for 24 hours prior to addition of NIH3T3-conditioned medium and/or goat IgG or Dlk1 antibody (0.5 or 1 μg/ml) (Santa Cruz Biotechnology, Santa Cruz, CA).
MTS cell assay
Cell number was measured using the Cell Titer 96 AQueous One Solution Cell Proliferation Assay (Promega UK, Southampton, UK). Cell Titer Solution was added to the cells in culture medium at a ratio of 1:6 and incubated at 37°C for 100 minutes followed by absorbance reading at 490 nm.
Cell infection with lentivirus
CAFs and BPH1 cells were seeded and, after 24 hours, cells were infected with lentivirus-containing media (EV, Dlk1-LV and SCUBE1-LV) with 0.6 μg/ml polybrene (Sigma-Aldrich, Dorset, UK).
Kidney capsule xenografts
Cell recombinants were prepared by mixing 100,000 epithelial (BPH1) cells with 250,000 CAFs in neutralised rat tail collagen prepared as described previously (Ao et al., 2007; Olumi et al., 1999). Recombinants were then grafted beneath the renal capsule of adult male SCID mice. Three months after grafting, hosts were sacrificed; BrdU was injected 2 hours before sacrifice and kidneys were excised and photographed. The graft dimensions were measured and tumour volume was calculated using the ellipsoidal formula: volume = width2 × length × 0.52, as described (Ao et al., 2007). Note that this formula underestimates the volume of large invasive tumours compared with smaller non-invasive tumours. Grafts were fixed in 10% formalin, embedded in paraffin and processed for immunohistochemistry.
Northern blotting analysis
Total RNA was extracted using the RNeasy Mini kit (Qiagen, Crawley, UK) as per the manufacturer's instructions. Northern blotting for DLK1, NOTCH2 and GAPDH was performed as described previously (Orr et al., 2009; Vanpoucke et al., 2007).
Immunohistochemistry
Histology of xenograft sections was examined by H&E staining. For immunostaining, sections were pressure-cooked in 10 mM citric acid, pH 6.0, for 5 minutes. Immunohistochemistry with DAB was performed using the Vision Biosystems Bond Immunostaining Robot using a secondary polymer (Leica Microsystems Wetzlar, Germany). Cells on chamber slides or six-well plates were fixed in methanol and acetone (1:1); antigen retrieval of nuclear protein or BrdU was performed by pre-treatment with 2M HCl. For fluorescent immunolocalisation in cells and antigen co-localisation, antibodies were visualised with species conjugated with either Alexa Fluor 488 or Alexa Fluor 546 (Molecular Probes, Eugene, OR), as appropriate. DAPI (Sigma-Aldrich, Dorset, UK) was used as a counterstain. To calculate the proliferation index, confocal images of sections of xenografts stained for BrdU and SV40T at 40× magnification were used to measure BrdU incorporation into BPH1 cells, as SV40T is a marker of BPH1 cells. The BrdU proliferation index was measured by cell counting of at least three confocal images of each xenograft, and calculated as the number of BrdU-and SV40T-positive cells as a percentage of the total number of SV40T cells. Statistical analysis was performed using Graphpad Prism software (San Diego, CA).
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mike Millar and Sheila McPherson for help with histology and confocal co-localisation, and Ronnie Grant for help with figure preparation. We thank Griet Vanpoucke for probe and vector construction, and Professor Nick Hastie for advice and encouragement. This work is dedicated to those who worked at the MRC Human Reproductive Sciences Unit before its closure on 31st March 2011.
FUNDING: Funding was provided by the Medical Research Council [grant number WBSe 1276.00.003.00004.01], The Prostate Cancer Charity [grant number 110702 to A.A.T.] and the National Cancer Institute [grant number CA151924 to S.W.H.].
Footnotes
COMPETING INTERESTS: The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS: B.O. and O.C.G. performed experimental work, P.B. constructed viral vectors and provided high titre viral stocks for infections, A.C.P.R. and G.D.S. consented and provided primary human prostate samples, O.E.F and S.W.H. performed xenografting experimental work. B.O. and A.A.T. analysed the data and wrote the manuscript, and A.A.T. supervised the project.
SUPPLEMENTARY MATERIAL: Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.010355/-/DC1
References
- Abbott D. E., Bailey C. M., Postovit L. M., Seftor E. A., Margaryan N., Seftor R. E., Hendrix M. J. (2008). The epigenetic influence of tumor and embryonic microenvironments: how different are they? Cancer Microenviron. 1, 13-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao M., Franco O. E., Park D., Raman D., Williams K., Hayward S. W. (2007). Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 67, 4244-4253 [DOI] [PubMed] [Google Scholar]
- Baladrón V., Ruiz-Hidalgo M. J., Nueda M. L., Díaz-Guerra M. J., García-Ramírez J. J., Bonvini E., Gubina E., Laborda J. (2005). dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp. Cell Res. 303, 343-359 [DOI] [PubMed] [Google Scholar]
- Barclay W. W., Woodruff R. D., Hall M. C., Cramer S. D. (2005). A system for studying epithelial-stromal interactions reveals distinct inductive abilities of stromal cells from benign prostatic hyperplasia and prostate cancer. Endocrinology 146, 13-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick N. A., Neilson E. G., Moses H. L. (2004). Stromal fibroblasts in cancer initiation and progression. Nature 432, 332-337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S. J., Takada S., Harrison E., Shen S. C., Ferguson-Smith A. C. (2008). The atypical mammalian ligand Delta-like homologue 1 (Dlk1) can regulate Notch signalling in Drosophila. BMC Dev. Biol. 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I., Schmidt M. H. (2010). Notch: Implications of endogenous inhibitors for therapy. BioEssays 32, 481-487 [DOI] [PubMed] [Google Scholar]
- Franco O. E., Jiang M., Strand D. W., Peacock J., Fernandez S., Jackson R. S., 2nd, Revelo M. P., Bhowmick N. A., Hayward S. W. (2011). Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 71, 1272-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmond S., Larder R., Van Hateren N., Siggers P., Hulsebos T. J., Arkell R., Greenfield A. (2000). Cloning, mapping, and expression analysis of a gene encoding a novel mammalian EGF-related protein (SCUBE1). Genomics 70, 74-81 [DOI] [PubMed] [Google Scholar]
- Hayashi N., Cunha G. R. (1991). Mesenchyme-induced changes in the neoplastic characteristics of the Dunning prostatic adenocarcinoma. Cancer Res. 51, 4924-4930 [PubMed] [Google Scholar]
- He Y., Franco O. E., Jiang M., Williams K., Love H. D., Coleman I. M., Nelson P. S., Hayward S. W. (2007). Tissue-specific consequences of cyclin D1 overexpression in prostate cancer progression. Cancer Res. 67, 8188-8197 [DOI] [PubMed] [Google Scholar]
- Joesting M. S., Perrin S., Elenbaas B., Fawell S. E., Rubin J. S., Franco O. E., Hayward S. W., Cunha G. R., Marker P. C. (2005). Identification of SFRP1 as a candidate mediator of stromal-to-epithelial signaling in prostate cancer. Cancer Res. 65, 10423-10430 [DOI] [PubMed] [Google Scholar]
- Kawakami A., Nojima Y., Toyoda A., Takahoko M., Satoh M., Tanaka H., Wada H., Masai I., Terasaki H., Sakaki Y., et al. (2005). The zebrafish-secreted matrix protein you/scube2 is implicated in long-range regulation of hedgehog signaling. Curr. Biol. 15, 480-488 [DOI] [PubMed] [Google Scholar]
- Kiskowski M. A., Jackson R. S., 2nd, Banerjee J., Li X., Kang M., Iturregui J. M., Franco O. E., Hayward S. W., Bhowmick N. A. (2011). Role for stromal heterogeneity in prostate tumorigenesis. Cancer Res. 71, 3459-3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nueda M. L., Baladrón V., Sánchez-Solana B., Ballesteros M. A., Laborda J. (2007). The EGF-like protein dlk1 inhibits notch signaling and potentiates adipogenesis of mesenchymal cells. J. Mol. Biol. 367, 1281-1293 [DOI] [PubMed] [Google Scholar]
- Olumi A. F., Grossfeld G. D., Hayward S. W., Carroll P. R., Tlsty T. D., Cunha G. R. (1999). Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 59, 5002-5011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A., Gupta P. B., Sgroi D. C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V. J., Richardson A. L., Weinberg R. A. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121, 335-348 [DOI] [PubMed] [Google Scholar]
- Orr B., Grace O. C., Vanpoucke G., Ashley G. R., Thomson A. A. (2009). A role for notch signaling in stromal survival and differentiation during prostate development. Endocrinology 150, 463-472 [DOI] [PubMed] [Google Scholar]
- Orr B., Riddick A. C., Stewart G. D., Anderson R. A., Franco O. E., Hayward S. W., Thomson A. A. (2011). Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene 31, 1130-1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler S. J., Rowley D. R. (2011). The WFDC1 gene: role in wound response and tissue homoeostasis. Biochem. Soc. Trans. 39, 1455-1459 [DOI] [PubMed] [Google Scholar]
- Santagata S., Demichelis F., Riva A., Varambally S., Hofer M. D., Kutok J. L., Kim R., Tang J., Montie J. E., Chinnaiyan A. M., et al. (2004). JAGGED1 expression is associated with prostate cancer metastasis and recurrence. Cancer Res. 64, 6854-6857 [DOI] [PubMed] [Google Scholar]
- Sugimoto H., Mundel T. M., Kieran M. W., Kalluri R. (2006). Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 5, 1640-1646 [DOI] [PubMed] [Google Scholar]
- Tuxhorn J. A., McAlhany S. J., Dang T. D., Ayala G. E., Rowley D. R. (2002). Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 62, 3298-3307 [PubMed] [Google Scholar]
- Vanpoucke G., Orr B., Grace O. C., Chan R., Ashley G. R., Williams K., Franco O. E., Hayward S. W., Thomson A. A. (2007). Transcriptional profiling of inductive mesenchyme to identify molecules involved in prostate development and disease. Genome Biol. 8, R213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods I. G., Talbot W. S. (2005). The you gene encodes an EGF-CUB protein essential for Hedgehog signaling in zebrafish. PLoS Biol. 3, e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yevtodiyenko A., Schmidt J. V. (2006). Dlk1 expression marks developing endothelium and sites of branching morphogenesis in the mouse embryo and placenta. Dev. Dyn. 235, 1115-11123 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


