Abstract
Delayed deterioration associated with vasospasm (DDAV) after subarachnoid hemorrhage (SAH), often called vasospasm, continues to be both a difficult entity to treat and a leading cause of morbidity in patients. Until recently, attention was focused at alleviating the vascular spasm. Recent evidence shows that vascular spasm may not account for all the morbidity of DDAV. There is renewed interest in looking for other potentially targets for therapy. Inflammation has become a promising area of research for new treatments. In this review, we will explore the evidence that inflammation is a driver of DDAV by asking three questions: 1) If inflammation is important in the pathogenesis of the disease, what part or parts of the inflammatory response are involved? 2) When does inflammation occur in the SAH? And 3) in what compartment of the skull does the inflammation occur, the cerebrospinal fluid and meninges, the cerebral arteries or the brain itself.
Keywords: Inflammation, Delayed Cerebral Vasospasm, Subarachnoid Hemorrhage, Neutrophils
Introduction
Delayed neurological deterioration after subarachnoid hemorrhage (SAH) was initially described before the advent of cerebral angiography. After the development cerebral angiography, the association with cerebral vasospasm became apparent prompting researchers to focus on reversal of the vascular spasm. Results of trials using the calcium channel blocker nicardipine and the endothelin-1 antagonist clazosentan revealed an unexpected finding; the cerebral vasculature can be dilated without improvement in patient outcomes(1, 2). Recently, there have been a number of investigators who have questioned whether vascular constriction is associated with ALL the morbidity of the syndrome(3–6). Over the last 15 years, there are a number of laboratories investigating the inflammatory underpinnings of both vascular constriction and the brain damage associated with SAH.
The names associated with delayed neurological deterioration after SAH have been a source of confusion. Over the years, the terms delayed cerebral vasospasm (DCV), delayed ischemic neurological deficits (DIND), and delayed cerebral ischemia (DCI) have all been employed to describe the syndrome. Unfortunately, all of these names assume that the cause of damage is due to vasospasm or ischemia. The studies of nicardipine and clazosentan would suggest that other mechanisms might be at play(1, 2). The ideal name should both appreciate the association with vasospasm and not making assumptions about the cause of damage. I favor the name delayed deterioration associated with vasospasm (DDAV) as it meets both criteria and keeps open the possibility that forces other than ischemia such as inflammation may play a role. In this review, I will use DDAV to describe the syndrome of delayed deterioration and vasospasm to denote specifically changes in vessel caliber.
In this review, we will ask a series of questions about the possible role of inflammation in DDAV. We will describe the research to date that supports the answers to the specific questions.
What type of inflammation is associated with DDAV?
The study of inflammation in SAH dates back more than 30 years with the finding that early elevations in white blood cell (WBC) cell counts are associated with the later development of vasospasm(7–10). A number of studies have investigated inflammation by examining cytokine levels in blood and CSF in patients with SAH. In addition, there have been a number of interesting animal studies that have suggested the involvement of the inflammatory system in DDAV.
We must first define what inflammation is in the context of SAH. The term inflammation very broadly describes a local response to tissue injury that is marked by a number of specific events: increased capillary permeability and fluid extravasation into the tissue, leukocyte infiltration, and the four cardinal signs described by Celsus over 2000 years ago of tumor, calor, rubor, and dolor. Inflammation is the final common pathway of processes directed by the immune system.
The immune system is a complex and tightly regulated system of effectors against external pathogens AND endogenous tissue damage. It can be broadly divided into two component parts, the innate immune system and the adaptive immune system. It is out of the scope of this review to discuss the intricacies of the two systems but a good review this was published in 2000(11). Although the immune system functions both to attack external pathogens and mitigate internal cell malfunctions, the roll of immunity against external pathogens has been better studied.
The innate immune system is the first line of defense against external pathogens that includes processes from the skin (preventing entry of foreign pathogens) to marrow derived innate immune cells that form the first responder corps against bacteria and viruses. In infection, the innate cell response, which consists of mainly neutrophils (also macrophages, natural killer cells and monocytes) enters an infected tissue and nonspecifically release tissue-destroying enzymes and reactive oxygen species. The signals to which innate immune cells respond were originally thought to be due to recognition of self versus non-self. This theory has been largely replaced by a newer theory that is supported by evidence that neutrophils respond to specific danger signals called pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) that can be either from pathogens or from release of intracellular contents when a cell is damaged or cancerous(12). When presented these signals, innate cells destroy the tissue and send signals to the adaptive immune system to develop a more specific response.
The adaptive immune system is a well-regulated set of processes that has as its end product a very specific response to a specific organism (or tissue). T cells and B cells are able to develop a specific response to cellular elements of an external pathogen by selection and clonal expansion. This response takes longer to develop than the innate system but has long lasting effect including immunity against pathogens years after initial exposure. The process of regulation of adaptive immunity is based on communication between antigen-presenting cells in the lymph nodes and naïve lymphocytes with signaling from numerous cytokines.
Given the time that it takes DDAV to develop, one would suspect that the actor in inflammation would be the adaptive immune system. Interestingly, there is mounting evidence in other critical illness associated injuries that suggests that in a non-infected environment, the innate immune system can have actions that are longer lasting than in infection(13, 14). Research from our laboratory suggests that neutrophils are associated with vasospasm in patients with SAH(15). This is consistent with studies done in animal models that suggest that interfering with the mechanism of neutrophil signaling or availability ameliorates vasospasm. Studies from the laboratory of Tamargo and colleagues have shown that blocking CD11b/CD18 complex (the cell surface ligand for on myeloid cells that pairs with ICAM-1 for trafficking out of the blood stream) prevents vasospasm(16). Ma and colleagues have shown that there is evidence of signaling of the largely neutrophil-associated TLR4 in the brain after subarachnoid hemorrhage(17). Minami and colleagues also found evidence of neutrophil interaction with basilar artery interstitium after SAH associated with vasospasm(18). The laboratory of Zhang and colleagues has been investigating early inflammation after SAH and its implications for vasospasm. They have found that administration of L-arginine decreases early inflammation after SAH (19). L-arginine is the substrate for NADPH oxidase, a neutrophil enzyme. This is evidence that innate mechanisms are active early after SAH.
There is little evidence from sampled CSF in patients with SAH that adaptive immune system cells (T-cells and B-cells) are associated with DDAV although definitive investigation of this is lacking (Provencio, JJ, data not published). There is some conflicting data in the field that decreases our certainty that innate immunity has a definite role. A study from patients with SAH who had surgical clipping showed that levels of IL-1β but not TNF-α were increased and correlated with the later development of vasospasm(20). If the innate immune system were the driver, one would expect that both IL-1β and TNF-α would correlate to vasospasm. A study by Oruckaptan and colleagues in Turkey found no role for neutrophils or myeloperoxidase (MPO, a neutrophil-derived effector enzyme(21). Although there were methodological issues that call into question the results of this study, replication of this study has not been published to my knowledge. These few contradictory studies do not dissuade my enthusiasm for inflammation as a reasonable area of research in SAH.
When does inflammation occur that leads to delayed deterioration associated with vasospasm?
One of the most tantalizing prospects for treatment of DDAV is its occurrence often a week after the onset of SAH. With so much time between ictus and onset of symptoms, it is hoped that treatments can be implemented in this window. The important question that arises is whether the processes that lead to DDAV and cerebral vasospasm develop at the time of clinical presentation or are the end product of a process that begins at the time of SAH and takes time to develop clinically.
Evidence from studies of early inflammation after SAH suggests that inflammatory processes begin early. Zhang and colleagues have shown that vascular endothelial growth factor (VEGF) and mitogen-activation protein kinase (MAPK) are up-regulated early after experimental SAH(22). Both are associated with innate inflammatory responses. Likewise, McGirt, Laskowitz and colleagues found that in patients with SAH, early, elevated levels of matrix-metalloproteinase-9 (MMP-9) and VEGF were associated with vasospasm(23). An interesting study by Clatterbuck and colleagues showed that inhibition of lymphocyte function-associated antigen-1 (LFA-1) within 3 hours of the hemorrhage prevents vasospasm of isolated femoral arteries suggesting that the process of inflammation occurs very early in the course of SAH(24). In our laboratory, we have found that neutrophils are elevated in the first three days after SAH; days before the onset of clinical symptoms or evidence of vasospasm(15). In a mouse model of vasospasm, depletion of myeloid cells (neutrophils, monocytes, and macrophages) prior to SAH prevents both the vascular manfestions of DDAV but also the behavioral deficits(25).
Where does the inflammation occur?
There are three possible sites of inflammation after SAH as it pertains to DDAV: the meninges and CSF space, the brain, and the cerebral arteries. It is possible that more than one compartment is affected in the process. Studies investigating the different compartments are lacking. One of the strongest criticisms of inflammation as a driver for vasospasm and DDAV is that there is not as robust a meningeal reaction to spilled blood in the CSF space as in bacterial meningitis, which is less associated with this syndrome. It is important to reiterate the caution that innate inflammatory responses in infected tissues appear to differ from those responses in sterile environments.
Work in our laboratory and others have suggested that inflammation does occur in the CSF of patients with SAH although not to the extent seen in meningitis(15, 26–31). Whether the inflammatory cells cause permanent cerebral damage is still unclear.
Damage to the blood vessels is the most studied of the inflammatory pathways. There is evidence of inflammation in blood vessels after SAH associated with vasospasm (32–35). Inflammatory cell infiltration of vascular intima has been described(36–38). Tamargo and colleagues described inflammation in blood vessels in conceptual terms as the “leukocyte-endothelial cell interaction”(39). This is supported by a number of studies that report endothelial dysfunction in animals models of vasospasm(40–42).
Inflammation of the brain directly causing both the vascular syndrome of vasospasm and the clinical syndrome of delayed deterioration is possibe based on the data but a sobering finding because the process seems to occur extremely early after SAH obliterating the window of treatment theory that is integral to our optimism for a cure (Sehba, Pluta and Zhang for an informative review(43)). A number of studies suggest that early inflammation leads to poor outcome after SAH(44–46). The innate brain inflammatory cell most likely to participate in early brain inflammation is the microglial cell. Results from our laboratory show that microglial activation in the first day of SAH in a murine model histologically correlates well with the presence of later vasospasm and behavioral deficits(25, 47). It is still unclear if microglia direct the inflammation from the brain or respond to outside influences. In brain trauma, microglia seem to play an important and direct role in the inflammation (48–50).
What are the drivers of inflammation?
It is well recognized that where there is cerebral ischemia, there is brain inflammation. The question at the root of DDAV is whether inflammation is the mechanism of cell injury in SAH, or whether other factors directly or indirectly (through vasoconstriction and stroke) lead to poor outcomes in patients. There are a number of putative agents that have been proposed to cause vasospasm and (by assumption) DDAV because most studies have not looked out long-term outcome from DDAV. The studies of clazosentan and nicardipine warn that the assumption that vasospasm and outcome are tightly linked may be falty(1, 2).
Early studies of vasospasm focused on hemoglobin and oxyhemoglobin as the causative agents(51–53). Two correlates of this theory are that blood breakdown produces either: 1) bilirubin oxidation products (BOX's) that lead to damage or 2) free hemoglobin which is a sump for nitric oxide, which leads to spasm based on relative over-expression of endothelin-1(54–60). There are number of genetic allele variants that seem to be important in the risk for the development of vasospasm in humans. They include the ApoE4 and the Haptoglobin α2/α2 genotype and are thought to code for products that alter the risk of vasopasm(61–64). Finally, other blood cell components such as platelets have been postulated to lead to direct damage to blood vessels and the cause of vasospasm(65, 66).
It is possible that all of these mediators are actually early triggers for inflammation either in the brain, the blood vessels or the meninges. It has been postulated for a number of the above-mentioned entities that inflammation may be the mechanism of injury.
Conclusion
A great deal of work is left to resolve whether inflammation is a driver of DDAV or a consequence of the ischemia that results from vasospasm. The failure of vasodilators to improve patient outcome despite improving vascular constriction, suggests that investigating other possible entities is warranted. Inflammation seems the most probable non-vascular research avenue to pursue. In the next few years, I look forward to new studies and mechanistic evaluations of inflammation in SAH based on the three questions we discussed here. When we know what cells, when and in what compartment of the cranium inflammation acts, we will hopefully be able to develop rational treatment strategies.
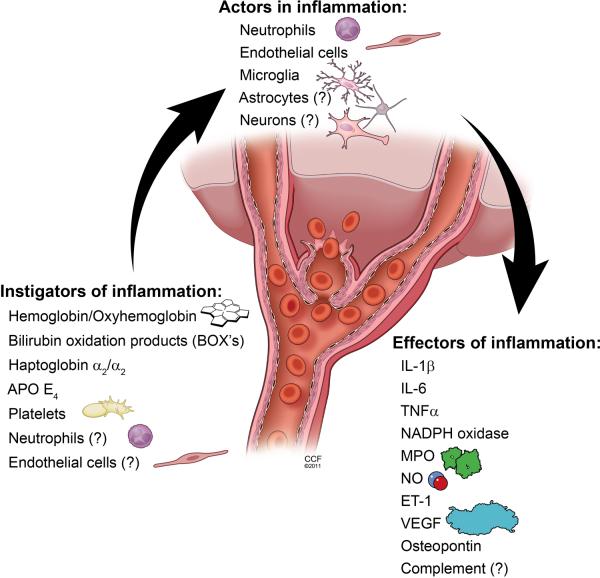
Figure 1.
Depiction of possible instigators, actors and effectors of inflammation that could contribute to both vascular changes and poor outcomes in patients with delayed deterioration associated with vasospasm (DDAV) after aneurismal SAH.
Acknowledgement
The author would like to acknowledge David Schumick for developing the illustration.
Financial Disclosure: This work was supported by a grant from the NIH (K08-NS051350 JJP) and the Cleveland Clinic Cerebrovascular Center (Institutional Support JJP).
Footnotes
The author has no conflicts to report.
References
- 1.Macdonald RL, et al. Clazosentan to Overcome Neurological Ischemia and Infarction Occurring After Subarachnoid Hemorrhage (CONSCIOUS-1): Randomized, Double-Blind, Placebo-Controlled Phase 2 Dose-Finding Trial. Stroke. 2008;39(11):3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 2.Haley EC, Jr., Kassell NF, Torner JC, Truskowski LL, Germanson TP. A randomized trial of two doses of nicardipine in aneurysmal subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. Journal of Neurosurgery. 1994;80(5):788–796. doi: 10.3171/jns.1994.80.5.0788. [DOI] [PubMed] [Google Scholar]
- 3.Pellettieri L, Carlson CA, Lindholm L. Is the vasospasm following subarachnoidal hemorrhage an immunoreactive disease? Experientia. 1981;37(11):1170–1171. doi: 10.1007/BF01989900. [DOI] [PubMed] [Google Scholar]
- 4.Stein SC, Levine JM, Nagpal S, LeRoux PD. Vasospasm as the sole cause of cerebral ischemia: how strong is the evidence? Neurosurgical Focus. 2006;21(3):E2. doi: 10.3171/foc.2006.21.3.2. [DOI] [PubMed] [Google Scholar]
- 5.Etminan N, Vergouwen MD, Ilodigwe D, Macdonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen-Schwartz J, Vajkoczy P, Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol Sci. 2007;28(6):252–256. doi: 10.1016/j.tips.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Neil-Dwyer G, Cruickshank J. The blood leucocyte count and its prognostic significance in subarachnoid haemorrhage. Brain. 1974;97(1):79–86. doi: 10.1093/brain/97.1.79. [DOI] [PubMed] [Google Scholar]
- 8.Niikawa S, Hara S, Ohe N, Miwa Y, Ohkuma A. Correlation between blood parameters and symptomatic vasospasm in subarachnoid hemorrhage patients. Neurol Med Chir (Tokyo) 1997;37(12):881–884. doi: 10.2176/nmc.37.881. [DOI] [PubMed] [Google Scholar]
- 9.Maiuri F, Gallicchio B, Donati P, Carandente M. The blood leukocyte count and its prognostic significance in subarachnoid hemorrhage. J Neurosurg Sci. 1987;31(2):45–48. [PubMed] [Google Scholar]
- 10.McGirt MJ, et al. Leukocytosis as an independent risk factor for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;98(6):1222–1226. doi: 10.3171/jns.2003.98.6.1222. [DOI] [PubMed] [Google Scholar]
- 11.Delves PJ, Roitt IM. The Immune System. New England Journal of Medicine. 2000;343(1):37–49. doi: 10.1056/NEJM200007063430107. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81(1):1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 13.Roy A, et al. Mice lacking in gp91 phox subunit of NAD(P)H oxidase showed glomus cell [Ca(2+)](i) and respiratory responses to hypoxia. Brain Res. 2000;872(1–2):188–193. doi: 10.1016/s0006-8993(00)02458-6. [DOI] [PubMed] [Google Scholar]
- 14.Sela S, et al. The involvement of peripheral polymorphonuclear leukocytes in the oxidative stress and inflammation among cigarette smokers. Israel Medical Association Journal: Imaj. 2002;4(11):1015–1019. [PubMed] [Google Scholar]
- 15.Provencio JJ, et al. CSF neutrophils are implicated in the development of vasospasm in subarachnoid hemorrhage. Neurocrit Care. 2010;12(2):244–251. doi: 10.1007/s12028-009-9308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clatterbuck RE, et al. Prevention of cerebral vasospasm by a humanized anti-CD11/CD18 monoclonal antibody administered after experimental subarachnoid hemorrhage in nonhuman primates. J Neurosurg. 2003;99(2):376–382. doi: 10.3171/jns.2003.99.2.0376. [DOI] [PubMed] [Google Scholar]
- 17.Ma CX, et al. Toll-like receptor 4/nuclear factor-kappa B signaling detected in brain after early subarachnoid hemorrhage. Chin Med J (Engl) 2009;122(13):1575–1581. [PubMed] [Google Scholar]
- 18.Minami N, Tani E, Yokota M, Maeda Y, Yamaura I. Immunohistochemistry of leukotriene C4 in experimental cerebral vasospasm. Acta Neuropathologica. 1991;81(4):401–407. doi: 10.1007/BF00293461. [DOI] [PubMed] [Google Scholar]
- 19.Yang MF, et al. Alleviation of brain edema by L-arginine following experimental subarachnoid hemorrhage in a rat model. Clinical Hemorheology & Microcirculation. 2003;29(3–4):437–443. [PubMed] [Google Scholar]
- 20.Nam DH, et al. Expression of interleukin-1 beta in lipopolysaccharide stimulated monocytes derived from patients with aneurysmal subarachnoid hemorrhage is correlated with cerebral vasospasm. Neuroscience Letters. 2001;312(1):41–44. doi: 10.1016/s0304-3940(01)02194-2. [DOI] [PubMed] [Google Scholar]
- 21.Oruckaptan HH, Caner HH, Kilinc K, Ozgen T. No apparent role for neutrophils and neutrophil-derived myeloperoxidase in experimental subarachnoid haemorrhage and vasospasm: a preliminary study. Acta Neurochirurgica. 2000;142(1):83–90. doi: 10.1007/s007010050011. [DOI] [PubMed] [Google Scholar]
- 22.Eiserich JP, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296(5577):2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 23.McGirt MJ, et al. Serum von Willebrand factor, matrix metalloproteinase-9, and vascular endothelial growth factor levels predict the onset of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;51(5):1128–1134. doi: 10.1097/00006123-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Clatterbuck RE, et al. Inhibition of vasospasm with lymphocyte function-associated antigen-1 monoclonal antibody in a femoral artery model in rats. Journal of Neurosurgery. 2002;97(3):676–682. doi: 10.3171/jns.2002.97.3.0676. [DOI] [PubMed] [Google Scholar]
- 25.Provencio JJ, Altay T, Smithason S, Moore SK, Ransohoff RM. Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. J Neuroimmunol. 2011;232(1–2):94–100. doi: 10.1016/j.jneuroim.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polin RS, et al. Detection of soluble E-selectin, ICAM-1, VCAM-1, and L-selectin in the cerebrospinal fluid of patients after subarachnoid hemorrhage. Journal of Neurosurgery. 1998;89(4):559–567. doi: 10.3171/jns.1998.89.4.0559. [DOI] [PubMed] [Google Scholar]
- 27.Gaetani P, et al. Cisternal CSF levels of cytokines after subarachnoid hemorrhage. Neurological Research. 1998;20(4):337–342. doi: 10.1080/01616412.1998.11740528. [DOI] [PubMed] [Google Scholar]
- 28.Hendryk S, Jarzab B, Josko J. Increase of the IL-1 beta and IL-6 levels in CSF in patients with vasospasm following aneurysmal SAH. Neuroendocrinology Letters. 2004;25(1–2):141–147. [PubMed] [Google Scholar]
- 29.Kikuchi T, Okuda Y, Kaito N, Abe T. Cytokine production in cerebrospinal fluid after subarachnoid haemorrhage. Neurol Res. 1995;17(2):106–108. doi: 10.1080/01616412.1995.11740296. [DOI] [PubMed] [Google Scholar]
- 30.Vieweg U, Schramm J, Urbach H. Platelet-derived growth factor (PDGF-AB) like immune reactivity in serum and in cerebral spinal fluid following experimental subarachnoid haemorrhage in dogs. Acta Neurochirurgica. 1999;141(8):861–865. doi: 10.1007/s007010050388. [DOI] [PubMed] [Google Scholar]
- 31.Ng WH, Moochhala S, Yeo TT, Ong PL, Ng PY. Nitric oxide and subarachnoid hemorrhage: elevated level in cerebrospinal fluid and their implications. Neurosurgery. 2001;49(3):622–626. doi: 10.1097/00006123-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Aihara Y, Kasuya H, Onda H, Hori T, Takeda J. Quantitative analysis of gene expressions related to inflammation in canine spastic artery after subarachnoid hemorrhage. Stroke. 2001;32(1):212–217. doi: 10.1161/01.str.32.1.212. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, et al. Expression change of interleukin-8 gene in rabbit basilar artery after subarachnoid hemorrhage. Neuroscience Bulletin. 2007;23(3):151–155. doi: 10.1007/s12264-007-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang ZW, et al. Hydrogen peroxide induces contraction and raises [Ca2+]i in canine cerebral arterial smooth muscle: participation of cellular signaling pathways. Naunyn-Schmiedebergs Archives of Pharmacology. 1999;360(6):646–653. doi: 10.1007/s002109900128. [DOI] [PubMed] [Google Scholar]
- 35.Ryba M, Jarzabek-Chorzelska M, Chorzelski T, Pastuszko M. Is vascular angiopathy following intracranial aneurysm rupture immunologically mediated? Acta Neurochirurgica. 1992;117(1–2):34–37. doi: 10.1007/BF01400632. [DOI] [PubMed] [Google Scholar]
- 36.Hughes JT, Schianchi PM. Cerebral artery spasm. A histological study at necropsy of the blood vessels in cases of subarachnoid hemorrhage. J Neurosurg. 1978;48(4):515–525. doi: 10.3171/jns.1978.48.4.0515. [DOI] [PubMed] [Google Scholar]
- 37.Crompton MR. The Pathogenesis of Cerebral Infarction Following the Rupture of Cerebral Berry Aneurysms. Brain. 1964;87:491–510. doi: 10.1093/brain/87.3.491. [DOI] [PubMed] [Google Scholar]
- 38.Pluta RM, Zauner A, Morgan JK, Muraszko KM, Oldfield EH. Is vasospasm related to proliferative arteriopathy? J Neurosurg. 1992;77(5):740–748. doi: 10.3171/jns.1992.77.5.0740. [DOI] [PubMed] [Google Scholar]
- 39.Gallia GL, Tamargo RJ. Leukocyte-endothelial cell interactions in chronic vasospasm after subarachnoid hemorrhage. Neurol Res. 2006;28(7):750–758. doi: 10.1179/016164106X152025. [DOI] [PubMed] [Google Scholar]
- 40.Iuliano BA, Pluta RM, Jung C, Oldfield EH. Endothelial dysfunction in a primate model of cerebral vasospasm. Journal of Neurosurgery. 2004;100(2):287–294. doi: 10.3171/jns.2004.100.2.0287. [DOI] [PubMed] [Google Scholar]
- 41.Kwan AL, Solenski NJ, Kassell NF, Lee KS. Inhibition of nitric oxide generation and lipid peroxidation attenuates hemolysate-induced injury to cerebrovascular endothelium. Acta Neurochir (Wien) 1997;139(3):240–247. doi: 10.1007/BF01844759. [DOI] [PubMed] [Google Scholar]
- 42.Handa Y, et al. The correlation between immunological reaction in the arterial wall and the time course of the development of cerebral vasospasm in a primate model. Neurosurgery. 1991;28(4):542–549. doi: 10.1097/00006123-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Sehba FA, Pluta RM, Zhang JH. Metamorphosis of subarachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol. 2011;43(1):27–40. doi: 10.1007/s12035-010-8155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Shi JX, Yin HX, Ma CY, Zhang QR. The influence of subarachnoid hemorrhage on neurons: an animal model. Ann Clin Lab Sci. 2005;35(1):79–85. [PubMed] [Google Scholar]
- 45.Yamaguchi M, Zhou C, Nanda A, Zhang JH. Ras protein contributes to cerebral vasospasm in a canine double-hemorrhage model. Stroke. 2004;35(7):1750–1755. doi: 10.1161/01.STR.0000129898.68350.9f. [DOI] [PubMed] [Google Scholar]
- 46.Yatsushige H, et al. Role of c-Jun N-terminal kinase in cerebral vasospasm after experimental subarachnoid hemorrhage. Stroke. 2005;36(7):1538–1543. doi: 10.1161/01.STR.0000170713.22011.c8. [DOI] [PubMed] [Google Scholar]
- 47.Smithason S, Moore SK, Provencio JJ. Systemic administration of LPS worsens delayed deterioration associated with vasospasm after subarchnoid hemorrhage through a myeloid cell dependent mechanism. Neurocrit Care. 2011 doi: 10.1007/s12028-011-9651-3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eugenin EA, et al. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fassbender K, et al. Temporal profile of release of interleukin-1beta in neurotrauma. Neuroscience Letters. 2000;284(3):135–138. doi: 10.1016/s0304-3940(00)00977-0. [DOI] [PubMed] [Google Scholar]
- 50.Gentleman SM, et al. Long-term intracerebral inflammatory response after traumatic brain injury. Forensic Science International. 2004;146(2–3):97–104. doi: 10.1016/j.forsciint.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 51.Boullin DJ, Tagari P, du Boulay G, Aitken V, Hughes JT. The role of hemoglobin in the etiology of cerebral vasospasm. An in vivo study of baboons. J Neurosurg. 1983;59(2):231–236. doi: 10.3171/jns.1983.59.2.0231. [DOI] [PubMed] [Google Scholar]
- 52.Pluta RM, Afshar JK, Boock RJ, Oldfield EH. Temporal changes in perivascular concentrations of oxyhemoglobin, deoxyhemoglobin, and methemoglobin after subarachnoid hemorrhage. J Neurosurg. 1998;88(3):557–561. doi: 10.3171/jns.1998.88.3.0557. [DOI] [PubMed] [Google Scholar]
- 53.Horky LL, Pluta RM, Boock RJ, Oldfield EH. Role of ferrous iron chelator 2,2'-dipyridyl in preventing delayed vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg. 1998;88(2):298–303. doi: 10.3171/jns.1998.88.2.0298. [DOI] [PubMed] [Google Scholar]
- 54.Clark JF, Reilly M, Sharp FR. Oxidation of bilirubin produces compounds that cause prolonged vasospasm of rat cerebral vessels: a contributor to subarachnoid hemorrhage-induced vasospasm. Journal of Cerebral Blood Flow & Metabolism. 2002;22(4):472–478. doi: 10.1097/00004647-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Clark JF, Sharp FR. Bilirubin oxidation products (BOXes) and their role in cerebral vasospasm after subarachnoid hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2006;26(10):1223–1233. doi: 10.1038/sj.jcbfm.9600280. [DOI] [PubMed] [Google Scholar]
- 56.Pyne-Geithman GJ, et al. Bilirubin production and oxidation in CSF of patients with cerebral vasospasm after subarachnoid hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2005;25(8):1070–1077. doi: 10.1038/sj.jcbfm.9600101. [DOI] [PubMed] [Google Scholar]
- 57.Pluta RM, Oldfield EH. Sodium nitrite as a therapeutic agent for central nervous system diseases. Surg Neurol. 2006;66(1):5–7. doi: 10.1016/j.surneu.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 58.Pluta RM, et al. Nitric oxide and vasospasm. Acta Neurochirurgica Supplement. 2001;77:67–72. doi: 10.1007/978-3-7091-6232-3_15. [DOI] [PubMed] [Google Scholar]
- 59.Pradilla G, et al. Delayed intracranial delivery of a nitric oxide donor from a controlled-release polymer prevents experimental cerebral vasospasm in rabbits. Neurosurgery. 2004;55(6):1393–1399. doi: 10.1227/01.neu.0000143615.26102.1a. [DOI] [PubMed] [Google Scholar]
- 60.Sabri M, Ai J, Macdonald RL. Nitric oxide related pathophysiological changes following subarachnoid haemorrhage. Acta Neurochir Suppl. 2011;110(Pt 1):105–109. doi: 10.1007/978-3-7091-0353-1_19. [DOI] [PubMed] [Google Scholar]
- 61.Chaichana KL, Levy AP, Miller-Lotan R, Shakur S, Tamargo RJ. Haptoglobin 2-2 Genotype Determines Chronic Vasospasm After Experimental Subarachnoid Hemorrhage. Stroke. 2007;38(12):3266–3271. doi: 10.1161/STROKEAHA.107.490003. [DOI] [PubMed] [Google Scholar]
- 62.Khurana VG, Fox DJ, Meissner I, Meyer FB, Spetzler RF. Update on evidence for a genetic predisposition to cerebral vasospasm. Neurosurgical Focus. 2006;21(3):E3. doi: 10.3171/foc.2006.21.3.3. [DOI] [PubMed] [Google Scholar]
- 63.Gao J, et al. A novel apoE-derived therapeutic reduces vasospasm and improves outcome in a murine model of subarachnoid hemorrhage. Neurocrit Care. 2006;4(1):25–31. doi: 10.1385/NCC:4:1:025. [DOI] [PubMed] [Google Scholar]
- 64.Mesis RG, et al. Dissociation between vasospasm and functional improvement in a murine model of subarachnoid hemorrhage. Neurosurg Focus. 2006;21(3):E4. doi: 10.3171/foc.2006.21.3.4. [DOI] [PubMed] [Google Scholar]
- 65.Hansen-Schwartz J. Cerebral vasospasm: a consideration of the various cellular mechanisms involved in the pathophysiology. Neurocritical Care. 2004;1(2):235–246. doi: 10.1385/NCC:1:2:235. [DOI] [PubMed] [Google Scholar]
- 66.Tekkok IH, Tekkok S, Ozcan OE, Erbengi T, Erbengi A. Preventive effect of intracisternal heparin for proliferative angiopathy after experimental subarachnoid haemorrhage in rats. Acta Neurochir (Wien) 1994;127(1–2):112–117. doi: 10.1007/BF01808557. [DOI] [PubMed] [Google Scholar]