Abstract
Innate immune responses are regulated by microorganisms and cell death, as well as by a third class of stress signal from the nervous and endocrine systems. The innate immune system also feeds back, through the production of cytokines, to regulate the function of the central nervous system (CNS), and this has effects on behaviour. These signals provide an extrinsic regulatory circuit that links physiological, social and environmental conditions, as perceived by the CNS, with transcriptional ‘decision-making’ in leukocytes. CNS-mediated regulation of innate immune responses optimizes total organism fitness and provides new opportunities for therapeutic control of chronic infectious, inflammatory and neuropsychiatric diseases.
Immune responses are mediated by the activation of immune response genes that encode regulatory and effector molecules, such as cytokines, antimicrobial peptides, antibodies and cytolytic molecules. Transcriptional activation in innate immune cells is triggered by two types of signal from the body’s internal environment: pathogen-associated molecular patterns (PAMPs) and ‘danger signals’ derived from host cell stress or death1. A growing body of research shows that a third class of stimulus, in the form of neural and endocrine signals that result from macroenvironmental sensing, also plays a significant role in modulating immune responses2. In addition, immune mediators such as cytokines feed back to the brain to regulate neural and endocrine activity3. The resulting neuro–immune circuit coordinates immune responses with other physiological processes — such as fightor-flight stress responses — to maximize the overall fitness of the organism within complex environments that bear multiple threats. Such threats can be microbial, physiological (such as trauma and sleep loss) or social–ecological (such as predation, conspecific violence and interpersonal loss) in nature4.
The neuro–immune circuit was initially discovered in the context of adaptive immune responses2, but recent findings suggest that this circuit originates with the innate immune system5,6. This article highlights emerging biological themes on the reciprocal regulation of immune response gene expression and central nervous system (CNS) function. We map the molecular signalling pathways involved in this reciprocal regulation, discuss their implications for social influences on disease7 and highlight new therapeutic approaches for inflammatory diseases and psychiatric syndromes such as major depression, insomnia and fatigue3. In addition, we discuss the evolutionary basis for the emergence of a third, CNS-derived signal that controls immune responses6.
Immune response gene regulation
Functional genomics studies have identified two broad gene expression programmes that can be induced in myeloid lineage cells by different types of microbial stimulus8. Extracellular pathogens, such as bacteria, activate a pro-inflammatory gene programme characterized by the expression of genes such as interleukin-1β (IL1B), IL6 and tumour necrosis factor (TNF) via transcription factors including nuclear factor-κB (NF-κB) and activator protein 1 (AP1). Intracellular pathogens, such as viruses, elicit a distinct antiviral gene programme that involves the induction of type I interferon (IFN) genes via transcription factors such as interferon regulatory factors (IRFs). These two gene expression programmes mediate fundamentally different effector responses8,9, but they have in common a substantial energetic cost and the potential for collateral damage (for example, septic shock, autoimmunity, fibrosis and the promotion of inflammation-associated diseases such as atherosclerosis, diabetes, neurodegeneration and neoplasia)10. To mitigate these autotoxic risks, an additional layer of regulation exists for the key genes mediating pro-inflammatory and antiviral responses. One type of licensing signal is mediated by ‘danger’ and requires the presence of signals that are induced by host cell stress, necrosis or apoptosis to generate high-level immune response gene transcription1. A second, macroenvironmental licensing signal allows the CNS to integrate information regarding general physiological conditions and the extra-organismal perceived environment to regulate immune response gene expression programmes via hormones and neurotransmitters2 (FIG. 1).
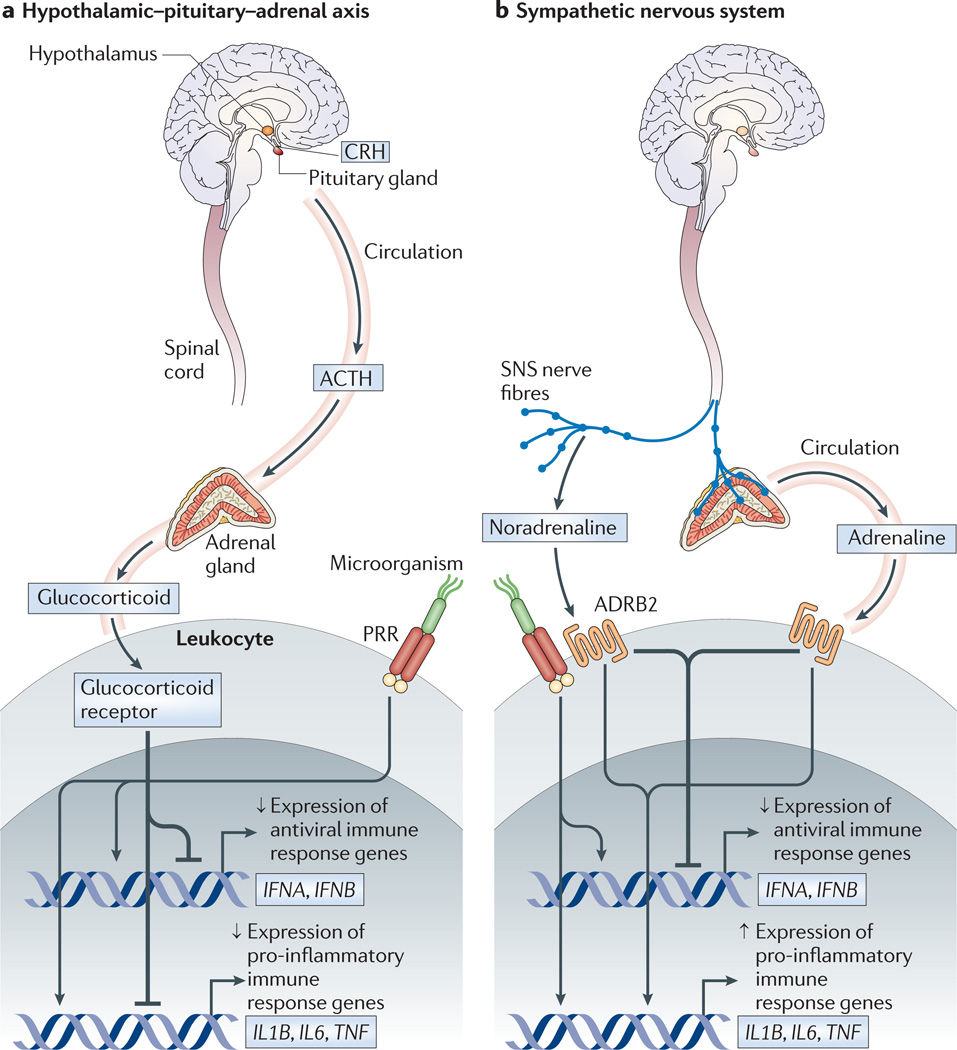
Figure 1. CNS regulation of innate immune response gene programmes.
a | The hypothalamic–pituitary–adrenal (HPA) axis distributes glucocorticoid hormones through the blood to regulate gene expression in virtually every cell of the body. Hormone activation of the glucocorticoid receptor in leukocytes results in profound suppression of both pro-inflammatory gene networks (for example, NF-κB-mediated transcription of pro-inflammatory cytokine genes, such as IL1B, IL6 and TNF) and antiviral gene programmes (for example, IRF-mediated transcription of type I interferon (IFN) genes, such as IFNA and IFNB). Activation of cytokine receptors in the hypothalamus triggers the production of glucocorticoids by the HPA axis. This constitutes the body’s primary systemic mechanism for negative feedback control of pro-inflammatory gene expression triggered by microbial pattern recognition receptors (PRRs). b | During fight-or-flight responses and acute injury, nerve fibres from the sympathetic nervous system (SNS) release the neurotransmitter noradrenaline into primary and secondary lymphoid organs, all other major organ systems (including the vasculature and perivascular tissues) and many peripheral tissues in which pro-inflammatory reactions occur. SNS nerve fibres can also stimulate the adrenal glands to release stored adrenaline into the systemic circulation. Both of these neuromediators regulate vascular function and stimulate leukocyte adrenergic receptors (for example, ADRB2) to activate transcription factors such as CREB and GATA family factors. SNS-induced transcriptional alterations can modulate haematopoiesis, redeploy leukocytes between tissue and blood, and repress IRF-mediated antiviral immune response gene programmes while enhancing many NF-κB-mediated pro-inflammatory programmes. ACTH, adrenocorticotropic hormone; ADRB2, β2-adrenergic receptor; CRH, corticotropin-releasing hormone; IL, interleukin; IRF, interferon regulatory factor; NF-κB, nuclear factor-κB; TNF, tumour necrosis factor.
The neural environment
The primary physiological role of the CNS is to perceive external physical and social conditions (the environment, broadly speaking), assess their implications for organismal well-being (fitness) and modulate the activity of internal physiological processes to optimally adapt to those external conditions4. In response to the perception of a threatening environment (for example, the appearance of a predator or hostile conspecifics), the CNS signals for adaptive changes in physiological function (for example, fight-or-flight stress responses). This signalling occurs via the release of neuroeffector molecules (such as noradrenaline) from nerves of the sympathetic nervous system (SNS) or of glucocorticoids from the hypothalamic–pituitary–adrenal (HPA) axis4. In addition to regulating virtually every other cell type in the body, these biochemical manifestations of CNS-perceived external conditions regulate the cells of the immune system (BOX 1).
Box 1 | Neural regulation of the innate immune response.
Several molecular pathways allow the central nervous system (CNS) to regulate the transcription of immune response genes in peripheral tissues3. These mechanisms include:
Hypothalamic–pituitary–adrenal axis production of glucocorticoids, which circulate throughout the body to alter a variety of metabolic and developmental processes, in addition to suppressing both pro-inflammatory and antiviral immune response gene programmes;
Sympathetic nervous system (SNS) innervation of primary and secondary lymphoid organs. This delivers the neurotransmitter noradrenaline directly into parenchymal tissues involved in haematopoiesis and interactions between antigen-presenting cells and lymphocytes18;
SNS innervation of the vasculature and peripheral organs and tissues. This releases noradrenaline into the local microenvironment of many acute and chronic inflammatory responses;
SNS innervation of the adrenal gland. This releases the hormone adrenaline into systemic circulation, which suppresses type I interferon-mediated antiviral responses17 and upregulates transcription of pro-inflammatory cytokines23;
Efferent neural distribution into peripheral tissues of pain-related neuropeptides, enteric system-regulating neuropeptides and a diverse array of other physiologically specialized neuromodulators that can stimulate receptors present on cells of the innate and adaptive immune systems;
CNS-mediated release of circulating mediators, such as growth hormone, insulin-like growth factor, endogenous opioids and a diverse range of other hormones that can affect cells of the innate and adaptive immune systems.
The hypothalamic–pituitary–adrenal axis
The earliest identified CNS-mediated immunoregulatory function involved the brain’s ability to suppress the transcription of both pro-inflammatory and antiviral gene programmes by stimulating glucocorticoid release from the HPA axis11–13. Activation of the glucocorticoid receptor inhibits the transcription of many immune response genes. This effect is mediated by three mechanisms, namely: suppressive binding of the glucocorticoid receptor to gene promoter sequences; glucocorticoid receptor-mediated transcriptional induction of anti-inflammatory genes (such as NFKBIA, which encodes IκBα); and nongenomic antagonism of pro-inflammatory transcription factors (such as NF-κB and AP1) via protein–protein interactions14.
The brain detects peripheral pro-inflammatory and antiviral cytokines via multiple pathways (BOX 2) and stimulates glucocorticoid release from the HPA axis to systemically inhibit immune response gene transcription when inflammation levels become damagingly high or energetic resources need to be shifted elsewhere3,15. Glucocorticoid-mediated feedback inhibition of immune response gene transcription is now recognized as the most fundamental physiological mechanism for protection against hyper-inflammatory disease and as a prototype of our most effective anti-inflammatory drugs14,15.
Box 2 | Inflammatory regulation of brain function.
Several molecular pathways allow peripherally generated pro-inflammatory signals to alter neural activity in the central nervous system (CNS). These mechanisms include:
Interaction of circulating cytokines with brain cytokine receptors in circumventricular organs that lack a functional blood–brain barrier;
Stimulation of brain vascular endothelial cells to release second messengers that stimulate subsequent cytokine production within the brain;
Active transport of cytokines across the blood–brain barrier via carrier molecules;
Peripheral inflammatory stimulation of afferent nerves that subsequently stimulate CNS tissues to produce cytokines.
Brain structures that show functional alterations in response to cytokine signalling include:
The hypothalamus, which has a key role in the regulation of systemic physiological function and organism-level biobehavioural dynamics (such as metabolism, sleep and feeding);
The amygdala, which mediates fear- or threat-related responses and processes social information;
The hippocampus, which has a key role in learning and short-term memory, general information processing, spatial information processing, and navigation and mobility;
The pre-frontal cortex, which is involved in complex information processing and planning;
The anterior cingulate cortex, which is involved in a diverse array of cognitive–emotional interactions;
The ventral striatum, which is involved in positive motivation and reward.
In many cases, cytokines directly interact with receptors in one brain structure (for example, the brainstem), which subsequently influences the functional activity of other brain structures (for example, the hypothalamus) via distant neural projections80.
The sympathetic nervous system
In addition to the global anti-inflammatory effect of glucocorticoids, a second neural pathway mediated by the SNS allows the CNS to ‘steer’ innate immune responses between pro-inflammatory and antiviral programmes16,17. The neural fibres of the SNS distribute the neurotransmitter noradrenaline into tissue microenvironments in which immune response gene transcription occurs, including all primary and secondary lymphoid organs, the vasculature and perivascular tissues, and most visceral organs and musculoskeletal structures18. Noradrenaline modulates leukocyte gene expression via stimulation of β-adrenergic receptors, which are associated with the signalling cascade that involves Gαs, adenylyl cyclase, cyclic AMP and protein kinase A18. β-adrenergic signalling was initially found to modulate adaptive immune responses by stimulating the transcription of T helper 2 (TH2)-type cytokine genes (such as IL4 and IL5) and suppressing the expression of TH1-type genes (such as IFNG and IL12B)19–21. Recent studies have discovered a similar SNS-mediated steering of innate immune response programmes, which involves suppression of type I IFN-mediated antiviral responses17 and upregulated transcription of pro-inflammatory cytokine genes (such as IL1B, IL6 and TNF)16,22.
Activation of the SNS has also been found to alter the production and trafficking of innate immune cells, for example through the upregulation of myelopoiesis and the mobilization of haematopoietic stem cells, natural killer cells and splenic neutrophils and monocytes18. Collectively, these studies of neuro–immune regulation have provided new insights into the mechanistic relationships between the cellular and microbial microenvironment in which immune responses have traditionally been analysed and the broader macroenvironment of the host body and its surrounding social and physical ecology, as perceived by the CNS6,23,24 (BOX 1).
Neural influences on disease
Epidemiological studies have long identified a link between adverse social and environmental conditions — such as the death of a spouse, low socio-economic status or social isolation — and an increased risk of infectious disease (presumably owing to insufficient expression of immune response genes). The risk of inflammation-associated cardiovascular, autoimmune, neurodegenerative and neoplastic diseases is also increased (presumably owing to excessive expression of immune response genes)7,16,25,26. Efforts to account for this pattern of infectious versus inflammation-associated disease risk based on glucocorticoid-mediated suppression of an immune response were obviously unsuccessful, but the discovery that the SNS could simultaneously inhibit antiviral genes and activate pro-inflammatory genes has provided a more plausible mechanistic explanation.
Laboratory animal and human studies have confirmed that experimental induction of acute psychological stress can increase circulating levels of IL-6 and IL-1β27, activate NF-κB in peripheral blood mononuclear cells27,28 and prime leukocytes for increased ex vivo production of pro-inflammatory cytokines in response to stimulation by the PAMP lipopolysaccharide (LPS) and other Toll-like receptor ligands5,29,30. Some of these effects may be mediated by mobilization of specific leukocyte subsets31, but their net effect is to acutely increase the pro-inflammatory potential of the circulating innate immune system.
Additional insights into the immunological effects of long-term social stress have come from transcriptional profiling of circulating leukocytes in human populations6,23,26,32 and experimental analyses of repeated social threat in animal models (such as encountering an aggressive intruder)5,33,34. People confronting long-term social adversities — such as the mortal illness of a spouse, low socio-economic status, post-traumatic stress disorder or long-term social isolation — have repeatedly been found to show increased expression of pro-inflammatory immune response genes, despite the presence of stable or elevated glucocorticoid levels6,23,26,32,35.
Promoter-based bioinformatic analysis of the transcription factors involved in these responses suggests a genome-wide reduction in glucocorticoid-mediated transcription (not just among immune response genes) as a mechanistic explanation for this apparent paradox. Reduced levels of glucocorticoid-mediated gene transcription despite elevated circulating glucocorticoid levels have also been observed in animal models of chronic social threat5,33,34 and appear to reflect a functional desensitization of the glucocorticoid receptor15. As a result of reduced glucocorticoid-mediated feedback inhibition, gene transcription shifts towards increased NF-κB- and AP1-mediated pro-inflammatory gene expression both under basal conditions and in response to stimulation by PAMPs. Recent analyses suggest that chronic threat-induced glucocorticoid receptor desensitization may stem from increased myelopoietic generation of immature LY6Chi monocytes (CD16– monocytes in humans) that express constitutively high levels of mitogen-activated protein kinases33,34, which constitutively inhibit glucocorticoid receptor function15. A similar desensitization of glucocorticoid receptor genomic signalling has been observed in monocytes from humans confronting extended social adversity26.
Transcriptome analyses of leukocytes from humans undergoing chronic social adversity have also begun to identify some of the specific neurobehavioural pathways through which stressful life circumstances affect innate immune responses. Wake–sleep cycles have emerged as prominent regulators of inflammatory biology, with experimental sleep restriction studies showing upregulated leukocyte expression of pro-inflammatory cytokine genes and increased NF-κB activity36,37. Observational studies have also identified elevated levels of C-reactive protein and other inflammation-related biomarkers in night-shift workers, insomniacs and people suffering poor sleep duration or quality38. Experimentally induced sleep loss (whole night) or restriction (partial reduction over several days) has also been found to increase circulating pro-inflammatory biomarkers39. These effects are especially pronounced in females37,40, possibly owing to sex difference in SNS upregulation of IL-6 production41, and this might contribute to sex differences in the incidence of inflammation-related behavioural and autoimmune diseases.
In sum, the CNS orchestrates the perception of the external physical and social environment and the evaluation of environmental conditions as threatening versus salutary. In cases of threat, the CNS activates stress signalling pathways (such as the HPA axis and the SNS) to regulate multiple internal physiological processes, including broad patterns of transcriptional activity in innate immune cells. Activation of the HPA axis inhibits both antiviral and pro-inflammatory gene modules, whereas SNS activation suppresses antiviral responses while stimulating pro-inflammatory genes. In turn, circulating pro-inflammatory cytokines evoke a glucocorticoid-mediated negative feedback response from the CNS, raising the possibility that other brain-mediated processes might also be altered.
Inflammatory regulation of behaviour
Several molecular signalling pathways have been identified to convey peripheral pro-inflammatory and antiviral signals into the brain3,42 (BOX 2). Within the brain, pro-inflammatory cytokines decrease the activity of key behaviour-modulating neurotransmitters, including noradrenaline, dopamine and serotonin43. Moreover, these cytokines activate physiological and behavioural responses, such as fever and social withdrawal, which function together with leukocyte activation dynamics to limit the spread of infectious disease both within and between individuals3,44,81.
Sickness
Prostaglandin E2 released from brain endothelial cells is well known to trigger CNS-mediated febrile and metabolic responses to infection3. Recent studies also show that pro-inflammatory cytokines can activate the CNS to produce a broader array of ‘sickness behaviours’, which include emotional alterations (anhedonia, fatigue and dysphoria), reductions in exploratory and reward-seeking motivation, altered cognitive and motor function, sleep alterations and reduced social and reproductive motivation3,44. These behaviours are triggered in part via IL-1 receptors in the hypothalamus and hippocampus3,44. Experimental administration of type I IFNs or pro-inflammatory cytokines in mice has been found to activate sickness behaviour syndromes3,44, although the exact behavioural dynamics vary somewhat with the specific triggering cytokine.
Functional neuroimaging studies in humans have begun to map the specific neural circuits associated with cytokine-induced sickness behaviours. The findings have shown altered connectivity between the subgenual anterior cingulate cortex, amygdala and medial prefrontal cortex45,46, and a reduced ventral striatum response to reward cues47. The functions of these areas of the brain are described in BOX 2. Given the strong evolutionary conservation of cytokine-induced sickness behaviour3,44, these dynamics appear to play a key role in coordinating the overall mammalian response to acute infection by regulating systemic physiological processes and altering behaviour. Such physiological or behavioural modifications include redirecting energy resources to the immune response, reducing circulating iron levels, raising the body temperature above the optimal levels for some pathogens, immobilization to conserve energy and avoid predation, and reducing social and/or reproductive contact to limit the spread of infection3,44,81.
Depression
Sickness behaviours are highly reminiscent of some common adverse behavioural syndromes with poorly understood aetiology, suggesting that dysregulated activation of cytokine-mediated sickness behaviours might underlie some cases of medically unexplained fatigue, sleep impairment or major depressive disorder (MDD)43. Consistent with this hypothesis, epidemiological studies have linked IL-6 and TNF levels with the risk of developing MDD48, and documented increased rates of MDD in clinical conditions involving high levels of inflammation (for example, in patients with cancer)43,49.
Pharmacological administration of IFNα to patients with cancer induces an array of MDD symptoms, including anhedonia and sadness, disturbed sleep, fatigue and loss of appetite43,50,51. Conversely, pharmacological antagonism of TNF has been shown to reduce depressive symptoms52. Gene polymorphisms that result in the increased expression of IL1B and TNF are associated with increased MDD risk and reduced clinical response to antidepressant medication43,53. Elevated circulating pro-inflammatory biomarkers such IL-6 and TNF also predict poor clinical response to antidepressant medications43,54.
These results suggest that blockade of pro-inflammatory cytokines might diminish the risk and improve the treatment response of depression. In addition, they raise the possibility that circulating pro-inflammatory biomarkers and inflammation-related genetic polymorphisms could be used to help guide targeted therapies for specific MDD subtypes that are thought to have an inflammatory component (such as depression in older adults or in those with inflammation-associated disease). Links between inflammation and depressive symptoms have also led to the hypothesis that contemporary increases in the prevalence of MDD may stem from dysregulated pro-inflammatory signalling that is due to the decreased exposure to tolerogenic microorganisms in modern industrialized societies (a psychiatric version of the ‘hygiene hypothesis’)55.
Sleep
Pro-inflammatory cytokines and type I IFNs also have a role in the homeostatic regulation of sleep56. Animal genetic studies and LPS administration studies in humans56,57 have linked changes in non-rapid eye movement sleep (NREM sleep) to elevated levels of circulating type I IFNs and pro-inflammatory cytokines. Moreover, pharmacological administration of IL-6 and IFNα in humans results in decreases in NREM slow-wave sleep and complementary increases in REM sleep56,58, although animals studies show that other cytokines (such as TNF) increase NREM sleep and decrease REM sleep56. Elevated daytime levels of TNF have also been linked with sleepiness, fatigue and altered sleep architecture, and pharmacological antagonism of TNF can reduce these effects59,60. TNF antagonism can also normalize REM sleep levels (for example, in abstinent alcohol-dependent individuals, who have elevated amounts of REM sleep)61.
Given the substantial fraction of time we spend asleep and the general immunological activation that occurs during sleep38, as well as the epidemiological links between abnormally high levels of REM sleep and mortality62, regulation of sleep architecture by the innate immune system may play a substantial role in structuring overall inflammatory homeostasis38,56.
Fatigue
Pro-inflammatory gene expression can induce profound fatigue during waking hours63 — one of the most debilitating burdens of chronic inflammatory disease. Clinical studies have documented links between inflammation-related biomarkers and the development of fatigue both in healthy older adults64 and in individuals with inflammatory diseases such as multiple sclerosis65, Sjogren’s syndrome66, rheumatoid arthritis67 and cancer68. Patients with cancer often experience substantial increases in NF-κB inflammatory signalling owing to tumour-derived cytokines, the effects of cancer treatment (for example, radiation and chemotherapy) on tissue69 and therapeutic administration of type I IFNs49.
Even when chemotherapy and/or radiation treatments are completed, approximately one-third of breast cancer survivors suffer from persistent, medically unexplained fatigue. This fatigue is associated with increased circulating biomarkers of IL-1β and IL-6 activity70, increased NF-κB-mediated gene transcription71 and enhanced IL-6 and TNF production in response to ex vivo LPS-mediated stimulation of circulating leukocytes70. Fatigue in breast cancer survivors is also particularly elevated among patients with IL1B, IL6 or TNF polymorphisms that cause high-level expression of the respective cytokines72. Pharmacological antagonism of TNF can reduce chemotherapy-related fatigue60, confirming a key functional role for pro-inflammatory cytokines in the aetiology of cancer-associated fatigue.
In sum, peripheral innate immune responses can influence CNS functions (including neurotransmitter metabolism, regional brain activity and sleep–wake cycles) and behavioural processes (including depression, sleep and fatigue), and this has implications for neuropsychiatric disease.
The neuro–immune circuit
Understanding and controlling innate immune responses is complicated by the fact that immune response genes are regulated by both external influences (through neural activity) and internal factors (such as pathogens and cell damage)16 (FIG. 2). Furthermore, the reciprocal regulation of neural activity by immune response genes would seem to hopelessly complicate matters. However, such reciprocal regulation provides exactly the feedback required by dynamic systems theory to stabilize the circuit as a whole, particularly given the fact that CNS function is itself regulated by both the internal (inflammatory) and external (ecological) environments simultaneously.
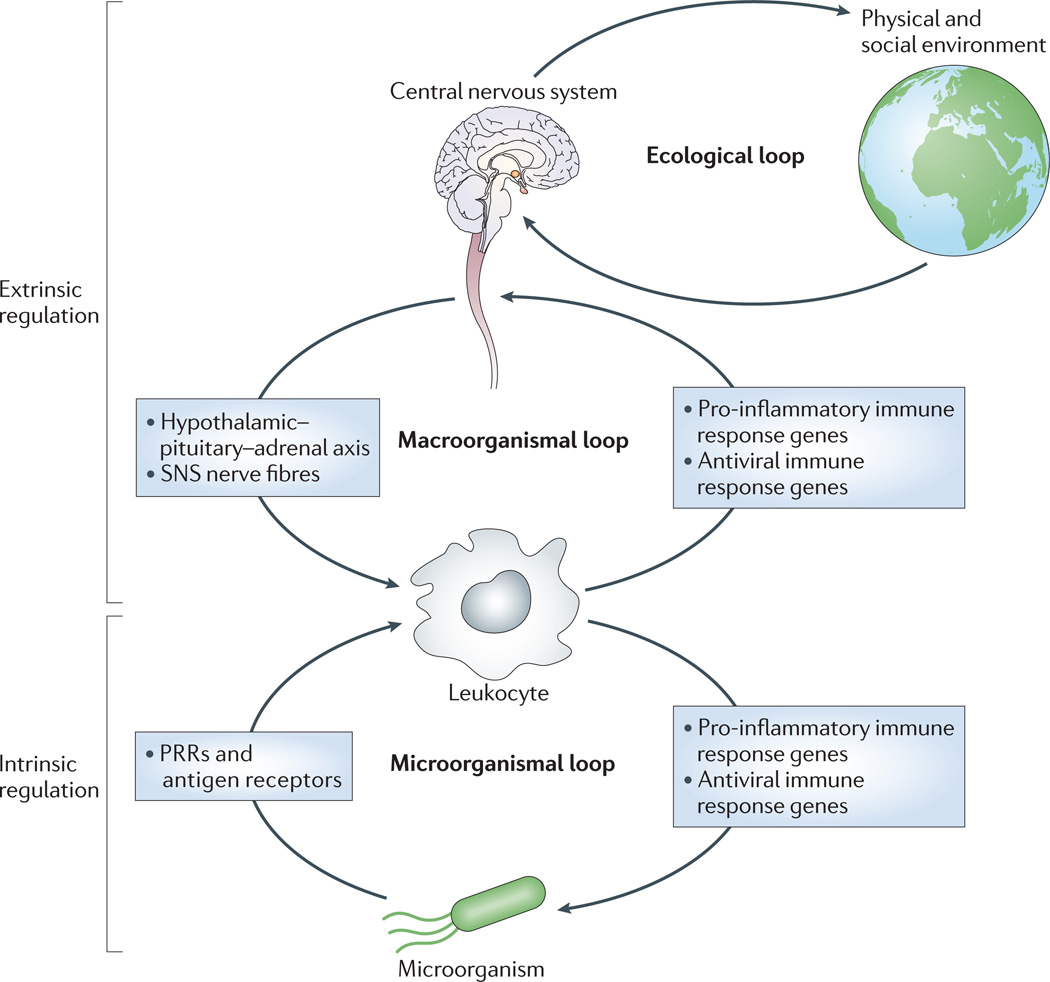
Figure 2. Multi-circuit control of the innate immune transcriptome.
Leukocyte transcription of immune response gene programmes is regulated by both intrinsic immunological signals representing local tissue and microbial conditions and extrinsic neural and endocrine signals representing global physiological and environmental conditions. Intrinsic circuits detect microorganisms via pattern recognition receptors (PRRs) and stimulate pro-inflammatory and antiviral immune response gene programmes via transcription factors such as nuclear factor-κB (NF-κB) and interferon regulatory factors (IRFs). The resulting production of innate immune effector molecules reduces microbial burden, and thereby feeds back to reduce PRR and antigen receptor signalling and immune response gene transcription. Extrinsic regulation of immune response gene transcription is mediated by central nervous system (CNS) integration of information regarding general physiological and ecological conditions. This can either globally suppress immune response gene transcription via the hypothalamic–pituitary–adrenal axis or steer immune response gene transcriptional profiles away from antiviral programmes and towards more robust pro-inflammatory gene expression. CNS-mediated transduction of information from the social and physical ecology allows extra-organismal environmental conditions to indirectly regulate the immune response gene transcriptional profiles of immune cells. SNS, sympathetic nervous system.
What the organism as a whole gains by superimposing the ‘extrinsic’ CNS–leukocyte–CNS regulatory circuit on the more immunologically fundamental ‘intrinsic’ microorganism–leukocyte–microorganism circuit is the opportunity to coordinate the microbiological battle between pathogen and immune response within the context of the broader macrobiological conditions that affect overall survival and natural selection. For example, this crosstalk allows the organism to suppress the immobilizing effects of inflammation and sickness behaviour to facilitate fight-or-flight responses to predation or conspecific aggression. There are lots of other ways to die or fail to reproduce besides infection, and an optimal immune response needs to be adapted to the total array of conditions that confront the organism rather than blindly pursuing its microbial antagonists. The fitness advantage of innate immune regulation by the extrinsic circuit is demonstrated by the reduced survival of organisms that are challenged with pathogens following blockade of CNS signalling to leukocytes, as well as by the reduced individual and population-level resistance to infectious disease in the absence of leukocyte signalling to the CNS44,81. These whole-organism fitness implications provide a teleological rationale for the evolution of CNS–immune interactions, as well as new opportunities for therapeutic intervention.
Biobehavioural control of inflammation
Several behavioural interventions have been applied to modulate pro-inflammatory signalling in the innate immune system. Randomized controlled trials have documented reductions in pro-inflammatory cytokine activity following several types of behavioural intervention (BOX 3), including cognitive behavioural therapy73, aerobic exercise74, meditation75 and Tai Chi76,77. The regulation of leukocytes by the CNS may also contribute to the neurobiological impact of behavioural interventions if, for example, CNS-mediated reductions in peripheral inflammation feed back to reciprocally reduce sickness behaviour. SNS-mediated regulation of pro-inflammatory and antiviral genes also suggests potential neuropharmacological strategies for mitigating the long-observed effects of environmental adversity on disease risk4. For example, β-adrenergic receptor blockade has been shown in animal models to reverse several stress-induced alterations in immune response gene transcription22,34,78. Therefore, it may provide a strategy for redirecting the leukocyte transcriptome via the induction of multiple trans-acting transcription factors by β-adrenergic receptors.
Box 3 | Behavioural interventions that modulate inflammation.
Several types of behavioural intervention have been found to reduce cellular and plasma biomarkers of inflammation.
Cognitive behavioural therapy (CBT)
CBT uses a programme of specific verbal and/or written protocols that guide participants through exercises designed to change specific targeted thoughts, feelings and behavioural patterns. As an example, CBT for depression involves a restructuring of dysfunctional thoughts and ruminations that contribute to negative perceptions of self and the environment. Benefits on the cellular expression of pro-inflammatory cytokines were found after 16 weeks in individuals whose depression was improved73.
Aerobic exercise
This involves moderate-intensity physical activities, such as walking for 20–30 minutes per day, which provides aerobic training without activating anaerobic respiration. Benefits on inflammatory markers were found after 12 months74.
Meditation
Meditation involves thought-focusing and relaxation exercises that calm cognitive and emotional processes and reduce sympathetic nervous system activity and related peripheral physiological processes. The practices found to alter inflammatory responses to stress used concentrative and mindfulness exercises to establish focus and awareness, followed by analytical exercises designed to challenge unexamined assumptions regarding feelings and actions towards others, with a focus on generating spontaneous empathy and compassion for themselves and others. Benefits on inflammatory markers were found after 6 weeks75.
Tai Chi Chih
Tai Chi Chih is a westernized form of the Chinese martial art Tai Chi, which involves slow movement and meditation. It combines the practice of 20 aerobic exercises with relaxed breathing, attentional focus and body awareness. Benefits on inflammatory markers were found after 25 weeks76.
Biomarkers affected by such interventions include indicators of pro-inflammatory cytokine activity (for example, C-reactive protein) and ex vivo cellular production of tumour necrosis factor and interleukin-1β in response to lipopolysaccharide stimulation.
Teleological considerations
Perhaps the most striking implication of neuro–immune circuitry is the possibility that the innate immune response may be controlled in part by an anticipation of future environmental conditions by the CNS, in addition to its regulation by the present microbial and host cell environment. Allostatic theories of physiology4 propose that natural selection favours ‘prepared’ physiological systems that actively anticipate homeostatic challenges and proactively alter their function to mitigate these challenges. Given that the HPA axis and the SNS both regulate leukocyte gene transcription, and both show CNS-mediated anticipatory activation4, CNS-mediated perceptions of potential threat may be sufficient to alter the basal leukocyte transcriptome in ways that subsequently affect responses to pathogens (for example, by altering inflammatory responses in myeloid lineage cells)5,6,16,26,78. This ‘forward-looking’ view of the immune system represents a significant departure from traditional pathogen- or damage-reactive models of immune regulation. But it does provide a parsimonious account of epidemiological data that link social–ecological conditions to immune response gene expression and disease resistance, as well as a plausible evolutionary rationale for the specific pattern of transcriptional alterations observed.
Social ecology of immune responses
Specific patterns of neural and endocrine activity are associated with distinctive social or ecological conditions, and these conditions affect the nature of the injuries and pathogens we confront. Thus, a natural selective pressure arises for immune response genes to develop a sensitivity to these neural and endocrine signals6. Such sensitivity could explain why, for example, the SNS has evolved the capacity to steer the innate immune transcriptome away from antiviral responses and towards pro-inflammatory responses. As threat-induced SNS signalling has historically been associated with a near-term increase in the likelihood of wound-mediated bacterial infection (for example, via predation of isolated organisms or conspecific hostility), SNS priming of the pro-inflammatory gene programme would seem to be highly adaptive.
By contrast, viral infections disseminate predominately through close social contact. Therefore, there would be little need for antiviral priming under hostile or isolated social conditions, but a much greater value in priming an antiviral transcriptional bias under long-term salutary social conditions (when SNS activity levels are generally low). Similarly, in response to more profound threats — such as trauma, starvation or exhaustion — that markedly activate the HPA axis, it makes good evolutionary sense for the body to redirect resources away from both types of long-term antimicrobial defence (antiviral and pro-inflammatory) in favour of more immediate physiological survival needs.
Conclusion
The whole-organism fitness advantages of a forward-looking, neurally regulated and neurally regulating innate immune response have clarified a variety of previously puzzling phenomena. These include: environmentally dependent variations in the basal transcriptome of unstimulated immune cells6,16,23,26,32,35,36,79; the striking co-morbidity of neuropsychiatric symptoms and inflammatory disease; the biological rationale for specific HPA- and SNS-related patterns of immune response gene transcription; and the distinctive impact of effector systems of the CNS (the HPA axis and the SNS) on peripheral myeloid lineage cells. As the relationship between environmental conditions and infectious disease has changed over the past century10, the historically beneficial crosstalk between the CNS and the immune system has become misaligned with our current ecology. Now, this crosstalk may allow abstract non-physical threats to induce inflammation-related cardiovascular, neurodegenerative and neoplastic diseases, while undermining our innate antiviral defences. Nevertheless, it may still be possible to harness reciprocal neural–immune regulation through pharmacological or behavioural interventions to redirect the basic transcriptional stance of the innate immune system and more effectively accommodate the health ecology that we now inhabit.
Acknowledgements
The authors are supported by grants R01-AG034588, R01-AG026364, R01-CA119159, R01-HL079955, P30-AG028748 and R01-MH091352 (to M.R.I.); grants R01-CA116778, R01-AG033590, R21-CA138687 and P30-AG028748 (to S.W.C.); and the Cousins Center for Psychoneuroimmunology.
Glossary
- Conspecific
Belonging to the same species.
- Glucocorticoids
A class of steroid hormones that are involved in carbohydrate, protein and fat metabolism. These hormones are anti-inflammatory and immunosuppressive.
- Hypothalamic–pituitary–adrenal
(HPA). This term refers to a complex set of direct influences and feedback interactions between the hypothalamus, the pituitary gland (a pea-shaped structure located below the hypothalamus) and the adrenal glands (small, conical organs on top of the kidneys).
- Non-rapid eye movement sleep
(NREM sleep). The sleep stages 1–3 (previously known as stages 1–4) are collectively referred to as NREM sleep. Rapid eye movement (REM) sleep is not included. There are distinct electroencephalographic and other characteristics seen in each stage, and there is usually little or no eye movement during NREM sleep. Dreaming is rare during NREM sleep, and muscles are not paralyzed as in REM sleep.
- Social ecology
A broad range of complex physical and symbolic features of the environment that are created by the presence of conspecifics (including social structures such as cultural systems or socio-economic status), as well as physical processes, such as transmission of communicable diseases, provision of medical care or physical aggression.
- Sympathetic nervous system
(SNS). One of three parts of the autonomic nervous system (along with the enteric and parasympathetic systems). The SNS serves to mobilize the body’s resources during flight-or-flight stress responses.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Michael R. Irwin and Steven W. Cole’s homepage: http://www.semel.ucla.edu/cousins
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Michael R. Irwin, Cousins Center for Psychoneuroimmunology, Semel Institute for Neuroscience, 300 UCLA Medical Plaza, University of California, Los Angeles, California 90095-7076, USA
Steven W. Cole, Cousins Center for Psychoneuroimmunology, Semel Institute for Neuroscience, 300 UCLA Medical Plaza, University of California, Los Angeles, California 90095-7076, USA Department of Medicine, Division of Haematology-Oncology, Jonsson Comprehensive Cancer Center, and the UCLA Molecular Biology Institute, University of California, Los Angeles, California, USA.
References
- 1.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nature Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 2.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nature Rev. Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 3.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 5.Powell ND, Mays JW, Bailey MT, Hanke ML, Sheridan JF. Immunogenic dendritic cells primed by social defeat enhance adaptive immunity to influenza A virus. Brain Behav. Immun. 2011;25:46–52. doi: 10.1016/j.bbi.2010.07.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc. Natl Acad. Sci. USA. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 8.Amit I, et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science. 2009;326:257–263. doi: 10.1126/science.1179050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nature Rev. Immunol. 2005;5:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 10.Finch CE. Evolution in health and medicine Sackler colloquium. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA. 2010;1:1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- 12.Berkenbosch F, VanOers J, DelRey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 13.Besedovsky H, del Rey A, Sorkin E, Dinarello CA. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986;233:652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- 14.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids — new mechanisms for old drugs. N. Engl. J. Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 15.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav. Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole S, et al. Computational identification of gene–social environment interaction at the human IL6 locus. Proc. Natl Acad. Sci. USA. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collado-Hidalgo A, Sung C, Cole S. Adrenergic inhibition of innate anti-viral response: PKA blockade of type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain Behav. Immun. 2006;20:552–563. doi: 10.1016/j.bbi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav. Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee HJ, et al. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J. Exp. Med. 2000;192:105–115. doi: 10.1084/jem.192.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panina-Bordignon P, et al. β2-agonists prevent Th1 development by selective inhibition of interleukin 12. J. Clin. Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effect on cytokine production. J. Immunol. 1998;161:610–616. [PubMed] [Google Scholar]
- 22.Grebe KM, et al. Cutting edge: sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J. Immunol. 2009;184:540–544. doi: 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole SW, et al. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:1–13. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sloan EK, et al. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J. Neurosci. 2007;27:8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiecolt-Glaser JK, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc. Natl Acad. Sci. USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller GE, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Biol. Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pace TW, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 28.Bierhaus A, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl Acad. Sci. USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goebel MU, Mills PJ, Irwin MR, Ziegler MG. Interleukin-6 and tumor necrosis factor-α production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom. Med. 2000;62:591–598. doi: 10.1097/00006842-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Bower JE, et al. Inflammatory responses to psychological stress in fatigued breast cancer survivors: relationship to glucocorticoids. Brain Behav. Immun. 2007;21:251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Richlin VA, Arevalo JM, Zack JA, Cole SW. Stress-induced enhancement of NF-κB DNA-binding in the peripheral blood leukocyte pool: effects of lymphocyte redistribution. Brain Behav. Immun. 2004;18:231–237. doi: 10.1016/j.bbi.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Miller GE, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl Acad. Sci. USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Wohleb ES, et al. β-adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen E, et al. Genome-wide transcriptional profiling linked to social class in asthma. Thorax. 2009;64:38–43. doi: 10.1136/thx.2007.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 37.Irwin MR, et al. Sleep loss activates cellular inflammatory signaling. Biol. Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motivala S, Irwin MR. Sleep and immunity: cytokine pathways linking sleep and health outcomes. Curr. Dir. Psychol. Sci. 2007;16:21–25. [Google Scholar]
- 39.Meier-Ewert HK, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 40.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav. Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R145–R151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- 42.Watkins LR, Maier SF. Implications of immune-to-brain communication for sickness and pain. Proc. Natl Acad. Sci. USA. 1999;96:7710–7713. doi: 10.1073/pnas.96.14.7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hart BL. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- 45.Harrison NA, et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenberger NI, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gimeno D, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009;39:413–423. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine–immune mechanisms of behavioral comorbidities in patients with cancer. J. Clin. Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capuron L, et al. Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 51.Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon α-2b therapy. J. Clin. Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- 52.Tyring S, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 53.Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol. Psychiatry. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benedetti F, Lucca A, Brambilla F, Colombo C, Smeraldi E. Interleukin-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:1167–1170. doi: 10.1016/s0278-5846(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 55.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nature Rev. Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullington J, et al. Dose-dependent effects of endotoxin on human sleep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- 58.Raison CL, et al. Chronic interferon-α administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol. Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vgontzas AN, et al. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J. Clin. Endocrinol. Metab. 2004;89:4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 60.Monk JP, et al. Assessment of tumor necrosis factor α blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J. Clin. Oncol. 2006;24:1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 61.Irwin MR, Olmstead R, Valladares EM, Breen EC, Ehlers CL. Tumor necrosis factor antagonism normalizes rapid eye movement sleep in alcohol dependence. Biol. Psychiatry. 2009;66:191–195. doi: 10.1016/j.biopsych.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dew MA, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom. Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 63.Thomas KS, Motivala S, Olmstead R, Irwin MR. Sleep depth and fatigue: role of cellular inflammatory activation. Brain Behav. Immun. 2011;25:53–58. doi: 10.1016/j.bbi.2010.07.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho HJ, Seeman TE, Bower JE, Kiefe CI, Irwin MR. Prospective association between C-reactive protein and fatigue in the coronary artery risk development in young adults study. Biol. Psychiatry. 2009;66:871–878. doi: 10.1016/j.biopsych.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heesen C, et al. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J. Neurol. Neurosurg. Psychiatry. 2006;77:34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harboe E, et al. Fatigue in primary Sjogren’s syndrome — a link to sickness behaviour in animals? Brain Behav. Immun. 2009;23:1104–1108. doi: 10.1016/j.bbi.2009.06.151. [DOI] [PubMed] [Google Scholar]
- 67.Davis MC, et al. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: implications for fatigue. Brain Behav. Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav. Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Bower JE, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 71.Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behav. Immun. 2011;25:147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early findings. Brain Behav. Immun. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zautra AJ, et al. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J. Consult. Clin. Psychol. 2008;76:408–421. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]
- 74.Nicklas BJ, et al. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J. Am. Geriatr. Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pace TW, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults. Am. J. Geriatr. Psychiatry. doi: 10.1097/JGP.0b013e3182330fd3. (in the press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lavretsky H, et al. Complementary use of Tai Chi Chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am. J. Geriatr. Psychiatry. 2011 Mar 6; doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sloan EK, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Idaghdour Y, et al. Geographical genomics of human leukocyte gene expression variation in southern Morocco. Nature Genet. 2010;42:62–67. doi: 10.1038/ng.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cole SW. In: Complex Systems Science in Biomedicine. Deisboeck TS, Kresh JY, editors. New York: Springer; 2006. pp. 605–629. [Google Scholar]