Abstract
Remodeling of ion channels is an important mechanism of arrhythmia induced by heart failure (HF). We investigated the expression of potassium channel encoding genes in the ventricles of rabbit established by volume-overload operation followed with pressure-overload. The reversible effect of these changes with bisoprolol was also evaluated. The HF group exhibited left ventricular enlargement, systolic dysfunction, prolongation of corrected QT interval (QTc), and increased plasma brain natriuretic peptide levels in the HF rabbits. Several potassium channel subunit encoding genes were consistently down-regulated in the HF rabbits. After bisoprolol treatment, heart function was improved significantly and QTc was shortened. Additionally, the mRNA expression of potassium channel subunit genes could be partially reversed. The down-regulated expression of potassium channel subunits Kv4.3, Kv1.4, KvLQT1, minK and Kir 2.1 may contribute to the prolongation of action potential duration in the heart of rabbits induced by volume combined with pressure overload HF. Bisoprolol could partially reverse these down-regulations and improve heart function.
Keywords: heart failure, potassium channel, down-regulation, animal models
INTRODUCTION
Heart failure is a clinical syndrome caused by various impairments in cardiac function. Sudden death, associated with arrhythmia, is responsible for approximately half of mortality rate among patients with heart failure[1]. Remodeling of ion channels and ion-transport function are an important mechanism of arrhythmia induced by diseased heart. The prolonged action potential duration (APD), associated with early after-depolarization (EAD), is a consistent finding in the ventricular myocytes of patients with heart failure or of animal models[2].
APD prolongation reflects an increased inward current or decreased outward current during the plateau of the action potential (AP)[3]. Potassium current as an outward current plays a pivotal role in APD. Transcriptional down-regulation underlies the molecular basis of changes in K+ currents. Down-regulation of Kv4.3 and Kv1.4 subunits underlies Ito reduction in human failing hearts and rabbit failing hearts induced by ventricular tachycardia[4]–[6]. Reduced IKs appears to be due to transcriptional down-regulation of the α-subunit (KvLQT1) and β-subunit (minK) in rabbit heart failure model induced by ventricular tachycardia[5]. The change of IKr units ERG has been consistent. Few studies have reported reduction of ERG[6]. Similar discrepancies exist in the study of IK1 unit Kir2.1[4],[7].
Furthermore, although the beneficial effects of β-blocker therapy have been known for quite a while[8]–[10], their exact mechanisms of action still remain incompletely understood. Studies report that most of ionic currents affected by β-adrenergic stimulation are also altered by β-blockers[11],[12]. In the present study, we explored the prolongation of the QT interval and down-regulation of K+ channel expression in rabbit heart failure model induced by volume-overload combined with pressure overload. The reversibility of these changes with bisoprolol was also demonstrated.
MATERIALS AND METHODS
Animal groups and materials
Thirty-one male New Zealand rabbits weighing 2.5 kg and 3.5 kg were obtained from Xi'an Jiaotong University Laboratorial Animal Center (Shaanxi, China). All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication, No. 85-23, revised in 1996). Additionally, all experimental procedures were approved by the Care of Experimental Animals Committee of the First Affiliated Hospital of Medical School, Xi'an Jiaotong University, Shaanxi, China. After evaluation by echocardiography, the rabbits were randomly divided into three groups, namely, sham-operation rabbits as the control group (CTL, n=10), heart failure group (HF, n=10) and heart failure with bisoprolol treatment group (Biso, n=11).
All supplements were purchased as follows: Trizol from Sigma (USA), SYBR® Premix Ex Taq™ II from Takara (Dalian, China), rabbit BNP ELISA kit from Xitang Shanghai Biological Technology Co., Ltd. (Shanghai, China), bisoprolol fumarate tablets from Merck Serono (Germany), and 5F catheter from Cordis (USA).
Establishment of heart failure model
Induction of heart failure model was performed according to the method of Den[13] with some modifications. In brief, following induction of anesthesia (sodium pentobarbital, 30 mg/kg, ip), a 5F catheter was introduced into the right carotid artery to induce aortic insufficiency by repeatedly moving the catheter to and fro across the aortic valve until the pulse pressure increased by >50%. After 3 w, rabbits were anesthetized to induce pressure overload as described above. Through a mid-abdominal incision the aorta was freed from the adjacent tissues at a point just above the renal arteries. A stainless steel rod (external diameter of 2.3 mm) was placed next to the aorta. A ligature was tied around the aorta and rod after which the rod was removed. This resulted in a reduction of the aortic diameter of approximately 50%. In sham operated rabbits, the right carotid artery and abdominal aorta was only freed from the adjacent tissues. After induction, the Biso group rabbits began to receive bisoprolol treatment (1 mg/kg/d) by gastric lavage for about 8 w.
Evaluation of heart function
After 8 w of induction of pressure overload, rabbit heart function of the three groups was monitored by echocardiographic measurements. Left arterial diameter (LAD), aortic diameter (AOD), left ventricular end-diastolic dimension (LVDD), left ventricular end-systolic dimension (LVDS), left ventricular posterior wall (LVPW), interventricular septal thickness (IVST), ejection fraction (EF), fractional shortening (FS), and early diastolic filling to atrial filling velocity ratio of mitral flow (E/A) were measured. QT interval was measured using a standard lead II electrocardiogram (ECG). The QT interval was corrected (QTc) according to Carlsson's formula[14] for rabbits with QTc= QT-0.175(RR-300),and brain natriuretic peptide (BNP) in blood was measured using enzyme-linked immunosorbent assay (ELISA) kit.
cDNA synthesis and quantitative real-time PCR analysis
Ventricular tissue RNA isolation and reverse transcription were carried out using the Superscript first-strand synthesis system (Takara, Dalian, China) according to the manufacturer's instructions. Specific primers for rabbit Kir2.1, Kv4.3, Kv1.4, KvLQT1, minK, ERG and GAPDH are shown in Table 1. Real-time PCR was performed on an iQ5 Real-Time PCR Detection System (BIO-RAD, USA) using the SYBR® Premix Ex Taq™ II (Takara, Dalian, China) with an initial cycle of 95°C for 10 s followed by 50 cycles of 95°C for 5 s and 60°C for 31 s. Relative quantitation of target gene expression normalized to GAPDH was calculated according to the 2−ΔΔCT method.
Table 1. Specific primers for rabbit target genes.
| Gene | Gene ID | Primer (5′-3′) | Size (bp) | TM (°C) |
| Kir2.1 | NM001082198 | F: ACTCCCCTGTGTAGTGCCAGA R: GCCTGGTTGTGAAGGTCAATG |
179 | 62.0 |
| Kv4.3 | NC013681 | F: GCCGCAGTAAGAAGACCACAC R: TTGGTCTCAGTCCGTCGTCTG |
165 | 62.0 |
| Kv1.4 | NC013669 | F: CAGCAGCAACAGGCCATGTC R: CTCCGCGAAATACACAGCCT |
204 | 63.6 |
| KvLQT1 | NW003159627 | F: GCCGCAGCAGTATGTCG R: CCTTCTCAGCAGGTACACGA |
317 | 58.6 |
| minK | NW003159355 | F: GAGACGGCCCACCTACGG R: CGAAGAAGCCGAGCACCAT |
112 | 57.0 |
| ERG | NW003159353 | F: TCGCACCATTAGCAAGATTC R: GGATGAGCCAGTCCCACA |
263 | 62.0 |
| GAPDH | NC013676 | F: CTCTGGGGCTGTGGCGT R: GCTCGGGGATGACCTTGC |
99 | 63.2 |
Statistical analysis
Data were expressed as mean±SEM. Statistical comparisons between two groups were performed with one-way ANOVA test. Differences were considered significant at P < 0.05.
RESULTS
Characteristics of heart function of the three groups
Heart failure was confirmed by both echocardiographic examination and plasma BNP level test at 8 weeks after the pressure overload induction. As shown in Table 2, compared with those of control rabbits, LAD, AOD, LVDD, LVDS, LVPW, IVST, and FS increased and EF decreased significantly in heart failure rabbits. Furthermore, plasma BNP level, a valuable marker of heart failure[15], also increased significantly in heart failure rabbits. After bisoprolol treatment, EF, FS and E/A of heart failure rabbits were increased significantly, compared with heart failure rabbits. In addition, BNP level decreased.
Table 2. Characteristics of heart function measurement in the three groups.
| CTL | HF | Biso | |
| LAD (mm) | 7.73±0.41 | 9.16±1.77* | 8.51±0.41*# |
| AOD (mm) | 7.25±0.34 | 8.52±0.59* | 8.20±0.64*# |
| LVDD (mm) | 11.68±1.09 | 19.67±3.98* | 15.10±1.21*# |
| LVDS (mm) | 7.39±1.89 | 13.33±3.64* | 7.70±1.32*# |
| LVPW (mm) | 2.41±0.72 | 3.23±0.37* | 2.70±0.27*# |
| IVST (mm) | 2.16±0.81 | 3.42±0.54* | 2.90±0.23*# |
| EF (%) | 71.10±1.73 | 60.20±0.39* | 67.20±0.25*# |
| FS (mm) | 37.00±2.33 | 32.83±4.53* | 34.00±4.57*# |
| E/A | 2.65±0.35 | 2.00±0.53* | 2.35±0.81*# |
| BNP (nmol/L) | 2.83±0.35 | 4.65±0.47* | 3.78±0.67*# |
*P < 0.05 vs control group (CTL); #P < 0.05 vs heart failure group (HF). AOD: aortic diameter; BNP: brain natriuretic peptide; E/A: early diastolic filling to atrial filling velocity ratio of mitral flow; EF: ejection fraction; FS: fractional shortening; IVST: interventricular septal thickness; LAD: left arterial diameter; LVDD: left ventricular end-diastolic dimension; LVDS: left ventricular end-systolic dimension; LVPW: left ventricular posterior wall.
ECG changes
Table 3 shows QT and QTc in electrocardiograhic results from the three groups. In the HF group, QT and QTc increased to (114±6) ms and (137±5) ms, respectively. However, QT and QTc increased to (117±5) ms and (130±4) ms, respectively, in Biso group.
Table 3. In vivo measurements.
| Body weight (kg) | Heart rate | QT interval (ms) | QTc (ms) | |
| CTL | 3.38±0.26 | 284±11 | 110±9 | 125±7 |
| HF | 3.29±0.36 | 335±13** | 114±6 | 137±5* |
| Biso | 3.32±0.37 | 310±16*# | 117±5 | 130±4*# |
*P < 0.05 vs control (CTL), #P < 0.05 vs heart failure (HF). QTc: corrected QT interval.
Expression of Ito subunit mRNA
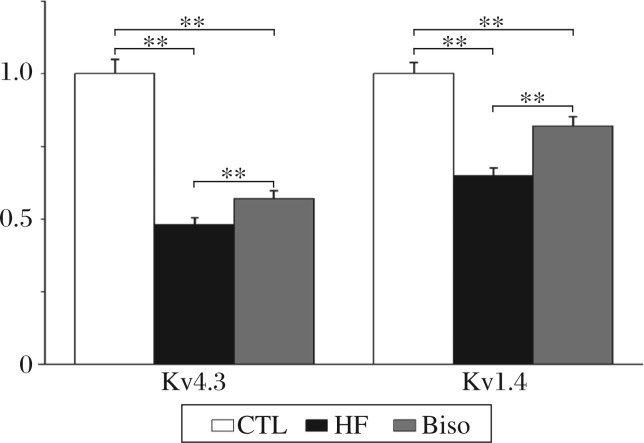
The expression of Ito subunit mRNA was evaluated. As shown in Fig. 1, the expression of Kv4.3 was significantly down-regulated by 52% in the HF group (P < 0.01). In the Biso group, expression of Kv4.3 was reduced by 44% (P < 0.01). Similar results were obtained for Kv1.4 mRNA expression, reduction was 34% in the HF group (P < 0.01), but 17% in the Biso group (P < 0.01).
Fig. 1. Histogram comparing relative amounts of the potassium channel isoform Kv4.3 and Kv1.4 mRNA in the ventricular myocardium of rabbits from the three groups.
**P < 0.01. CTL: the control group; HF: the heart failure group; Biso: the bisoprolol group.
Expression of Iks and Ikr subunit mRNA
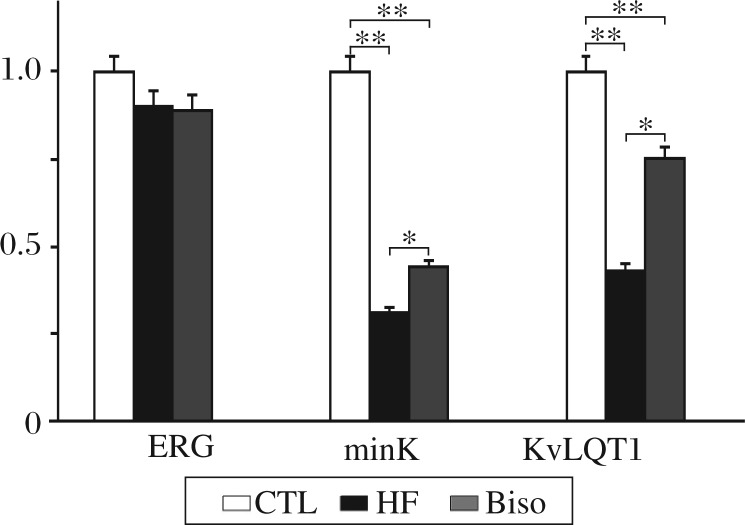
Fig. 2 shows ERG, minK and KvLQT1 expression in the left ventricles of rabbits from the three groups. KvLQT1 expression was reduced by 69% in the HF group and in the Biso by 56%. In addition, minK expression was reduced by 57% in the heart failure rabbits but 25% in bisoprolol rabbits. However, there were no significant differences in ERG expression among rabbits from the three groups.
Fig. 2. Real-time PCR results of KvLQT1, minK and ERG.
Expression of KvLQT1 was 0.28±0.11 in the HF rabbits, 0.40±0.10 in the Biso group. Expression of minK was 0.39±0.09 in the HF rabbits, 0.68±0.08 in the Biso group. *P < 0.05, **P < 0.01. CTL: the control group; HF: the heart failure group; Biso: the bisoprolol group.
Expression of Ik1 subunit mRNA
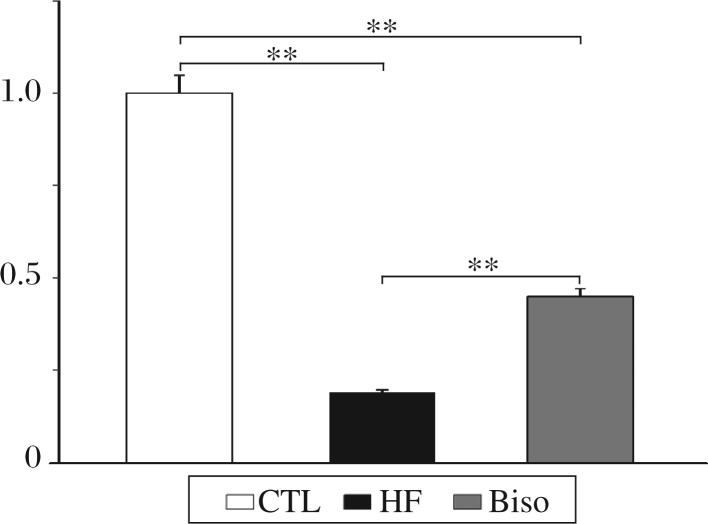
Kir2.1 expression in the left ventricles of rabbits from the three groups is shown in Fig. 3. Kir2.1 mRNA levels exhibited a trend toward reduced expression in failing ventricles. However, bisoprolol could reverse the change significantly. Compared with that of the control rabbits, the expression of Kir2.1 was down-regulated by 81% in HF rabbits and by 55% in Biso group.
Fig. 3. Real-time PCR results of Kir2.1.
Relative fold of expression on Kir2.1 was 0.24±0.08 and 0.57±0.09 in the HF group and Biso group, respectively. **P < 0.01. CTL: the control group; HF: the heart failure group; Biso: the bisoprolol group.
DISCUSSION
In this study, we found that the expression of several genes encoding potassium channel isoforms (Ito, IKs and IK1 subunits) was down-regulated in the heart of rabbits with volume combined with pressure overload heart failure. This may account for the prolongation of APD of ventricular myocytes. Treatment with bisoprolol, a β-blockers, could partially reverse these down-regulations and improve heart function.
Rabbits have a distinct plateau stage in action potential and a similar expression of K+ channels to human's. In our study, the heart failure model was induced in rabbits by volume-overload combined with pressure-overload procedure and confirmed by echocardiographic examination and plasma BNP level test. The model with both systolic and diastolic dysfunctions has a similar pathophysiology to a human one, including ventricular hypertrophy and dilatation, combined with decreased EF and FS 8 weeks after surgery by echocardiography. Plasma BNP was increased significantly and QTc was prolonged in ECG of failing heart.
Potassium currents play a key role in the formation of APD[2]. Transient outward current (Ito, encoded by Kv4.3 and Kv1.4) is the main current in phase 1 early rapid repolarization. Phase 3 is a late repolarization phase of APD dominated by IKr (encoded by ERG and minK) and Iks (encoded by KvLQT1 and minK). IK1 (encoded by Kir2.1) is a determinant factor for maintenance of resting potential in phase 4. APD is shown as QT in ECG clinically. Prolonged QTc is consistent with findings in studies of human and animal heart failure models[16],[17].
Studies have shown that repolarization abnormalities and QT/APD prolongation may be due to changes of K+ currents induced by heart failure[6],[18]. In our study, there was a reduction in mRNA level of Kv4.3 by 52%, Kv1.4 by 34 %, KvLQT1 by 69%, minK by 57% and Kir2.1 by 81% in the left ventricles from the heart failure rabbit. However, ERG mRNA was not observed with great changes. Such remodeling of K+ channel expression was accompanied with QTc prolongation. Our data are in agreement with those of previous studies in the failing ventricles from human and animal models. In rabbit failing heart induced by tachypacing, studies showed that Kv4.3, Kv1.4, KvLQT1 and minK mRNA were reduced[6]. In canine heart failure model induced by tachypacing, a significant decrease in Kv4.3 mRNA[19] has been found. In the human failing heart, Kir2.1 and Kv4.3 mRNA was measured with a striking decrease by gene microarray analysis, while KvLQT1, minK and ERG mRNA were found[4].
Prolongation of QT or APD, reflecting impaired repolarization, is a consistent finding in previous studies[20],[21]. Early after-depolarizations (EADs) and delayed after-depolarizations (DAD) are the heart failing-induced arrhythmias which are associated with remodeling of K+ currents. EAD commonly occurs in phase 2 and phase 3 of APD. EAD is the result of reduction of IKs, IKr, Ito and IK1[2],[21]. DADs are favored by increased Na+-Ca2+ exchange and reduced IK1. Remodeling of K+ currents plays a key role in cardiac arrhythmias associated with heart failing. The decrease of mRNA expression of K+ channels subunits underlies the molecular down-regulation of K+ currents[2].
Bisoprolol has been confirmed to be beneficial for shortening prolonged QT induced by heart failure in clinical trials and animal experiments[23],[24]. Our present study is in agreement with previous reports. Our previous study on heart failure rats showed a significant reduction of Ito and IK1 and expression of Ito and IK1 at the protein level. We also found that bisoprolol could reverse the down-regulation of IKr, Ito and IK1. The interesting findings showed in our study are that bisoprolol could reverse down-regulation of K+ current expression. These findings may underlie the molecular mechanism for antiarrhythmia of bisoprolol.
Ion-channel function is regulated by channel phosphorylation state, neurotransmitters, and interactions with other channels and transporters. In classic β1-adrenoceptor-mediated signaling in cardiomyocyte, protein kinase A (PKA) passway activated via secondary messenger cAMP can phosphorylate many proteins involved in ion channels resulting in changes of their kinetic properties.β-Adrenergic stimulation via cAMP-dependent PKA mechanism enhances IK1 and IKs current[25]. while decreases HERG(α-subunit of IKr) current by 19%-40%[26]. However, exact mechanism of β-blockers modulating the remodeling of potassium channels in cardiomyocyte were still unclear. Experiments with isoproterenol and β-blockers demonstrated that IK is affected by PKA phosphorylation[27]. The results also suggested β-blockers suppress adrenergic stimulation and alter PKA to regulate potassium channels.
In summary, our data demonstrated that down-regulated expression of potassium channel isoforms Kv4.3 and Kv1.4 (encoding Ito subunit), KvLQT1 and minK (for IK) and Kir 2.1 (for IK1) may contribute to the prolongation of APD in the heart of rabbits with volume combined with pressure overload heart failure. Additionally, treatment with bisoprolol, a β-blockers, can partially reverse these down-regulations and improve heart function.
Acknowledgments
We are grateful for Dr. Jian Lin for his help in hemodynamic measurement. We also thank Dr. Xiaohui Fan for revision of the manuscript on English writing.
Footnotes
This work was supported by the State Key Program of the National Natural Science Foundation of China (No. 30830051).
References
- 1.Ramani GV, Uber P A, Mehra MR. Chronic heart failure: contemporary diagnosis and management. Mayo Clin Proc. 2010;85:180–95. doi: 10.4065/mcp.2009.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–56. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 3.Michael G, Xiao L, Qi XY, Dobrev D, Nattel S. Remodelling of cardiac repolarization: how homeostatic responses can lead to arrhythmogenesis. Cardiovasc Res. 2009;81:491–9. doi: 10.1093/cvr/cvn266. [DOI] [PubMed] [Google Scholar]
- 4.Borlak J, Thum T. Hallmarks of ion channel gene expression in end-stage heart failure. FASEB J. 2003;17:1592–608. doi: 10.1096/fj.02-0889com. [DOI] [PubMed] [Google Scholar]
- 5.Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le Bouter S, et al. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res. 2008;103:733–42. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- 6.Tsuji Y, Zicha S, Qi XY, Kodama I, Nattel S. Potassium channel subunit remodeling in rabbits exposed to long-term bradycardia or tachycardia discrete arrhymogenic consequences related to differential delayed-rectifier changes. Circulation. 2006;113:345–55. doi: 10.1161/CIRCULATIONAHA.105.552968. [DOI] [PubMed] [Google Scholar]
- 7.Akar FG, Wu RC, Juang GJ, Tian Y, Disilvestre D, Xiong W, et al. Molecular mechanisms underlying K+ current downregulation in canine tachycardia-induced heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H2887–96. doi: 10.1152/ajpheart.00320.2004. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, et al. Optimize-HF investigators and coordinators. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2008;52:190–9. doi: 10.1016/j.jacc.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Anoymous The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomized trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 10.De Peuter OR, Souverein PC, Klungel OH, Büller HR, de Boer A, Kamphuisen PW. Non-selective vs selective beta-blocker treatment and the risk of thrombo-embolic events in patients with heart failure. Eur J Heart Fail. 2011;13:220–6. doi: 10.1093/eurjhf/hfq176. [DOI] [PubMed] [Google Scholar]
- 11.Workman AJ, Pau D, Redpath CJ, Marshall GE, Russell JA, Kane KA, et al. Post-operative atrial fibrillation is influenced by beta-blocker therapy but not by preoperative atrial cellular electrophysiology. J Cardiovasc Electrophysiol. 2006;17:1230–8. doi: 10.1111/j.1540-8167.2006.00592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savelieva I, Camm J. Anti-arrhythmic drug therapy for atrial fibrillation: current anti-arrhythmic drugs, investigational agents, and innovative approaches. Europace. 2008;10:647–65. doi: 10.1093/europace/eun130. [DOI] [PubMed] [Google Scholar]
- 13.Den Ruijter HM, Berecki G, Verkerk AO, Bakker D, Baartscheer A, Schumacher CA, et al. Acute administration of fish oil inhibits triggered activity in isolated myocytes from rabbits and patients with heart failure. Circulation. 2008;117:536–44. doi: 10.1161/CIRCULATIONAHA.107.733329. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson L, Abrahamsson C, Andersson B, Duker G, Schiller-Linhardt G. Proarrhythmic effects of the class III agent almokalant: importance of infusion rate, QT dispersion, and early afterdepolarisations. Cardiovasc Res. 1993;27:2186–93. doi: 10.1093/cvr/27.12.2186. [DOI] [PubMed] [Google Scholar]
- 15.Boerrigter G, Costello-Boerrigter LC, Burnett JC., Jr Natriuretic peptides in the diagnosis and management of chronic heart failure. Heart Fail Clin. 2009;5:501–14. doi: 10.1016/j.hfc.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vrtovec B, Ryazdanbakhsh AP, Pintar T, Collard CD, Gregoric ID, Radovancevic B. QTc interval prolongation predicts postoperative mortality in heart failure patients undergoing surgical revascularization. Tex Heart Inst J. 2006;33:3–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Arsenos P, Gatzoulis KA, Dilaveris P, Gialernios T, Sideris S, Lazaros G, et al. The rate-corrected QT interval calculated from 24-hour Holter recordings may serve as a significant arrhythmia risk stratifier in heart failure patients. Int J Cardiol. 2011;147:321–3. doi: 10.1016/j.ijcard.2010.12.076. [DOI] [PubMed] [Google Scholar]
- 18.Kolo PM, Opadijo OG, Omotoso AB, Katibi IA, Balogun MO, Araoye MA. Prognostic significance of QT interval prolongation in adult Nigerians with chronic heart failure. Niger J Clin Pract. 2008;11:336–41. [PubMed] [Google Scholar]
- 19.Zicha S, Xiao L, Stafford S, Chan TJ, Han W, Varro A, Nattel S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol. 2004;561:735–48. doi: 10.1113/jphysiol.2004.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rose J, Armoundas AA, Tian Y, DiSilvestre D, Burysek M, Halperin V, et al. Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am J Physiol Heart Circ Physiol. 2005;288:H2077–87. doi: 10.1152/ajpheart.00526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Kim KH, London B, Morales MJ, Backx PH. Dissection of the voltage-activated potassium outward currents in adult mouse ventricular myocytes: I (to,f), I (to,s), I (K,slow1), I (K,slow2), and I (ss) Basic Res Cardiol. 2011;106:189–204. doi: 10.1007/s00395-010-0134-z. [DOI] [PubMed] [Google Scholar]
- 22.Corrias A, Giles WR, Rodriguez B. Ionic mechanisms of electrophysiological properties and repolarization abnormalities in rabbit Purkinje fibers. Am J Physiol Heart Circ Physiol. 2011 Feb 18; doi: 10.1152/ajpheart.01170.2010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol. 2010;641:187–92. doi: 10.1016/j.ejphar.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 24.Grandi E, Pasqualini FS, Pes C, Corsi C, Zaza A, Severi S. Theoretical investigation of action potential duration dependence on extracellular Ca2+ in human cardiomyocytes. J Mol Cell Cardiol. 2009;46:332–42. doi: 10.1016/j.yjmcc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Zicha S, Tsuji Y, Shiroshita-Takeshita A, Nattel S. Beta-blockers as antiarrhythmic agents. Handb Exp Pharmacol. 2006;171:235–66. [PubMed] [Google Scholar]
- 26.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1–KCNE1 potassium channel. Science. 2002;295:496–9. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 27.Li GR, Feng J, Wang Z, Fermini B, Nattel S. Adrenergic modulation of ultrarapid delayed rectifier K+ current in human atrial myocytes. Cir Res. 1996;78:903–15. doi: 10.1161/01.res.78.5.903. [DOI] [PubMed] [Google Scholar]