Abstract
Doxorubicin (Dox) is a major anticancer chemotherapeutic agent. However, it causes cardiomyopathy due to the side effect of cardiomyocyte apoptosis. We have previously reported that angiopoietin-1 significantly reduced myocardial infarction after ischemic injury and protected cardiomyocytes from oxidative stress-induced apoptosis. It is hypothesized that angiopoietin-1 may protect cardiomyocytes from Dox-induced apoptosis. Cardiomyocytes H9C2 were transfected with adenovirus expressing angiopoietin-1 (Ad5-Ang-1) 24 h before the cells were challenged with Dox at a concentration of 2 µmol/L. Ad5-GFP served as the vector control. Cardiomyocyte apoptosis was evaluated using Annexin V-FITC staining and caspase-3 and caspase-8 activity was determined by Western blotting. The results showed that Dox treatment significantly induced cardiomyocyte apoptosis as evidenced by the greater number of Annexin V-FITC stained cells and increases in caspase-3 and caspase-8 activity. In contrast, overexpression of angiopoietin-1 significantly prevented Dox-induced cardiomyocyte apoptosis. To elucidate the mechanisms by which angiopoietin-1 protected cells from Dox-induced apoptosis, we analyzed both extrinsic and intrinsic apoptotic signaling pathways. We observed that angiopoietin-1 prevented Dox-induced activation of both extrinsic and intrinsic apoptotic signaling pathways. Specifically, angiopoietin-1 prevented DOX-induced increases in FasL and Bax levels and cleaved caspase-3 and caspase-8 levels in H9C2 cells. In addition, overexpression of angiopoietin-1 also activated the pro-survival phosphoinositide-3 kinase (PI3K)/Akt signaling pathway and decreased Dox-induced nuclear factor-kappaB (NF-κB) activation. Our data suggest that promoting the expression of angiopoietin-1 could be a potential approach for reducing Dox-induced cardiomyocyte cytoxicity.
Keywords: cardiomyocyte, doxorubicin, apoptosis, angiopoietin-1, phosphoinositide-3 kinase (PI3K), nuclear factor-kappaB (NF-κB)
INTRODUCTION
Doxorubicin (Dox) is one of the most potent and effective chemotherapeutic agents for the treatment of various types of cancers[1]. However, the dose-dependent chronic cardiac toxicity of Dox limits its clinical application and may ultimately lead to cardiomyopathy and heart failure[2]. It has been reported that reactive oxygen species generation and myocardial apoptosis may be responsible for the pathogenesis of Dox-induced cardiac toxicity[3],[4]. At present, the exact mechanisms of Dox-induced cardiac toxicity remain unclear. Therefore, the search for effective and safe agents that will reduce Dox-induced cardiac toxicity may provide a new approach for promoting Dox in clinical applications.
Angiopoietin-1 (Ang-1) is a growth factor protein, which plays an important role in vascular development and angiogenesis[5]. Recent studies have shown that Ang-1 significantly promoted cardiac survival after myocardial ischemic injury and attenuated cell apoptosis[6]. It has been reported that Ang-1 inhibits Dox-induced human umbilical vein endothelial cell death by modulating Fas expression via the phosphoinositide-3 kinase (PI3K)/Akt pathway[7]. However, the role of Ang-1 in Dox-induced myocardial apoptosis has not been investigated. This study was designed to determine the protective effect of Ang-1 on Dox-induced apoptosis in cardiomyocytes. We observed that Ang-1 overexpression significantly attenuated Dox-induced apoptosis in H9C2 cardiomyocytes. The mechanisms involved blunting Dox-activated Fas-mediated apoptotic signaling pathway and activation of the pro-survival Akt signaling pathway.
METHODS AND MATERIALS
Cell culture and reagents
We employed rat embryonic heart-derived myoblast cell line H9C2 (American Type Culture Collection). The cells were maintained in RPMI-1640 medium (Mediatech, Washington, DC, USA) supplemented with 10% newborn calf serum (HyClone, Logan, UT, USA) in a humidified atmosphere containing 5% CO2 at 37°C. Dox and LY294002 were purchased from Sigma (St. Louis, MO, USA) and FITC-conjugated Annexin V from BD Biosciences (Mountain View, CA, USA).
Cell treatment and gene transfection
Replication-deficient adenoviruses encoding human Ang-1 (Ad5-Ang1) were generated by homologous recombination as described previously[8]. Gene expression was driven by a cytomegalovirus promoter/enhancer. Ad5-green fluorescence protein (GFP) was used as a parallel control during gene transfection. Cardiomyocytes H9C2 were transfected with Ad5-Ang1 or Ad5-GFP at a multiplicity of infection (MOI; 20 plaque-forming units (PFUs)/cell). Twenty-four h after transfection, the cells were challenged with Dox at a concentration of 2 µmol/L. The cells were harvested for analysis of apoptosis and Western blot. There were three replicates in each group.
Western blotting assays
Cytoplasmic proteins were prepared from harvested cells. Western blotting was performed as described previously[9]. Briefly, the cytoplasmic proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto Hybond ECL nitrocellulose membranes (Amersham Pharmacia, San Francisco, CA, USA). The ECL membranes were incubated with the appropriate primary antibodies followed by incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology Inc., Beverly, MA, USA). The signals were detected with the ECL system (Amersham Pharmacia). The same membranes were probed with antibody for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Biodesign International Inc., Kennebunk, Maine, USA), and then washed with stripping buffer. The signals were quantified by scanning densitometry and computer-assisted image analysis. The primary antibodies used in the study were anti-cleaved caspase-3, anti-cleaved caspase-8, anti-FasL, anti-Bax, anti-phospho-Akt (Ser473), and anti-Akt (t-Akt) antibodies (Cell signaling Technology, USA).
Flow cytometric analysis
The assay was performed according to the manufacturer's instruction. Briefly, both treated and untreated cells were harvested, washed with PBS, suspended in Annexin V binding buffer (10 mmol/L HEPES, pH 7.4; 2.5 mmol/L CaCl2, 140 mmol/L NaCl), stained with Annexin V-FITC, and determined by flow cytometry using the FACScalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Electrophoretic mobility shift assay (EMSA)
Nuclear proteins were isolated from H9C2 cells as described previously[9]. Nuclear factor-kappaB (NF-κB) binding activity was examined by EMSA in a 15 µL of binding reaction mixture containing 15 µg of nuclear proteins and 35 fmol of 32P-labeled double-stranded NF-κB consensus oligonucleotide. The signals were quantified by scanning densitometry and computer-assisted image analysis. The results were expressed as the ratio of the integrated density volume (IDV) of NF-κB to the average IDV of background.
Statistical analysis
All results were expressed as mean±standard deviation (SD). For testing the differences of statistical significance between groups, one-way analysis of variance (ANOVA) and Student-Newnan-Keuls test were performed. A P-value < 0.05 was considered statistically significant.
RESULTS
Overexpression of Ang-1 suppresses DOX-induced apoptosis of cardiomyocytes
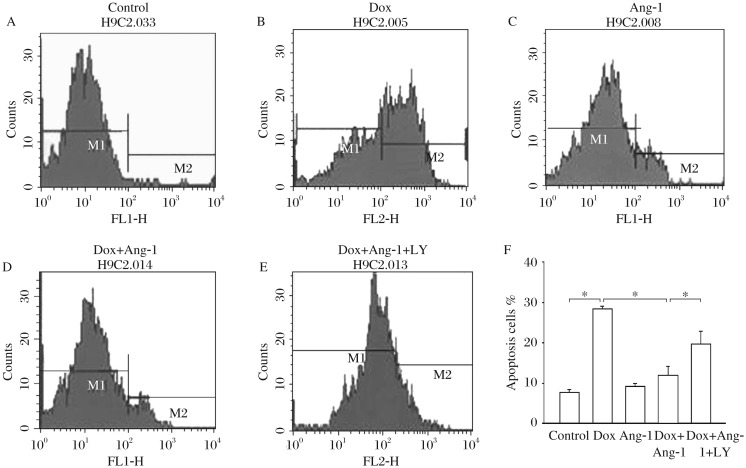
To investigate the effect of overexpression of Ang-1 on DOX-induced apoptosis in cardiomyocytes, we employed flow cytometry to detect apoptotic cells stained with Annexin V-FITC. Fig. 1 showed that Dox treatment significantly increased the proportion of apoptotic H9C2 cells (i.e., Annexin V positive staining cells) by 271% compared with the untreated cells. Consistently, Ad5-Ang-1 transfection significantly attenuated DOX-induced apoptosis by 59%. LY294002, a specific inhibitor of PI3-Kinase, abolished Ang-1-induced increase of apoptosis in H9C2 cardiomyocytes. There was no significant difference in the rate of apoptosis between the Ad5-Ang-1 and control groups.
Fig. 1. Overexpression of angiopoietin-1 (Ang-1) in cardiomyocytes attenuates doxorubicin (Dox)-induced apoptosis.
H9C2 cells were transfected with adenovirus expressing Ang-1 (Ad5-Ang-1) and Ad5-GFP 24 h before the cells were treated with 2 µmol/L Dox. Untransfected H9C2 cells were treated with Dox (2 µmol/L) for 24 h. Percentage of apoptotic populations is represented as the M2-gated population. A: control group; B: Dox; C: Ang-1; D: Dox and Ang-1; E: Dox, Ang-1 and LY. LY: LY294002. *P < 0.05, n = 3/group.
Overexpression of Ang-1 attenuated DOX-induced caspase-3 activity in cardiomyocytes
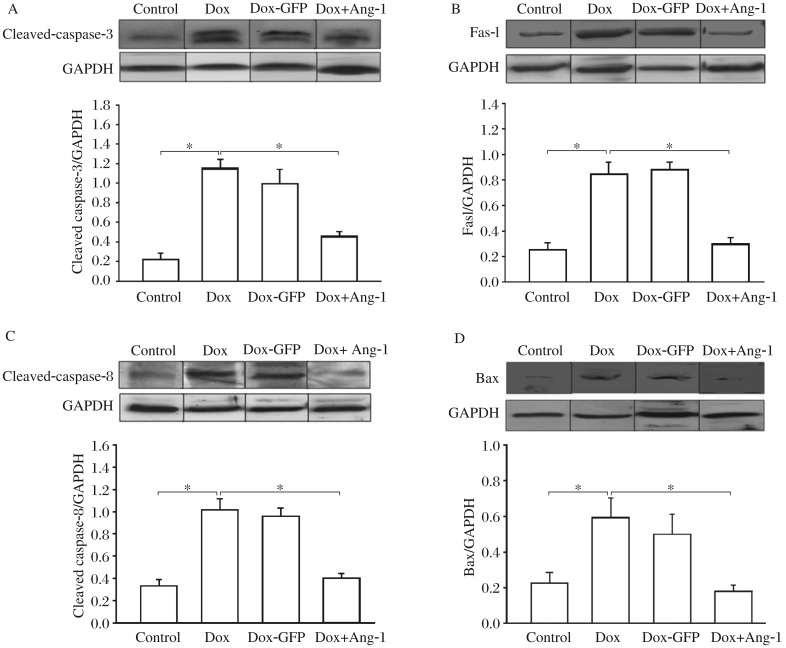
Increased caspase-3 activity is an established marker for cell apoptosis[10]. We examined caspase-3 activity in Dox-treated H9C2 cells in the presence or absence of Ang-1. As shown in Fig. 2A, Dox treatment significantly increased cleaved caspase-3 level by 380% compared with the control cells. However, overexpression of Ang-1 significantly attenuated Dox-activated increase in cleaved caspase-3 level by 55.5%. Transfection of cells with Ad5-GFP did not affect Dox-induced increases in cleaved caspase-3 level.
Fig. 2. Overexpression of angiopoietin-1 (Ang-1) on doxorubicin (DOX)-induced caspase-3, FasL, caspase-8, and Bax in cardiomyocytes.
H9C2 cells were transfected with adenovirus expressing Ad5-Ang-1 and Ad5-GFP 24 h before the cells were treated with 2 µmol/L Dox. Untransfected H9C2 cells were treated with Dox (2 µmol/L) for 24 h. Proteins were extracted for Western blot analysis with specific antibodies. A: overexpression of Ang-1 prevents DOX-induced caspase-3 activity. B: overexpression of Ang-1 attenuats Dox-induced increase in FasL level. C: overexpression of Ang-1 attenuats Dox-induced increase in cleaved caspase-8 level. D: overexpression of Ang-1 attenuated Dox-increased Bax. *P < 0.05. n = 3/group.
Overexpression of Ang-1 attenuated Dox-increased FasL and cleaved caspase-8 levels in cardiomyocytes
Fas is a cell surface receptor that recognizes Fas ligand (FasL), leading to the activation of caspase-8 mediated apoptotic signaling[11]. We examined the effect of Ang-1 overexpression on Dox-induced activation of FasL and its downstream caspase-8 activity in H9C2 cells. Fig. 2B showed that Dox treatment significantly increased FasL level by 230% compared with the untreated cells. In addition, Dox treatment significantly increased cleaved caspase-8 level by 207% compared with the untreated cells (Fig. 2C). Identically, transfection of the cells with Ad5-Ang-1 significantly prevented Dox-activated increases in FasL and caspase-8 levels. Transfection of the cells with Ad5-GFP did not affect Dox-activated increases in FasL and cleaved caspase-8 levels. These data suggested that Dox-induced apoptosis of H9C2 cells was mediated, in part, by activation of Fas-mediated apoptotic signaling pathway. Overexpression of Ang-1 significantly prevented Dox-activated increases in FasL and cleaved caspase-8 levels.
Overexpression of Ang-1 attenuated Dox-induced abundance of Bax in cardiomyocytes
We also examined the effect of Ang-1 on mitochondria-dependent apoptotic signaling pathway in Dox-treated H9C2 cells. In mitochondria-dependent apoptotic signaling pathway, cytochrome c, which is released by the mitochondria in response to pro-apoptotic stimuli, activates caspase-9 followed by activation of caspase-3, resulting in apoptosis. Bax is a pro-apoptotic protein, which can induce the release of cytochrome c from mitochondria[12]. Therefore, we examined the levels of Bax in Dox-treated H9C2 cells in the presence or absence of Ad5-Ang-1. Fig. 2D showed that Bax level was significantly increased by 159% compared with the untreated cells. Overexpression of Ang-1 significantly prevented Dox-induced increase in Bax level. Ad5-GFP transfection did not affect Dox-induced increase in Bax level in H9C2 cells. The data suggested that prevention of Dox-induced activation of mitochondria-mediated apoptotic signaling pathway could be one of the mechanisms by which Ang-1 protected cells against Dox-induced apoptosis.
Overexpression of Ang-1 upregulated PI3K/AKT activity in DOX-treated H9C2 cardiomyocytes
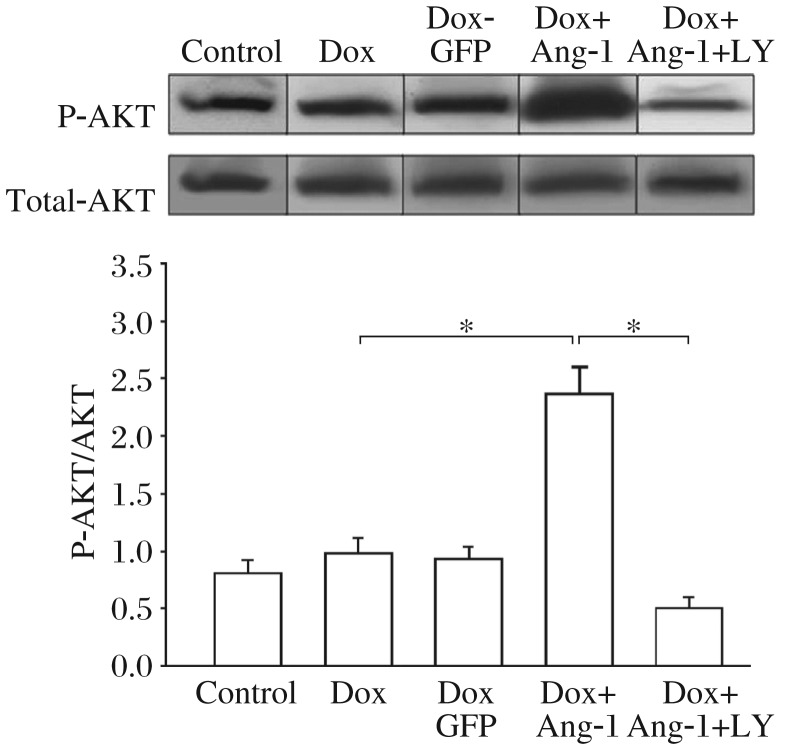
It has been shown that activation of the PI3K/Akt signaling pathway plays an important role in regulating cell survival[13]. Ang-1 is a growth factor which activates the PI3K/Akt signaling pathway[14]. We examined the role of Ang-1 in activating the PI3K/Akt signaling pathway in Dox-treated H9C2 cells. Fig. 3 showed that Dox treatment alone did not alter phospho-Akt level in H9C2 cells. However, overexpression of Ang1 significantly increased the level of phospho-Akt by 140% in Dox-treated cells compared with Dox-treated cells without Ang-1. Transfection of Ad5-GFP did not alter Akt level in Dox-treated cells. To determine whether increased Akt phosphorylation by Ang-1 is involved in the activation of PI3K, we employed LY294002, a specific inhibitor for PI3K in Ad5-Ang-1 expressed cells. As shown in Fig. 3, LY294002 administration abolished Ang-1-induced increases in phospho-Akt level in H9C2 cardiomyocytes. The data suggested that Ang-1 upregulated the pro-survival PI3K/Akt signaling pathway, which could be an additional mechanism of Ang-1 protection against Dox-induced apoptosis.
Fig. 3. Overexpression of angiopoietin-1 (Ang-1) increases the levels of phosphorylated Akt (p-Akt) in doxorubicin (Dox)-treated cardiomyocytes.
H9C2 cells were transfected with adenovirus expressing Ad5-Ang-1, Ad5-GFP 24 h before the cells were treated with 2 µM Dox. Untransfected H9C2 cells were treated with Dox (2 µM) for 24 h. Proteins were extracted for Western blot analysis with a specific antibody against p-Akt (Ser437) and total Akt. LY, LY294002. *P < 0.05, compared with indicated groups. n = 3/group.
Overexpression of Ang-1 prevented Dox-induced increase in NF-κB binding activity in H9C2 cardiomyocytes
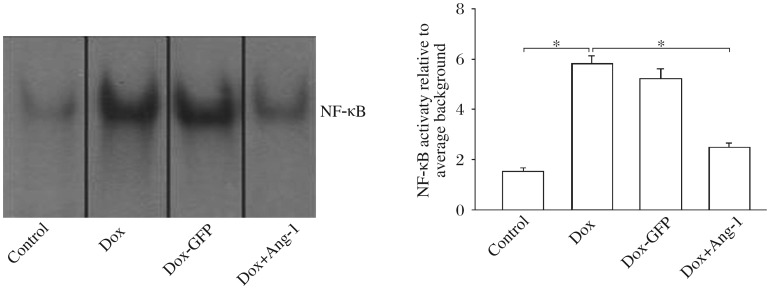
NF-κB plays a key role in Dox-induced apoptosis[15]. Therefore, we investigated the effect of Ang-1 overexpression on Dox-induced NF-κB activation. As shown in Fig. 4, NF-κB binding activity was significantly increased by 228% in Dox-treated cells compared with the untreated cells. Overexpression of Ang-1 significantly prevented Dox-induced increase in NF-κB binding activity (Fig. 4). Transfection of the cells with Ad5-GFP did not affect Dox-induced increases in NF-κB binding activity.
Fig. 4. Overexpression of angiopoietin-1 (Ang-1) decreases the NF-κB binding activity in doxorubicin (Dox)-treated cardiomyocytes.
H9C2 cells were transfected with adenovirus expressing Ad5-Ang-1 and Ad5-GFP 24 h before the cells were treated with 2 µmol/L Dox. Untransfected H9C2 cells were treated with Dox (2 µmol/L) for 24 h. The nuclear proteins were extracted for EMSA. *P < 0.05. n = 3/group.
DISCUSSION
In this study, we aimed to determine the effect of Ang-1 overexpression on Dox-induced apoptosis in H9C2 cardiomyocytes. We observed that overexpression of Ang-1 protected H9C2 cells from Dox-induced apoptosis. The mechanisms involved attenuation of Dox-induced both Fas-mediated and mitochondria-mediated apoptotic signaling pathways. In addition, overexpression of Ang-1 activated the pro-survival PI3K/Akt signaling pathway and decreased Dox-induced NF-κB activation. Our data suggest that promoting expression of Ang-1 could be a potential approach for reducing Dox-induced cardiomyocyte cytotoxicity.
Ang-1 is one type of angiopoietins, which are protein growth factors promoting angiogenesis, the formation of blood vessels from pre-existing blood vessels[16]. Ang-1 binds to its receptor Tie-2, a receptor tyrosine kinase expressed primarily on vascular endothelial cells[17]. Ang-1/Tie-2 signaling promotes angiogenesis during the development, remodeling, and repair of the vascular system[18]. Recent studies have shown that Ang-1 can improve cardiac function after myocardial ischemic injury[19] and sepsis and septic shock[20]. It has been previously reported that overexpression of Ang-1 significantly reduces myocardial infarction after myocardial ischemia injury and protects cardiac myocytes against oxidative stress-induced apoptosis[21]. Taken together, it is hypothesized that Ang-1 would protect cardiomyocytes against Dox-induced apoptosis.
Dox has been used for chemotherapy of cancer for at least three decades. However, its clinical application is limited because of its dose-dependent and progressive cardiomyopathy[22]. Cardiomyocyte apoptosis plays a critical role in Dox-induced cardiomyopathy[23]. The present study observed that Dox-induced apoptosis of H9C2 cardiomyocytes via activation of both Fas-mediated (extrinsic) and mitochondria-mediated (intrinsic) apoptotic signaling pathways. Indeed, it has been well documented that Dox-induced cardiomyocyte apoptosis involves activation of both extrinsic and intrinsic apoptotic signaling pathways[24]. In extrinsic apoptotic signaling pathway, the cell surface receptor Fas recruits a cytosolic FAS-associated death domain (FADD) protein after FasL stimulation. Thus, the Fas, FasL and FADD become a cytosolic complex, which subsequently activates caspase-8[25]. In the intrinsic apoptotic signaling pathway, damaged mitochondria release cytochrome c, a 13-kDa heme-containing protein, leading to caspase-9 activation via the formation of the apoptosome complex containing cytochrome c, pro-caspase-9 and apoptosis activating factor (Apaf-1)[26]. Both apoptotic signaling pathways finally activate common effector caspase-3 that executes the apoptosis process. Importantly, overexpression of Ang-1 by transfection of Ad5-Ang-1 into H9C2 cells significantly prevented Dox-induced activation of both extrinsic and intrinsic apoptotic signaling pathway. Thus, promotion of Ang-1 expression could be a potential approach for treating Dox-induced cardiomyopathy.
It has been demonstrated that activation of the PI3K/Akt signaling pathway plays an important role in regulating of cell growth and survival[27]. Ang-1/Tie-2 activates downstream signaling pathways, including PI3K/Akt signaling. In the present study, we observed that overexpression of Ang-1 significantly increased phosphorylated Akt level with the presence of Dox. The data suggest that activation of the PI3K/Akt signaling pathway may be responsible for the protection against Dox-induced apoptosis. To evaluate this hypothesis, we administered LY294002, a specific PI3K inhibitor, to the cells, and observed that Ang-1-induced protection against Dox was abrogated. Thus, it is concluded that overexpression of Ang-1 protects H9C2 cells against Dox-induced apoptosis, which is mediated, in part, via activation of the PI3K/Akt signaling pathway.
Activation of NF-κB plays a critical role in the induction of immune and inflammatory responses[28]. NF-κB activation also regulates the expression of Fas, FasL, and p53, which are important mediators for apoptosis[29],[30]. We observed in the present study that Dox treatment significantly increased NF-κB binding activity. However, overexpression of Ang-1 prevented Dox-induced NF-κB binding activity in H9C2 cells. At present, the mechanism by which overexpression of Ang-1 prevents Dox-induced increase in NF-κB binding activity remains unclear. However, it has been shown that activation of the PI3K/Akt signaling pathway negatively regulates NF-κB activation[31].
In summary, we observed that overexpression of Ang-1 significantly attenuated Dox-induced apoptosis in H9C2 cells. Further studies will focus on in-vivo experiments to demonstrate the protective effect of Ang-1 on Dox-induced cardiomyopathy.
Footnotes
This study was supported by the National Natural Science Foundation of China (No. 30971258), the National 973 project of China (NO.2012CB517503), the Key Project of Natural Science Foundation of Jiangsu Province (No. 11KJA310004), and Jiangsu “Six Personnel Peak” Talent-Funded Projects.
References
- 1.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Christiansen S, Autschbach R. Doxorubicin in experimental and clinical heart failure. Eur J Cardiothorac Surg. 2006;30:611–6. doi: 10.1016/j.ejcts.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002;234-235:119–24. [PubMed] [Google Scholar]
- 4.Simunek T, Sterba M, Popelova O, Adamcova M, Hrdina R, Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Re. 2009;61:154–71. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 5.Chen JX, Stinnett A. Ang-1 gene therapy inhibits hypoxia-inducible factor-1alpha (HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha expression, and normalizes immature vasculature in db/db mice. Diabetes. 2008;57:3335–43. doi: 10.2337/db08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96:e8–21. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- 7.Yin D, Li C, Kao RL, Ha T, Krishnaswamy G, Fitzgerald M, et al. Angiopoietin-1 inhibits doxorubicin-induced human umbilical vein endothelial cell death by modulating fas expression and via the PI3K/Akt pathway. Endothelium. 2004;11:247–52. doi: 10.1080/10623320490904115. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Ma W, Yang Z, Zhang F, Lu L, Ding Z, et al. VEGF165 and angiopoietin-1 decreased myocardium infarct size through phosphatidylinositol-3 kinase and Bcl-2 pathways. Gene Ther. 2005;12:196–202. doi: 10.1038/sj.gt.3302416. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Browder W, Kao RL. Early activation of transcription factor NF-kappaB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–52. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- 10.Liu XD, Fan RF, Zhang Y, Yang HZ, Fang ZG, Guan WB, et al. Down-regulation of telomerase activity and activation of caspase-3 are responsible for tanshinone I-induced apoptosis in monocyte leukemia cells in vitro. Int J Mol Sci. 2010;11:2267–80. doi: 10.3390/ijms11062267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaoka M, Yamaguchi S, Suzuki T, Okuyama M, Nitobe J, Nakamura N, et al. Apoptosis in rat cardiac myocytes induced by Fas ligand: priming for Fas-mediated apoptosis with doxorubicin. J Mol Cell Cardiol. 2000;32:881–9. doi: 10.1006/jmcc.2000.1132. [DOI] [PubMed] [Google Scholar]
- 12.Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J. 2006;395:57–64. doi: 10.1042/BJ20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan GC, Zhou X, Wang X, Song G, Qian J, Nicolaou P, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 2008;103:1270–9. doi: 10.1161/CIRCRESAHA.108.182832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parborell F, Abramovich D, Irusta G, Tesone M. Angiopoietin 1 reduces rat follicular atresia mediated by apoptosis through the PI3K/Akt pathway. Mol Cell Endocrinol. 2011;343:79–87. doi: 10.1016/j.mce.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Gangadharan C, Thoh M, Manna SK. Inhibition of constitutive activity of nuclear transcription factor kappaB sensitizes doxorubicin-resistant cells to apoptosis. J Cell Biochem. 2009;107:203–13. doi: 10.1002/jcb.22115. [DOI] [PubMed] [Google Scholar]
- 16.Chakroborty D, Sarkar C, Yu H, Wang J, Liu Z, Dasgupta PS, et al. Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci USA. 2011;108:20730–5. doi: 10.1073/pnas.1108696108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1606–13. doi: 10.1161/ATVBAHA.108.169235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Bae JO, Tsai JP, Kadenhe-Chiweshe A, Papa J, Lee A, et al. Angiopoietin-1/Tie-2 activation contributes to vascular survival and tumor growth during VEGF blockade. Int J Oncol. 2009;34:79–87. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SW, Won JY, Lee HY, Lee HJ, Youn SW, Lee JY, et al. Angiopoietin-1 protects heart against ischemia/reperfusion injury through VE-cadherin dephosphorylation and myocardiac integrin-beta1/ERK/caspase-9 phosphorylation cascade. Mol Med. 2011;17:1095–106. doi: 10.2119/molmed.2011.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David S, Park JK, Meurs M, Zijlstra JG, Koenecke C, Schrimpf C, et al. Acute administration of recombinant Angiopoietin-1 ameliorates multiple-organ dysfunction syndrome and improves survival in murine sepsis. Cytokine. 2011;55:251–9. doi: 10.1016/j.cyto.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Chen Y, Zhang F, Chen L, Ha T, Gao X, et al. Synergistically therapeutic effects of VEGF165 and angiopoietin-1 on ischemic rat myocardium. Scand Cardiovasc J. 2007;41:95–101. doi: 10.1080/14017430701197593. [DOI] [PubMed] [Google Scholar]
- 22.Takemura G, Fujiwara H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis. 2007;49:330–52. doi: 10.1016/j.pcad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Konorev EA, Vanamala S, Kalyanaraman B. Differences in doxorubicin-induced apoptotic signaling in adult and immature cardiomyocytes. Free Radic Biol Med. 2008;45:1723–8. doi: 10.1016/j.freeradbiomed.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira AL, Matsubara LS, Matsubara BB. Anthracycline-induced cardiotoxicity. Cardiovasc Hematol Agents Med Chem. 2008;6:278–81. doi: 10.2174/187152508785909474. [DOI] [PubMed] [Google Scholar]
- 25.Lee SD, Kuo WW, Ho YJ, Lin AC, Tsai CH, Wang HF, et al. Cardiac Fas-dependent and mitochondria-dependent apoptosis in ovariectomized rats. Maturitas. 2008;61:268–77. doi: 10.1016/j.maturitas.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Sun SY. Understanding the role of the death receptor 5/FADD/caspase-8 death signaling in cancer metastasis. Mol Cell Pharmacol. 2011;3:31–4. [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama S, Shibata R, Ohashi K, Ohashi T, Daida H, Walsh K, et al. Adiponectin ameliorates doxorubicin-induced cardiotoxicity through Akt protein-dependent mechanism. J Biol Chem. 2011;286:32790–800. doi: 10.1074/jbc.M111.245985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valen G. Innate immunity and remodelling. Heart Fail Rev. 2011;16:71–8. doi: 10.1007/s10741-010-9187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Hou J, Yin Y, Jang J, Zheng Z, Fan H, et al. alpha-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFkappaB. Biochem Pharmacol. 2010;80:247–54. doi: 10.1016/j.bcp.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu H, Bolati D, Adijiang A, Muteliefu G, Enomoto A, Nishijima F, et al. NF-kappaB plays an important role in indoxyl sulfate-induced cellular senescence, fibrotic gene expression, and inhibition of proliferation in proximal tubular cells. Am J Physiol Cell Physiol. 2011;301:1201–12. doi: 10.1152/ajpcell.00471.2010. [DOI] [PubMed] [Google Scholar]
- 31.Iyer AK, Azad N, Talbot S, Stehlik C, Lu B, Wang L, et al. Antioxidant c-FLIP inhibits Fas ligand-induced NF-kappaB activation in a phosphatidylinositol 3-kinase/Akt-dependent manner. J Immunol. 2011;187:3256–66. doi: 10.4049/jimmunol.1002915. [DOI] [PMC free article] [PubMed] [Google Scholar]