Abstract
This study was designed to determine the levels of early endothelial progenitor cells (EPCs), apelin, vascular endothelial growth factor (VEGF) and stromal cell-derived growth factor-1 (SDF-1) after acute myocardial infarction (AMI), and to investigate the relationships between these cytokines and early EPCs. Early EPCs, defined as CD133+, KDR+, and CD34+ cells, were quantified by flow cytometry. The levels of early EPCs and those cytokines in AMI patients were significantly different from those with coronary artery disease or controls (P < 0.05). Plasma apelin levels were inversely correlated with Gensini score and early EPCs (both P < 0.01). Early EPCs, VEGF and SDF-1 showed different patterns of changes in AMI patients during the first 24 h. The trend in the change of early EPCs was proportionally correlated with that of VEGF (P < 0.05). AMI patients exhibited increased early EPCs with remarkably decreased apelin levels and enhanced VEGF levels.
Keywords: apelin, vascular endothelial growth factor (VEGF), stromal cell-derived growth factor-1 (SDF-1), endothelial progenitor cells (EPCs)
INTRODUCTION
Endothelial progenitor cells (EPCs) are circulating angioblasts derived from human bone marrow with a potential to differentiate into endothelial cells[1],[2]. Circulating EPCs are defined as cells positive for haematopoietic stem cell markers, such as CD34, and endothelial markers, such as vascular endothelial growth factor (VEGFR) 2[3]. Early EPCs in the bone marrow, or immediately after their migration into the systemic circulation, are positive for CD133/ CD34/ VEGFR2 (KDR), whereas circulating EPCs are positive for CD34/ VEGFR2/ CD31/ VE-cadherin. They obviously lose CD133 and begin to express von Willebrand factor[4]. Increased EPCs in the peripheral blood have been reported in various pathologic conditions involving severe endothelial perturbation, including inflammatory disease, acute myocardial infarction (AMI), unstable angina (UA), and critical limb ischemia[5]–[7]. The elevation of early EPCs in peripheral blood represents the ability to assess endothelial damage. Early EPCs possess the capacity to home to sites of vascular injury, to produce neoangiogenesis in vivo and to maintain the homeostasis of vascular endothelia[8]. In some pathologic conditions, various cytokines, particularly vascular endothelial growth factor (VEGF), stromal cell-derived growth factor-1 (SDF-1), granulocyte colony stimulating factor (G-CSF), and interleukin-8 (IL-8)[9]–[11] have been shown to have beneficial effects on the mobilization of EPCs, by increasing their number and functionality[12]–[16].
Apelin is a peptide which appears to act as an endogenous ligand for the G-protein-coupled angiotensin II protein J (APJ) receptor that is expressed at the surface of some cell types[17]. In the vascular system, APJ and apelin are known to be expressed in endothelia and vascular smooth muscle cells[18]. In vitro and in vivo studies have demonstrated that, apelin is upregulated in response to hypoxia in peripheral and cardiac tissues[19],[20]. In rat experimental AMI, an increase in apelin and APJ expression was observed[21]. Apelin/APJ deficiency is preventative against oxidative stress-linked atherosclerosis, and the apelin/APJ system works as a mediator of oxidative stress in vascular tissue[22]. Activation of apelin, like VEGF[23], promotes the formation of new blood vessels and the proliferation of endothelial cells, umbilical endothelial cells and gastric cells. Thus, we hypothesized that a correlation may exist between early EPCs and apelin. We performed this study to investigate the mobilization of early EPCs marked as CD45low/− CD34+KDR+CD133+ and evaluate the correlation of cytokines with the level of early EPCs during the acute phase of cardiac disease.
SUBJECTS AND METHODS
Patients
The protocol was approved by the Institutional Ethics Committee in accordance with the principles outlined in the Declaration of Helsinki, with informed consent provided by each patient before enrollment. This was an observational study, and none of the participants could influence clinical practice. A total of 159 patients, who underwent diagnostic angiography, and some of whom received percutaneous coronary intervention (PCI) (including percutaneous transluminal coronary angioplasty), were enrolled at a single facility between June 2010 and March 2011. Control subjects (Control) were defined as those having angiographically normal coronary arteries. Coronary artery disease (CAD) patients enrolled should meet the following criteria: (1) history of transient episodes of typical exertional chest pain which remains unchanged for more than 3 months before blood sample collection (excluding rest angina); (2) at least one coronary stenosis producing ≥50% diameter reduction. AMI patients (AMI) enrolled were defined as those with symptom onset within the past 24 h. The diagnostic criteria of AMI were defined as follows: (1) creatine kinase MB (CK MB) fraction elevated above twice the upper normal limit with raised troponin T (TNT) levels; (2) concurrent prolonged chest pain or discomfort; (3) persistent ST-segment or T-wave changes or with presumed new left bundle-branch block. All AMI patients received a reasonable loading clopidogrel and aspirin on admission. Further analysis was given to AMI patients on the trend of EPCs and plasma concentration of cytokines during the first 24 h after admission. All patients received proper therapy according to their syndromes and diseases. Inclusion criteria included the ability to give informed consent and referral for coronary angiography for the evaluation of CAD. Exclusion criteria included cardiogenic shock, AMI in preceding years, malignancies, diabetes mellitus, current smoking, acute or chronic inflammatory diseases, infection with human immunodeficiency virus, and treatment with immunosuppressive medications including steroids, pregnancy, or any hematological disorders, and history of blood-transfusion within the past 2 weeks. Patients with thrombocytopenia (< 100×103 cells/mm3), anemia (Hb < 10 mg/dL) or renal failure (Cr > 2.5 mg/dL), were also excluded from the study.
Angiographic assessment
Standardized angiographic projections that showed coronary artery stenosis at its highest severity were recorded in every patient. Coronary angiograms were interpreted by two independent cardiologists. Percentage of coronary narrowing was calculated by comparison of the minimum diameter of the segment with the diameter of an adjacent angiographically normal coronary segment. Gensini score, which considers both the extent and the severity of the lesions at coronary angiography, was calculated for each patient and used for the assessment of coronary narrowing severity[24]. Gensini score system also grades the stenosis in the epicardial coronary arteries (1 for 1%-25% stenosis, 2 for 26%-50% stenosis, 4 for 51%-75% stenosis, 8 for 76%-90% stenosis, 16 for 91%-99% stenosis, and 32 for total occlusion), which is then multiplied by a constant number determined according to the anatomical position of the lesion.
Study protocol
Peripheral blood samples were collected on admission of all patients (baseline), and were also collected for AMI patients receiving primary PCI before the procedure. All stents used were rapamycin-eluting stents. For patients who received primary PCI according to guidelines, samples were drawn at 3, 6, 12, and 24 h after successful recanalization of the occluded infarct-related coronary artery (post-PCI 3 h, post-PCI 6 h, post-PCI 12 h, and post-PCI 24 h), respectively. For patients who did not receive primary PCI, peripheral blood samples were collected at 3, 6, 12, and 24 h after admission (non-PCI 3 h, non-PCI 6 h, non-PCI 12 h, and non-PCI 24 h), respectively.
Collection of blood samples
Peripheral blood samples were collected into a 4-mL Vacutainer (Becton Dickinson, Basel, Switzerland) tube containing liquid EDTA as an anticoagulant. Plasma was separated by prompt centrifugation (Histopaque-1077, Sigma-Aldrich, St. Louis, MO, USA) at 400 g at 4°C for 10 min and then stored at -80°C immediately. All procedures including EPC isolation, flow cytometric analysis and blood plasma centrifugation were performed within 2 h after blood collection.
Monoclonal antibodies
The following directly conjugated mouse anti-human monoclonal antibodies were used for flow cytometry: fluorescein isothiocyanate (FITC)-labeled anti-CD34 (eBioscience, San Diego, CA, USA), peridinim chlorophyll (PerCP)-cy5.5-labeled anti-CD45 (eBioscience), allophycocianin (APC) -labeled anti-VEGFR2 (KDR) (R&D Systems, Minneapolis, MN, USA), and phycoerthrin (PE)-labeled anti-CD133 (Miltenyi Biotec, Auburn, MN, USA).
Multicolor staining and flow analysis
Mononuclear cells were isolated from 1 mL peripheral blood with the use of a Ficoll density gradient (Biocoll, Biochrom, USA) by a density-gradient centrifugation (Histopaque-1077, Sigma-Aldrich) according to standard protocols, and then cells were centrifuged and resuspended in 1 mL phosphate-buffered saline (PBS), pH 7.2. A panel of monoclonal antibodies including anti-CD45 (leukocyte common antigen; to exclude haematopoietic cells), anti-CD34 (marker for bone marrow-derived progenitor stem cells), CD133 (immature haematopoietic stem cell marker), KDR (endothelial marker) and appropriate analysis gates were used to enumerate EPCs. To quantify the content of CD45low/−CD34+ KDR+CD133+cells, 100 µL of sample was incubated for 20 min at 4°C in the dark with 5 µL of PerCP-cy5.5-CD45 and FITC-CD34, 10 µL of PE-CD133 and APC-KDR. Cells labeled with PE-, FITC-, PerCP-cy5.5 and APC-conjugated isotypic monoclonal antibodies were used as controls to determine the background of fluorescence. After staining, red blood cell components in cells were lysed with 2 mL lysing solution (Biolegend, San Diego, CA, USA) according to the manufacturer's instruction. The remaining cells were washed in 1 mL PBS (300 g, 5 min) and finally resuspended in 500 µL PBS. Then, analysis was performed within 30 min. Nucleated cells from specimens were evaluated using a FACS Canto flow cytometer (BD Biosciences CA) as described previously[25], with identical set-up parameters between samples. The data was analyzed using BD FACSDiva software. All samples were measured in duplicate. For fluorescence-activated cell sorting analysis, 1×106 mononuclear cells were acquired and scored. Samples were subjected to a 2D side scatter-fluorescence dot plot analysis. The same trained operator blinded to this study performed all the tests.
Plasma concentration of cytokines
The plasma levels of apelin, VEGF, and SDF-1 were respectively determined in duplicate using commercially available ELISA kits according to the manufacturers' guidelines (Apelin: Phoenix Pharmaceuticals, Belmont, CA, USA; VEGF and SDF-1: R&D Systems).
Statistical analysis
Data were presented as mean±standard deviation (SD). The numbers of Gensini were considered as a continuous variable. Normally distributed variable were compared by means of independent t test. Correlations were assessed with linear regression analysis and Spearman's rank correlation test. All tests were two-sided, and statistical significance was considered for P values < 0.05. All statistical analyses were performed using SPSS version 17.0 for Windows (Chicago, IL, USA).
RESULTS
Baseline characteristics
This study included 87 consecutive patients with AMI (67 men and 20 women; age 69±13 years). Thirty-three patients with angiographically documented stable CAD (24 men and 9 women, age 64±10 years) and 39 control subjects (30 men and 9 women, age 66±8 years) were enrolled in the study. The patients' baseline demographic characteristics are shown in Table 1. There were no differences in age, body mass index (BMI), cardiovascular risk factors, and current medications (β-adrenoceptor blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) among the control, CAD and AMI groups (all P > 0.05). Additionally, there were no differences in age, infarct-related coronary vessel, NYHA-classification, hemoglobin, and red blood cells among these groups (all P > 0.05).
Table 1. Baseline characteristics and admission data of the studdy population.
| Characteristics | AMI (n = 87) | CAD (n = 33) | Control (n = 39) | P value |
| General data | ||||
| Age (years) | 69±13 | 64±10 | 66±8 | 0.07 |
| Gender (female/male) | 20/67 | 9/24 | 9/30 | 0.88 |
| Body mass index (kg/m2) | 24.08±3.21 | 24.33±2.47 | 24.45±2.93 | 0.88 |
| Systolic blood pressure (mmHg) | 137.65±21.14 | 129.32±17.60 | 127.41±18.40 | 0.087 |
| Gensini | 067.53±37.24 | 045.93±29.17 | 02.57±1.67 | P < 0.001 |
| Cardiovascular risk factors [n(%)] | ||||
| Hypercholesterolemia | 09 (10.3%) | 03 (9.09%) | 02 (5.13%) | 0.634 |
| Hypertension | 70 (80.5%) | 29 (87.9%) | 33 (84.6%) | 0.600 |
| Laboratory parameters | ||||
| CK-MB (U/L) | 048.71±89.64 | 12.09±8.74 | 10.45±2.43 | *0.010* |
| Troponin T (µg/L) | 00.35±0.53 | 00.05±0.00 | 00.05±0.01 | *0.000* |
| Triglycerides (mmol/L) | 01.81±1.20 | 01.96±1.29 | 01.52±0.71 | 0.333 |
| Medication [n(%)] | ||||
| Beta-blocker | 21 (24.1%) | 05 (15.2%) | 3 (7.7%) | 0.077 |
| Calcium channel blockers | 25 (28.7%) | 11 (33.3%) | 05 (12.8%) | 0.092 |
| Diuretics | 4 (4.6%) | 0 (-)000 | 1 (2.6%) | 0.426 |
| Statin | 19 (21.8%) | 05 (15.2%) | 3 (7.7%) | 0.142 |
| ACEI or ARB | 46 (52.9%) | 13 (39.4%) | 19 (48.7%) | 0.421 |
| Medical history [n(%)] | ||||
| stroke | 4 (4.6%) | 1 (3.0%) | 0 (-) | 0.395 |
| PCI | 8 (9.2%) | 1 (3.0%) | 0 (-) | 0.092 |
AMI: acute myocardial infarction; CAD: coronary artery disease; CK-MB: creatine kinase MB fraction; ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers; PCI: percutaneous coronary intervention. The body-mass index is the weight in kilograms divided by the square of the height in meters. Data are presented as number (percentage) or as mean±SD. *P < 0.05.
Early EPCs and plasma levels of apelin, VEGF and SDF-1 at baseline
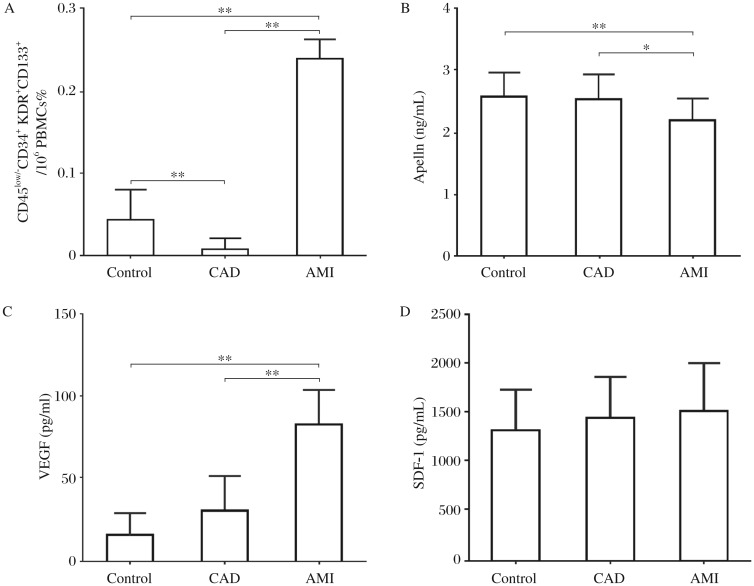
The number of CD45low/−CD34+ KDR+CD133+ early EPCs in AMI patients was 6-fold higher than that of controls, and 24-fold higher than that of CAD patients (Control vs CAD/AMI, 0.04%±0.03% vs 0.01%±0.01%/0.24%±0.02%, PControl vs CAD < 0.01, PControl vs AMI < 0.01, PCAD vs AMI < 0.01) (Fig. 1A). Plasma apelin levels in AMI patients were 0.85-fold lower than those of controls, and 0.87-fold lower than those of CAD patients (Control vs CAD/AMI, 2.60±0.36 vs 2.54±0.47/2.21±0.34 ng/mL, PControl vs CAD > 0.05, PControl vs AMI < 0.01, PCAD vs AMI < 0.05) (Fig. 1B). Plasma VEGF levels in AMI were 5.1-fold higher than those of controls, and 2.8-fold higher than those of CAD patients (Control vs CAD/AMI, 16.04±12.05 vs 29.93±27.02/82.58±32.38 pg/mL, PControl vs CAD > 0.05, PControl vs AMI < 0.01, PCAD vs AMI < 0.01) (Fig. 1C). There was no difference in plasma SDF-1 levels among AMI, Control and CAD (Control vs CAD/AMI, 1316.27±653.01 vs 1442.47±446.89/1511.59±528.94 pg/mL, PControl vs CAD > 0.05, PControl vs AMI > 0.05, PCAD vs AMI > 0.05) (Fig. 1D).
Fig. 1. Early EPCs and plasma apelin, VEGF and SDF-1 at baseline.
A: Early EPCs marked as CD45low/−CD34+ KDR+CD133+ were measured by flow cytometry. B, C and D: the level of apelin, VEGF and SDF-1 were determined using ELISA kits. **P < 0.01, *P < 0.05. Results are represented as mean±SD. AMI: acute myocardial infarction; CAD: coronary artery disease. PBMCs: peripheral blood mononuclear cells. SDF-1: stromal cell-derived factor-1; VEGF: vascular endothelial growth factor. AMI n = 87; Controls n = 39; CAD n = 33.
The plasma level of apelin at baseline was inversely correlated with Gensini score (Table 1) (r = -0.47, P < 0.01) and early EPCs levels (%) (r = -0.41, P < 0.01).
Acute changes in early EPCs and plasma VEGF and SDF-1 levels during the first 24 h in AMI
CD45low/–CD34+ KDR+CD133+EPCs
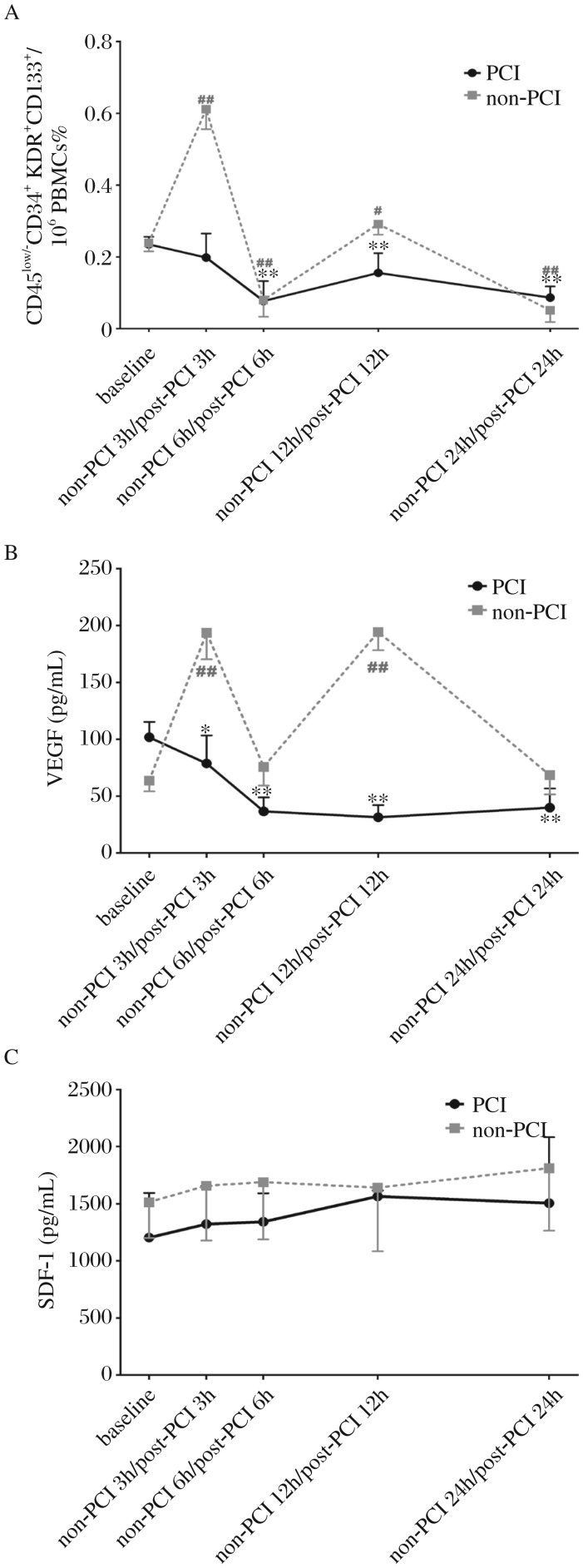
Since the mobilization of EPCs may be affected by the PCI procedure[26], we investigated early EPCs of AMI in the two groups during the first 24 h. For AMI patients who received primary PCI (PCI, n = 43), early EPCs reached the peak at the baseline, and declined in the next 24 h, without significant difference in the levels of early EPCs at baseline and post-PCI 3 h (baseline vs post-PCI 3 h, 0.24%±0.02% vs 0.20%±0.07%, Pbaseline vs post-PCI 3 h > 0.05). EPCs at baseline was 3-, 1.5-, and 2.7-folds higher than those at post-PCI 6, 12 and 24 h, respectively (baseline vs post-PCI 6 h/post-PCI 12 h/post-PCI 24 h, 0.24%±0.02% vs 0.08%±0.06%/0.16%±0.06%/0.09%±0.03%, Pbaseline vs post-PCI 6 h < 0.01, Pbaseline vs post-PCI 12 h < 0.01, and Pbaseline vs post-PCI 24 h < 0.01).
For AMI patients who did not receive primary PCI (non-PCI, n = 44), the level of early EPCs (3 h) was maximized during the first 24 h and reached another high spot at 12 h later (baseline vs non-PCI 3 h/non-PCI 12 h, 0.24%±0.02% vs 0.61%±0.06%/0.29%±0.03%, Pbaseline vs non-PCI 3 h < 0.01, Pbaseline vs non-PCI 12 h < 0.05). The early EPCs level at baseline was 3- and 5-folds higher than that at 6 h and 24 h, respectively (baseline vs 6 h/24 h, 0.24%±0.024% vs 0.08%±0.047%/0.052%±0.033%, Pbaseline vs non-PCI 6 h < 0.01, and Pbaseline vs non-PCI 24 h < 0.01) (Fig. 2A).
Fig. 2. Acute changes of early EPCs and plasma VEGF and SDF-1 levels during the first 24 h in AMI.
A: Early EPCs marked as CD45low/−CD34+ KDR+CD133+ measured by flow cytometry. B and C: the level of VEGF and SDF-1 was determined using ELISA kits. PBMCs: peripheral blood mononuclear cells. SDF-1: stromal cell-derived factor-1; VEGF: vascular endothelial growth factor. PCI (n = 43), non-PCI (n = 44). Compared with the baseline of receiving primary PCI (PCI), **P < 0.01, *P < 0.05. Compared with the baseline of not receiving primary PCI (non-PCI), ##P < 0.01, #P < 0.05.
Plasma concentrations of cytokines
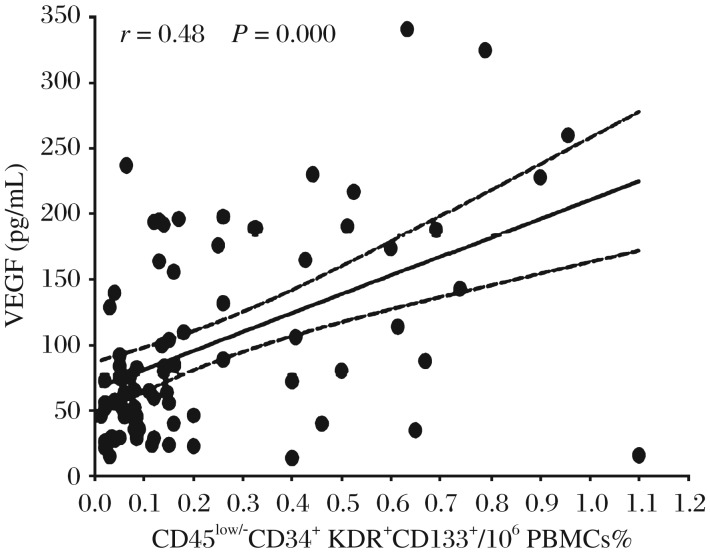
The pro-angiogenic factor VEGF level in plasma followed a similar pattern to early EPCs. In the PCI group, VEGF peaked at the baseline and then significantly declined during the next 24 h (baseline vs post-PCI 3 h/post-PCI 6 h/post-PCI 12 h/post-PCI 24 h, 101.69±13.53 vs 78.60±24.64/36.46±12.36/ 31.36±10.71/39.72±16.97 pg/mL, Pbaseline vs post-PCI 3 h < 0.05, Pbaseline vs post-PCI 6 h < 0.01, Pbaseline vs post-PCI 12 h < 0.01, and Pbaseline vs post-PCI 24 h < 0.01). An M-shaped pattern with a significant increase at 3 and 12 h after admission emerged in non-PCI patients (baseline vs 3 h/12 h, 63.63±9.52 vs 193.74±23.46/194.32±15.97 pg/mL, Pbaseline vs non-PCI 3 h < 0.01, Pbaseline vs non-PCI 12 h < 0.01). There was no significant difference in the levels of EPCs at baseline and at non-PCI 6 and 24 h (baseline vs non-PCI 6 h/non-PCI 24 h, 63.63±9.52 vs 75.64±16.53/68.40±16.85 pg/mL, Pbaseline vs non-PCI 6 h > 0.05, Pbaseline vs non-PCI 24 h > 0.05) (Fig. 2B). Plasma SDF-1 in the two groups remained at similar levels during the first 24 h (baseline vs post-PCI 3 h/post-PCI 6 h/post-PCI 12 h/post-PCI 24 h, 1203.12±389.50 vs 1321.41±333.52/1341.00±248.89/1563.57±51.01/1503.66±578.19 pg/mL, Pbaseline vs post-PCI 3 h > 0.05, Pbaseline vs post-PCI 6 h > 0.05, Pbaseline vs post-PCI 12 h > 0.05, and Pbaseline vs post-PCI 24 h > 0.05), (baseline vs non-PCI 3 h/non-PCI 6 h/non-PCI 12 h/non-PCI 24 h, 1512.13±313.83 vs 1656.39±479.47/1688.04±501.95/1639.56±556.17/ 1810.67±546.88 pg/mL, Pbaseline vs non-PCI 3 h > 0.05, Pbaseline vs non-PCI 6 h > 0.05, Pbaseline vs non-PCI 12 h > 0.05, and Pbaseline vs non-PCI 24 h > 0.05) (Fig. 2C). The level of early EPCs (%) was proportionally correlated with the plasma VEGF (pg/mL) (r = 0.48, P < 0.01) (Fig. 3).
Fig. 3. The correlation of early EPCs with VEGF During the first 24 h in AMI.
Spearman's correlation coefficients (r) and P value are indicated. Pair of dotted lines near the fitted line is the 95% median confidence limits for the fitted line. VEGF: vascular endothelial growth factor. PBMCs: peripheral blood mononuclear cells. Early EPCs marked as CD45low/−CD34+ KDR+CD133+.
DISCUSSION
Cardiovascular diseases are the leading cause of death worldwide and corresponding risk assessment has become mandatory in the adult population[27]. Over the past decade, it has become clear that patients with a given combination of risk factors clustered in the so-called metabolic syndromes are more prone to CAD[28]. Apart from classic risk factors, novel biomarkers have recently received much attention[29] and an extreme plasticity is being attributed to apelin and EPCs.
In the present study, we found that the levels of apelin were lower in the AMI group than the CAD group and the control. This result suggested that apelin may be involved in the pathophysiological process of coronary artery stenosis. which is consistent with the findings of a previous study in which apelin may be a new plasma marker for cardiovascular disease in vivo[30],[31]. In this study, the findings also revealed increased level of early EPCs in AMI patients, which means enhanced mobilization of EPCs after AMI. This result agrees with the findings of EPCs participating in angiogenesis and reendothelialization after vascular injury[1],[5]−[7].
Moreover, correlation analysis showed a significant relationship between plasma apelin level, Gensini score and early EPC level. The higher Gensini score is accompanied by a lower apelin level. Gensini score is a useful tool to estimate both the extent and the severity of the lesions of coronary arteries in CAD[32]. So our result suggested that apelin was associated with the extent of coronary stenosis[33],[34]. This finding is consistent with previous reports that the apelin/APJ system played certain pathophysiological roles in vascular disease[5],[35], and apelin and APJ together may promote the formation of new blood vessels[36],[37]. The mechanisms for the asosication of reduced apelin levels with the severity of coronary artery stenosis have not been clarified. The biological roles of apelin are only beginning to be explored. An important view is that apelin directly activates the vascular L-Arg/NOS/NO pathway, which could be one of the most important mechanisms of apelin-regulated vascular function in a physiological condition[38].
EPCs in peripheral blood are thought to participate in postnatal vasculogenesis and ongoing repair of vascular endothelium. Enumeration and functional assessment of EPCs has been considered a novel technique for the assessment of vascular damage and reparative capacity. Elevated levels of early EPCs in peripheral blood may represent the ability of the human body to repair endothelial injuries. Early EPCs possess the capacity to home to sites of vascular injury, to perform neoangiogenesis in vivo and to maintain the homeostasis of vascular endothelium[8]. The level of early EPCs is also related to the degree of endothelial damage and coronary stenosis. The angiogenic activity of apelin may result from the combination of the molecule to APJ, playing a role in the proliferation, migration, and tube formation of endothelial cells[39]. EPCs are circulating angioblasts derived from human bone marrow with a potential to differentiate into endothelial cells[1],[2]. Therefore, our result suggested that apelin may be related to the level of early EPCs. This finding is consistent with previous reports that the apelin/APJ system played certain pathophysiological roles in vascular disease. Apelin activates cell transduction cascades such as serine/ threonine kinase (Akt) and extracellular signal-regulated kinases (ERKs)[23],[40], which lead to the proliferation of endothelial cells and the formation of new blood vessels[2]. Interestingly, knocking out of the apelin gene is associated with a delay in the development of retinal vasculature[41].
The strength of our findings is reflected by the precise methodology for the evaluation of EPCs. In the present study, cells analyzed by the flow cytometry analysis were "true" early EPCs, characterized as CD45low/−CD34+ KDR+CD133+ cells, rather than undefined progenitor cells[42],[43]. The level of EPCs measured by flow cytometry was previously suggested as a biomarker to assess cardiovascular disease risk[44],[45]. It is now accepted that EPCs may exert an important function as an endogenous repair mechanism to maintain the integrity of the endothelial monolayer by replacing lost cells in the denuded part of the artery[46]. Thus, the greater the number of early EPCs is, the better mobilization of EPCs from the bone marrow is, which may enhance more therapeutic properties.
This study provides new insights into the relationships between change in cytokines and mobilizations of EPCs by showing the change patterns of cytokines and early EPCs during the first 24 h after admission. In this study population, this association may be ascribed to the extent of vascular tree under ischemia, which is particularly large in this clinical condition and may represent a continuous stimulus for EPC mobilization from the bone marrow into peripheral blood. High VEGF levels were significantly associated with increased early EPC levels, suggesting that this cytokine may contribute to EPC mobilization. Two studies that support this result were conducted by Scheubel et al.[47] and Asahara et al.[48] who demonstrated a significant association between circulating VEGF and EPCs. Furthermore, our data were different from those obtained by Roberts et al.[49] and Francesca et al.[50], who failed to demonstrate a correlation between EPCs and VEGF after cardiac surgery, and by Shomig et al. in AMI patients, in whom high levels of IL-8, but not of VEGF, were associated with progenitor mobilization[11]. These may be related to different defining characteristics of target progenitor cells, different types of progenitor cell analysis as well as different methods used for determining VEGF and IL-8 levels.
Local delivery of SDF-1 can enhance EPC recruitment and neovascularization. Combined delivery of SDF-1 and EPCs into sites of limb ischemia promotes local EPC-mediated vasculogenesis. SDF-1 has also been shown to promote bone marrow cell proliferation and angiogenesis[51]. Although effects of SDF-1 have been relatively extensively studied in vitro and in animal models, the role of SDF-1 in humans and especially in patients with myocardial ischemia has been poorly described so far. The present findings fail to show enhanced plasma SDF-1 in the cases of acute tissue ischemia. This study included a small number of patients, and therefore the ability to generalize the differences was limited. Larger study population would provide higher statistical power. Besides, local other than plasma SDF-1 may contribute to enhance progenitor cell recruitment and neovascularization. Therefore, although the exact clinical significance of SDF-1 in patients with AMI remains to be elucidated, it is tempting to speculate that SDF-1 may play a role in vascular and myocardial remodeling or regeneration in patients with AMI. Further studies are needed to elucidate this pathophysiological mechanism. Understanding the role of VEGF, SDF-1 and apelin in EPCs function and tissue regeneration may help to develop novel therapeutic strategies in treatment of patients with myocardial damage.
This study indicated the possible correlation of plasma apelin levels with the severity of coronary artery stenosis and early EPCs in humans, and plasma VEGF with early EPCs. Endogenous apelin and EPCs are required for the repair of endothelial damage, and apelin[52] and early EPC induction together with VEGF significantly restore ischemic damages by formation of new blood vessels. From the pathophysiological point of view, it might be speculated that apelin acts as a vasopressor in damaged vasculature (e.g., atherosclerosis) and plasma apelin levels may be a useful indicator for the severity of coronary artery stenosis. The measurement of plasma apelin levels may be useful for predicting the severity of coronary artery stenosis.
Acknowledgments
All authors appreciate participating nurses and physicians for their excellent work with patients, and technicians for their skillful technical assistance.
Footnotes
This work was supported by the program (No. CX10B_421Z to Jiaxin Ye) for Postgraduate Research Innovation in Universities of Jiangsu Province; the grants (No. 81070195) and (No. 81000055) from Chinese National Science Fund of China (all to Biao Xu), and grant (No. KF200938 to Lina Kang) from Jiangsu Province.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 3.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34 (+) cells identifies a population of functional endothelial precursor. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 4.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185–9. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 5.Dignat-George F, Sampol J. Circulating endothelial cells in vascular disorders: new insights into an old concept. Eur J Haematol. 2000;65:215–20. doi: 10.1034/j.1600-0609.2000.065004215.x. [DOI] [PubMed] [Google Scholar]
- 6.Mutin M, Canavy I, Blann A, Bory M, Sampol J, Dignat-George F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood. 1999;93:2951–8. [PubMed] [Google Scholar]
- 7.Makin A, Chung NAY, Silverman SH, Blann A, Lip GY. Assessment of endothelial damage in atherosclerotic vascular disease by quantification of circulating endothelial cells. Relationship with von Willebrand factor and tissue factor. Eur Heart J. 2004;25:371–6. doi: 10.1016/j.ehj.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Insner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–6. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, et al. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann NY Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. [DOI] [PubMed] [Google Scholar]
- 10.Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–60. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 11.Schömig K, Busch G, Steppich B, Sepp D, Kaufmann J, Stein A, et al. Interleukin-8 is associated with circulating CD133+ progenitor cells in acute myocardial infarction. Eur Heart J. 2006;27:1032–7. doi: 10.1093/eurheartj/ehi761. [DOI] [PubMed] [Google Scholar]
- 12.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 13.Werner N, Priller J, Laufs U, Endres M, Böhm M, Dirnagl U, et al. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–72. doi: 10.1161/01.atv.0000036417.43987.d8. [DOI] [PubMed] [Google Scholar]
- 14.Heeschen C, Aicher A, Lehmann R, Fichtlscherer S, Vasa M, Urbich C, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–6. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 15.Pistrosch F, Herbrig K, Oelschlaegel U, Richter S, Passauer J, Fischer S, et al. PPARγ-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183:163–7. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 16.Bahlmann FH, de Groot K, Mueller O, Hertel B, Haller H, Fliser D. Stimulation of endothelial progenitor cells: a new putative therapeutic effect of Angiotensin II receptor antagonists. Hypertension. 2005;45:526–9. doi: 10.1161/01.HYP.0000159191.98140.89. [DOI] [PubMed] [Google Scholar]
- 17.Falcão-Pires I, Leite-Moreira AF. Apelin: a novel neurohumoral modulator of the cardiovascular system. Pathophysiologic importance and potential use as a therapeutic target. Rev Port Cardiol. 2005;24:1263–76. [PubMed] [Google Scholar]
- 18.Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Sheikh AY, Chun HJ, Glassford AJ, Kundu RK, Kutschka I, Ardigo D, et al. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol. 2008;294:H88–98. doi: 10.1152/ajpheart.00935.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronkainen VP, Ronkainen JJ, Hänninen SL, Leskinen H, Ruas JL, Pereira T, et al. Hypoxia inducible factor regulates the cardiac expression and secretion of apelin. FASEB J. 2007;21:1821–30. doi: 10.1096/fj.06-7294com. [DOI] [PubMed] [Google Scholar]
- 21.Atluri P, Morine KJ, Liao GP, Panlilio CM, Berry MF, Hsu VM, et al. Ischemic heart failure enhances endogenous myocardial apelin and APJ receptor expression. Cell Mol Biol Lett. 2007;12:127–38. doi: 10.2478/s11658-006-0058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashimoto T, Kihara M, Imai N, Yoshida S, Shimoyamada H, Yasuzaki H, et al. Requirement of apelin-apelin receptor system for oxidative stress-linked atherosclerosis. Am J Pathol. 2007;171:1705–12. doi: 10.2353/ajpath.2007.070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masri B, Morin N, Cornu M, Knibiehler B, Audigier Y. apelin (65-77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J. 2004;18:1909–11. doi: 10.1096/fj.04-1930fje. [DOI] [PubMed] [Google Scholar]
- 24.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51:606. doi: 10.1016/s0002-9149(83)80105-2. [DOI] [PubMed] [Google Scholar]
- 25.Rustemeyer P, Wittkowski W, Jurk K, Koller A. Optimized flow cytometric analysis of endothelial progenitor cells in peripheral blood. J Immunoassay Immunochem. 2006;27:77–88. doi: 10.1080/15321810500403789. [DOI] [PubMed] [Google Scholar]
- 26.Lev EI, Kleiman NS, Birnbaum Y, Harris D, Korbling M, Estrov Z. Circulating Endothelial Progenitor Cells and Coronary Collaterals in Patients with Non-ST Segment Elevation Myocardial Infarction. J Vasc Res. 2005;42:408–14. doi: 10.1159/000087370. [DOI] [PubMed] [Google Scholar]
- 27.Buchwald H. Risk reduction and the program on the surgical control of the hyperlipidemias. Circulation. 2001;104:E47. doi: 10.1161/01.cir.104.9.e47. [DOI] [PubMed] [Google Scholar]
- 28.Avogaro P, Crepaldi G, Enzi G, Tiengo A. Associazione di iperlipidemia, diabete mellito ed obesita` di medio grado. Acta Diabetol Lat. 1967;4:572–90. [Google Scholar]
- 29.Fruchart J, Nierman M, Stroes E, Kastelein J, Duriez P. New risk factors for atherosclerosis and patient risk assessment. Circulation. 2004;109(23 S1):III15–9. doi: 10.1161/01.CIR.0000131513.33892.5b. [DOI] [PubMed] [Google Scholar]
- 30.Goetze JP, Rehfeld JF, Carlsen J, Videbaek R, Andersen CB, Boesgaard S, et al. Apelin: a new plasma marker of cardiopulmonary disease. Regul Pept. 2006;133(1-3):134–8. doi: 10.1016/j.regpep.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Bai Y, Hu J. Reduced apelin levels in stable angina. Intern Med. 2008;47:1951–5. doi: 10.2169/internalmedicine.47.1287. [DOI] [PubMed] [Google Scholar]
- 32.Ringqvist I, Fisher LD, Mock M, Davis KB, Wedel H, Chaitman BR, et al. Prognostic value of angiographic indices of coronary artery disease from the Coronary Artery Surgery Study (CASS) J Clin Invest. 1983;71:1854–66. doi: 10.1172/JCI110941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest. 2008;118:3343–54. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadoglou NP, Lampropoulos S, Kapelouzou A, Gkontopoulos A, Theofilogiannakos EK, Fotiadis G, et al. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease--KOZANI STUDY. Transl Res. 2010;155:238–46. doi: 10.1016/j.trsl.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Berry MF, Pirolli TJ, Jayasankar V, Burdick J, Morine KJ, Gardner TJ, et al. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation. 2004;110(11 S1):II187–93. doi: 10.1161/01.CIR.0000138382.57325.5c. [DOI] [PubMed] [Google Scholar]
- 36.Saint-Geniez M, Masri B, Malecaze F, Knibiehler B, Audigier Y. Expression of the murin msr/apj receptor and its ligand apelin is upregulated during formation of the retinal vessels. Mech Dev. 2002;110(1-2):183–6. doi: 10.1016/s0925-4773(01)00558-5. [DOI] [PubMed] [Google Scholar]
- 37.Cox CM, D'Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006;296:177–89. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 38.Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, et al. Apelin activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides. 2007;28:2023–9. doi: 10.1016/j.peptides.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 39.Kasai A, Shintani N, Oda M, Kakuda M, Hashimoto H, Matsuda T, et al. Apelin is a novel angiogenic factor in retinal endothelial cells. Biochem Biophys Res Commun. 2004;325:395–400. doi: 10.1016/j.bbrc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 40.Masri B, Lahlou H, Mazarguil H, Knibiehler B, Audigier Y. Apelin (65-77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem Biophys Res Commun. 2002;290:539–45. doi: 10.1006/bbrc.2001.6230. [DOI] [PubMed] [Google Scholar]
- 41.Kasai A, Shintani N, Kato H, Matsuda S, Gomi F, Haba R, et al. Retardation of retinal vascular development ion apelin-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1717–22. doi: 10.1161/ATVBAHA.108.163402. [DOI] [PubMed] [Google Scholar]
- 42.Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133+ cells. Br J Haematol. 2001;115:186–94. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 43.Leone AM, Valgimigli M, Giannico MB, Zaccone V, Perfetti M, D'Amario D, et al. From bone marrow to the arterial wall:the ongoing tale of endothelial progenitor cells. Eur Heart J. 2009;30:890–9. doi: 10.1093/eurheartj/ehp078. [DOI] [PubMed] [Google Scholar]
- 44.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 45.Briguori C, Testa U, Riccioni R, Colombo A, Petrucci E, Condorelli G, et al. Correlations between progression of coronary artery disease and circulating endothelial progenitor cells. FASEB J. 2010;24:1981–8. doi: 10.1096/fj.09-138198. [DOI] [PubMed] [Google Scholar]
- 46.Ross R. Atherosclerosis-- an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 47.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, et al. Age dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–80. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 48.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts N, Xiao Q, Weir G, Xu Q, Jahangiri M. Endothelial progenitor cells are mobilized after cardiac surgery. Ann Thorac Surg. 2007;83:598–605. doi: 10.1016/j.athoracsur.2006.09.087. [DOI] [PubMed] [Google Scholar]
- 50.Cesari F, Caporale R, Marcucci R, Caciolli S, Stefano PL, Capalbo A, et al. NT-proBNP and the anti-inflammatory cytokines are correlated with endothelial progenitor cells' response to cardiac surgery. Atherosclerosis. 2008;199:138–46. doi: 10.1016/j.atherosclerosis.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 51.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 52.Kidoya H, Naito H, Takakura N. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood. 2010;115:3166–74. doi: 10.1182/blood-2009-07-232306. [DOI] [PubMed] [Google Scholar]