Abstract
STUDY DESIGN
Descriptive prospective cohort study.
OBJECTIVES
To investigate the relationships between knee joint effusion, quadriceps activation, and quadriceps strength. These relationships may help clinicians better identify impaired quadriceps activation.
BACKGROUND
After anterior cruciate ligament (ACL) injury, the involved quadriceps may demonstrate weakness. Experimental data have shown that quadriceps activation and strength may be directly mediated by intracapsular joint pressure created by saline injection. An inverse relationship between quadriceps activation and the amount of saline injected has been reported. This association has not been demonstrated for traumatic effusion. We hypothesized that traumatic joint effusion due to ACL rupture and postinjury quadriceps strength would correlate well with quadriceps activation, allowing clinicians to use effusion and strength measurement as a surrogate for electrophysiological assessment of quadriceps activation.
METHODS
Prospective data were collected on 188 patients within 100 days of ACL injury (average, 27 days) referred from a single surgeon. A complete clinical evaluation of the knee was performed, including ligamentous assessment and assessment of range of motion and effusion. Quadriceps function was electrophysiologically assessed using maximal volitional isometric contraction and burst superimposition techniques to quantify both strength and activation.
RESULTS
Effusion grade did not correlate with quadriceps central activation ratio (CAR) (zero effusion: mean ± SD CAR, 93.5% ± 5.8%; trace effusion: CAR, 93.8% ± 9.5%; 1+ effusion: CAR, 94.0% ± 7.5%; 2+/3+ effusion: CAR, 90.6% ± 11.1%). These values are lower than normative data from healthy subjects (CAR, 98% ± 3%).
CONCLUSION
Joint effusion after ACL injury does not directly mediate quadriceps activation failure seen after injury. Therefore, it should not be used as a clinical substitute for electrophysiological assessment of quadriceps activation. Patients presenting to physical therapy after ACL injury should be treated with high-intensity neuromuscular electrical stimulation to help normalize this activation.
Keywords: ACL, effusion, electrophysiological assessment, swelling
The quadriceps of the involved limb weakens profoundly after anterior cruciate ligament (ACL) rupture.23 Even early after injury, quadriceps weakness can be substantial, despite little time for atrophy.3,11 These strength deficits can persist in patients long after ACL reconstruction.1,2 Preoperative quadriceps weakness predicts poor quadriceps strength and low self-reported function on validated outcome measures after surgery.6 The cycle of impaired quadriceps strength, therefore, begins at the time of injury and persists long after reconstruction, with significant implications for function.9
One reason for rapid weakening of the quadriceps after ACL rupture is an inability to fully activate the quadriceps due to structural changes in the joint, a condition termed “arthrogenic muscle inhibition.”3,7,9,27 The average volitional activation of individuals with an ACL injury is less than 93% in both limbs.3 Changes in the stimulation of joint receptors after injury lead to altered quadriceps function. Surgical intervention to address joint pathology does not immediately or reliably restore quadriceps activation.27,29 Activation failure can contribute to the profound strength deficits in patients with knee joint pathology and may serve as a barrier to rehabilitation of the quadriceps.7,18,27 Knee pain and joint effusion have both been implicated in the development of early activation failure. The mean ± SD volitional activation of healthy young adults in our laboratory and clinic is 98% ± 3%.26 These data are not normally distributed, with most subjects having 100% activation. Based on these data, we have operationally defined activation failure as volitional activation below 95%. The relationship between activation failure and maximal volitional isometric contraction (MVIC) is curvilinear and best fit by a second-order polynomial, so a small change in activation (near 100%) results in a substantial change in force.25
Failure of voluntary activation of the quadriceps can be induced by experimentally creating an effusion (eg, injecting saline into the joint).9,14 In the knee, a very large experimental effusion decreases the voluntary activity of the involved quadriceps, the effect of which is linked to the pressure-sensitive mechanoreceptors in the joint capsule.13,15,24 Large, experimentally induced effusions also alter joint mechanics.16 Early after ACL injury, effusion is nearly ubiquitous and the resolution of effusion is one of the primary goals of preoperative rehabilitation.8,20 We have previously studied quadriceps activation in a consecutive sample of patients before surgery, when their impairments were resolved (no effusion).3 However, the relationships among quadriceps activation, quadriceps strength, and knee joint effusion prior to the impairment resolution after ACL rupture have not been fully explored. Because there is a threshold effect of effusion on activation in experimental models, it is plausible that this may be true in clinical effusion. Therefore, quadriceps strength and activation failure may be more strongly correlated at higher levels of effusion. It is clear that experimentally induced effusion can be used to induce transient activation failure in an otherwise normal knee; however, there is no evidence that effusion from an acute knee injury induces or mediates quadriceps activation failure.
In addition to impaired activation of the involved-limb quadriceps, there is potential for activation failure in the uninvolved-limb quadriceps.30 Twenty-one percent of patients with an ACL-deficient knee demonstrated uninvolved-limb activation failure in a previous investigation.3 Arthrogenic muscle inhibition of the contralateral limb has not been produced with experimental joint effusions.13 Uninvolved-limb activation failure has the potential to confound strength measurements, making the injured limb appear stronger than it actually is. The appearance of symmetrical strength in some patients may result in insufficient dosages of strengthening before reconstructive surgery.30
The purpose of the present study was to examine activation changes and clinical correlates of these changes in a large, consecutive clinical sample, as patients presented for initial physical therapy evaluation after diagnosis of ACL rupture by an orthopaedic surgeon (the typical referral pattern for our large outpatient clinic).
With knowledge of the relationships between easily measured clinical variables and activation failure, clinical decisions may be made to optimize the function of the quadriceps after injury. We hypothesized that more patients with large effusions would demonstrate activation less than 95%. We also hypothesized that because arthrogenic muscle inhibition can cause quadriceps strength deficits, these measures would be strongly correlated. Last, because of these relationships, the combination of quadriceps strength, knee joint effusion, and other clinical variables would be able to reliably predict patients with quadriceps activation less than 95%.
METHODS
This is a secondary analysis of data collected prospectively from several ongoing observational studies of ACL injury. The use of these deidentified data for this purpose was approved by the Human Subjects Review Board at the University of Delaware. One hundred eighty-eight consecutive patients who had an ACL injury were evaluated an average of 27 days (range, 3–98 days) after injury. Twelve patients were evaluated within 1 week of injury, and an additional 50 in the second week from injury. One hundred twenty males (63%) and 68 females (37%), ranging in age from 13 to 63 years (mean ± SD, 29.3 ± 12.0 years), were referred by a single surgeon for preoperative evaluation and impairment resolution. A complete clinical evaluation of the patient’s knee was performed, including ligamentous testing and measures of knee joint effusion, range of motion, and quadriceps function. The patients included in this study had no formal physical therapy interventions before being evaluated in our clinic.
Inclusion and Exclusion Criteria
ACL injury was operationally defined as having a positive Lachman test and a 3-mm or greater difference in anterior excursion with instrumented arthrometry (KT1000; MEDmetric Corporation, San Diego, CA).4 All patients included in this sample had a positive Lachman test and a 3-mm difference in instrumented testing. Ruptures and concomitant injuries (medial collateral ligament and meniscus tears) were confirmed with magnetic resonance imaging and clinical examination. Patients who presented for initial evaluation within 100 days of their injury and had valid measures taken of quadriceps central activation ratio (CAR) and knee joint effusion were included in this analysis.
Effusion
Effusion was determined using a modified stroke test, which has been used clinically for more than 15 years.28 The stroke test is performed with the patient in supine and with the knee in full extension and relaxed. Starting at the medial tibio-femoral joint line, the examiner strokes upward 2 or 3 times toward the suprapatellar pouch, in an attempt to move the swelling within the joint to the suprapatellar pouch. The examiner then strokes downward on the distal lateral thigh, just superior to the suprapatellar pouch, toward the lateral joint line. A wave of fluid may be observed within seconds on the medial side of the knee. Effusion of the knee joint is quantified using a 5-point scale (TABLE 1). A zero grade is given when no wave is produced with the downward stroke. If the downward stroke produces a small wave on the medial side of the knee, the effusion is graded as trace, and a larger bulge is given a grade of 1+. If the effusion returns to the medial side of the knee without a downward stroke, the effusion is given a grade of 2+. The inability to move the effusion out of the medial aspect of the knee is graded as 3+. We recently reported a kappa value of 0.75 in our clinic, indicating very good agreement between raters.28
TABLE 1.
Effusion Grading Scale of the Knee Joint Based on the Stroke Test
| Grade | Test Result |
|---|---|
| Zero | No wave produced on downstroke |
| Trace | Small wave on medial side with downstroke |
| 1+ | Larger bulge on medial side with downstroke |
| 2+ | Effusion spontaneously returns to medial side after upstroke (no downstroke necessary) |
| 3+ | So much fluid that it is not possible to move the effusion out of the medial aspect of the knee |
Reprinted from Sturgill et al.28
Due to a threshold effect of the experimental effusions, clinical effusions were categorized as large effusions (grades 2+ and 3+) and small effusions (1+ or less) for secondary analysis.
Quadriceps Strength and Activation
The function of the quadriceps was investigated with burst superimposition testing during an MVIC.19,23,25,26 This evaluation method allows for calculation of both volitional quadriceps activation and quadriceps strength. The patient was positioned in an electromechanical dynamometer (Kin-Com; DJO Global, Vista, CA) in isometric mode, at 90° of hip and knee flexion, with the force transducer placed just proximal to the talocrural joint, and the pelvis and the thigh secured to the dynamometer using 2 straps. Two 3 × 5-in (7.6 × 12.7-cm) self-adhesive electrodes were placed over the quadriceps, proximally over the muscle belly of the vastus lateralis and distally over the vastus medialis. Patients performed sub-maximal (50%–75% of perceived maximum) and maximal (100% of perceived maximum) warm-up trials to familiarize themselves with the testing protocol. Patients were familiarized with the stimulation sensation using brief stimulations of 10, 30, and 60 V, which also allowed the tester to ensure that the electrodes were placed correctly. Three to 5 minutes of rest were given between the warm-up and test trials. During the test trials, a supramaximal 10-pulse (600 microseconds, 135 V), 100-pulse-per-second train of electrical stimulation was administered while the patient performed an MVIC. Pain during contraction was rated on a verbal analog scale, with a score range of 0 to 10, where 0 was no pain and 10 was the worst pain imaginable. If pain exceeded 2/10, actions were taken to change the tibiofemoral joint angle or to apply tape to the patellofemoral joint to decrease pain to allow successful completion of the task. The data of individuals with joint angles of less than 75° of knee flexion were not included in this analysis.
The CAR is a measurement of the patient’s ability to volitionally activate the quadriceps, differentiating weakness from impaired activation. The CAR is used as a measure of the presence of arthrogenic muscle inhibition. The strength obtained with voluntary contraction is compared to the strength obtained with the electrically elicited contraction and expressed as a ratio.
The CAR was determined through the following equation: CAR= (MVIC/electrically elicited isometric contraction) × 100%.
A CAR of 95% or higher is readily achievable in a variety of muscles in healthy individuals; therefore, our laboratory and clinic have used this criterion to indicate full activation, with activation failure operationally defined by a value below 95%.9,25,26 This threshold is greater than 1 standard deviation (±3%) less than the average activation (98%) of healthy control subjects tested with identical methodology in our laboratory.26 If activation was less than 95%, up to 2 additional trials were performed, with a 5-minute rest between trials. The trial with the greatest activation was used in the analysis.
The quadriceps index is a measure of the relative strength of the involved quadriceps compared to the uninvolved quadriceps. The quadriceps index was expressed as a ratio of the MVIC of the involved limb compared to the MVIC of the uninvolved limb. This method allowed for each individual’s uninvolved quadriceps to serve as a control for the involved quadriceps. The quadriceps index was determined using the following equation: quadriceps index = (involved-limb MVIC/uninvolved-limb MVIC) × 100%.
Statistical Measures
Nominal data were compared using chi-square tests. Differences in CAR and quadriceps index between grades of effusion were explored with 1-way analysis of variance with least significant difference post hoc testing. A 1-way analysis of variance was also used to determine differences in time from injury to initial evaluation between the effusion groups. Correlation coefficients were used to determine relationships between the quadriceps index and CAR. Alpha levels were set a priori at a significance level of .05.
Finally, logistic regression was used to explore how combinations of effusion grades, quadriceps index, and time from injury could be used to clinically predict which patients would demonstrate a CAR of less than 95%. To attempt to predict those patients who would demonstrate a CAR of less than 95%, effusion grades (small or large), quadriceps index, and time from injury were entered into a logistic regression in a block pattern as independent variables. Activation was classified as either a CAR of 95% or greater or a CAR of less than 95% and entered as the dependent variable. Quadriceps index was entered in 5 different models: (1) as a continuous variable; (2) dichotomized into “weak” (less than 90%) and “strong” (90% or greater); (3) dichotomized into “weak” (less than 80%) and “strong” (80% or greater); (4) dichotomized into “very strong” (90% or greater) and “very weak” (less than 80%); and (5) divided into 3 groups: “very strong” (90% or greater), “moderate” (80% or greater and less than 90%), and “very weak” (less than 80%). All statistics were run using PASW Statistics 18 (SPSS Inc, Chicago, IL).
RESULTS
Knee Joint Effusion
Eighty-five percent of the patients had a measurable effusion (trace or higher; TABLE 2). Only 3 patients had 3+ effusion; therefore, the 2+ and 3+ effusion groups were combined for the analysis of variance. The frequency of effusion grades was not evenly distributed, with a greater proportion of grades 2+/3+ (n = 62) and a minority of effusion-free patients (n = 29) (χ2 = 11.78, P = .008). Patients who presented to physical therapy sooner after injury had higher effusion grades (F = 8.509, P<.001).
TABLE 2.
Number of Days From Injury to Initial Evaluation for Each Level of Effusion
| Effusion Grade | n | Mean ± SD | Range | Median |
|---|---|---|---|---|
| Zero | 29 | 38 ± 24*† | 9–98 | 31 |
| Trace | 49 | 33 ± 24* | 4–88 | 26 |
| 1+ | 48 | 26 ± 21* | 4–88 | 19 |
| 2+/3+ | 62 | 18 ± 13 | 3–55 | 13 |
Significantly greater time from injury compared to 2+/3+ group, P<.001.
Significantly greater time from injury compared to 1+ group, P<.001.
Quadriceps Activation
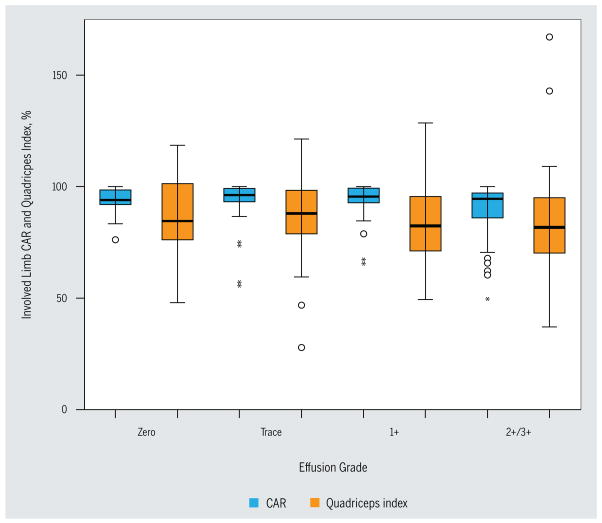
A CAR of less than 95% was identified in both limbs of 48 patients (25.5%). An additional 32 (17.1%) patients had a CAR of less than 95% in the involved quadriceps only, and 15 (8.0%) had a CAR of less than 95% in the uninvolved quadriceps only. Ninety-three (49.4%) patients had a CAR greater than 95% in both quadriceps muscles (FIGURE 1). As time between injury and evaluation increased, CAR also increased (r = 0.216, P = .003). Mean involved and uninvolved quadriceps activation did not differ by level of effusion (involved quadriceps: F = 0.710, P = .167; uninvolved quadriceps: F = 1.786, P = .151) (TABLE 3, FIGURE 2). There was no difference in the frequency of CAR less than 95% among the effusion grades (χ2 = 6.26, P = .100) (TABLE 3). When the effusion grades were dichotomized, the group with the large effusion had a lower CAR in the involved quadriceps (t = −2.028, P = .045) (TABLE 3).
FIGURE 1.
Distribution of individuals based on CAR after ACL injury. Abbreviations: ACL, anterior cruciate ligament; CAR, central activation ratio.
TABLE 3.
Quadriceps Function by Effusion Grade*
| Involved Limb | Uninvolved Limb | ||||
|---|---|---|---|---|---|
| CAR <95%, n | CAR ≥95%, n | CAR, % | CAR, % | Quadriceps Index | |
| Effusion grade | |||||
| Zero | 16 | 13 | 93.5 ± 5.8 | 94.8 ± 4.7 | 86.1 ± 17.0 |
| Trace | 16 | 33 | 93.8 ± 9.5 | 95.4 ± 7.1 | 87.0 ± 17.0 |
| 1+ | 17 | 31 | 94.0 ± 7.5 | 95.6 ± 5.4 | 83.9 ± 17.1 |
| 2+/3+ | 31 | 31 | 90.6 ± 11.1 | 92.8 ± 9.0 | 82.5 ± 21.8 |
| Effusion group | |||||
| Small | 49 | 77 | 93.8 ± 8.0 | 95.4 ± 5.9 | 85.6 ± 16.9 |
| Large | 31 | 31 | 90.6 ± 11.1 | 92.8 ± 9.0 | 82.5 ± 21.8 |
Abbreviation: CAR, central activation ratio.
Data presented in the table are mean ± SD unless indicated otherwise. Comparisons among effusion grades: involved CAR did not differ by effusion grade (analysis of variance: P = .167, F = 1.710); uninvolved CAR did not differ by effusion grade (analysis of variance: P = .151, F = 1.786); quadriceps index did not differ by effusion grade (analysis of variance: P = .600, F = 0.625); distribution of CAR below 95% did not differ by effusion grade (χ2 = 6.26, P = .100). Comparisons among effusion groups: involved CAR was significantly greater in small effusion group (t test: P = .045, t = −2.028); uninvolved CAR did not differ by effusion group (t test: P = .051, t = −1.981); quadriceps index did not differ by effusion group (t test: P = .282, t = −1.080); distribution of CAR below 95% did not differ by effusion group (χ2 = 1.669, P = .196).
FIGURE 2.

Interaction of CAR less than 95% and quadriceps index greater than 90%. Patients (n = 53) with quadriceps index less than 90% and bilateral CAR greater than or equal to 95% are not included in this diagram. Abbreviation: CAR, central activation ratio.
Quadriceps Strength
The CAR and quadriceps index were significantly correlated (r = 0.323, P<.001). This correlation was higher when the 63 patients with a CAR of less than 95% in the uninvolved quadriceps were excluded from the analysis (r = 0.518, P<.001). One quarter of patients presenting with a normal quadriceps index (90% or greater) had a bilateral CAR of less than 95% and over one third had a CAR of less than 95% in the uninvolved limb (FIGURE 2). The quadriceps index did not differ among the grades of effusion (F = 0.625, P = .600) (TABLE 3, FIGURE 3).
FIGURE 3.
Effusion and CAR. Boxes represent 25th to 75th percentile. The line in the middle represents the 50th percentile. The whiskers represent the range of values not including outliers. Open circles represent values between 1.5 and 3 times the interquartile range (outliers). Asterisks (*) represent values greater than 3 times the interquartile range (extreme values). Abbreviation: CAR, central activation ratio.
Logistic Regression
Each combination of variables produced a statistically significant model (P<.016) (TABLE 4). However, the amount of variance explained by each model was small (R2 values ranged from 0.076 to 0.101). A small majority of patients (57.4%) demonstrated a CAR greater than 95%, and none of the models produced greatly improved the percentage of correctly identified cases with a CAR less than 95% (61.7%-64.2%). Effusion was not a significant predictor of arthrogenic muscle inhibition in any iteration of the regression model. The quadriceps index was a significant predictor in each model (P<.05), and time from injury (P = .043) was a significant predictor of a CAR less than 95% in model 2.
TABLE 4.
Predicting Patients With a Central Activation Ratio Less Than 95% From Clinical Variables*
| Model | Nagelkerke R2 | Percentage of Correctly Identified Cases | P Value |
|---|---|---|---|
| 1 | .077 | 64.2% | .012 |
| 2 | .078 | 62.8% | .011 |
| 3 | .076 | 61.7% | .012 |
| 4 | .101 | 63.6% | .012 |
| 5 | .084 | 62.8% | .016 |
All models included effusion dichotomized into 2 groups (large versus small) and time from injury. In addition, each model included a different cutoff for quadriceps index: model 1, quadriceps index (continuous); model 2, dichotomized quadriceps index (<90% and ≥90%), time from injury; model 3, dichotomized quadriceps index (<80% and ≥80%); model 4, dichotomized quadriceps index (<80% and ≥90%); model 5, quadriceps strength (<80%, ≥80%, <90%, and ≥90%). All models included significant predictors.
DISCUSSION
A consecutive sample of individuals after unilateral ACL rupture was tested to determine the effects of knee joint effusion on quadriceps activation. Based on these results, the size (grade) of the knee joint effusion after injury does not predict quadriceps activation failure after ACL injury. In this sample of patients with an ACL-injured knee, while CAR was lower in those patients with a 2+ or 3+ effusion compared to those with a 1+ or less effusion, a substantial clinical error occurred if the size of the effusion was used to predict the presence or absence of activation failure. A CAR of less than 95% (operational definition of activation failure) occurred as often in those without a measurable effusion as it did in those with a large effusion. Finally, clinical measures (quadriceps index, effusion grade, and time from injury) cannot be used as surrogate measures to predict the presence or absence of a CAR less than 95%.
Knee joint effusion, quadriceps activation deficits, and quadriceps strength deficits are all prevalent after ACL injury and have a large impact on function and postoperative outcomes. In this sample, 59% of patients had an effusion grade of at least 1+, 43% had a CAR less than 95% in the involved quadriceps, and 63% demonstrated poor quadriceps strength (quadriceps index less than 90%). The use of 95% as the threshold to identify patients with activation failure is based on previous data collected from healthy control subjects by an identical methodology, with an average ± SD activation of 98% ± 3%.26 Therefore, 95% represents a value greater than 1 standard deviation less than the mean, truly identifying those patients with poor activation compared to healthy subjects. Despite the high prevalence of these impairments and the statistical significance of the relationships between them, the hypothesized relationships were not clinically meaningful, which did not allow for the simplification of clinical decision making.
Previous studies of experimentally induced effusion have demonstrated that knee joint effusion is a direct cause of activation failure when reaching a 60-cc saline injection threshold, as measured with H-reflexes (a nonclinical measurement of muscle inhibition).15,24 In our sample, those with a large effusion demonstrated a mean CAR of 3% lower than those with a small effusion, replicating this threshold effect. However, half of those with a large effusion still had full activation, indicating that the presence of a large joint effusion does not presage activation failure. Although the effects of experimentally induced effusions have been linked to pressure-sensitive mechanisms, it is possible that in the case of longer-duration effusion (lasting for hours), these mechanisms adapt to the presence of the effusion.
Another potential reason for the discrepancy between the findings of injected-saline studies and this investigation is the amount of time between the onset of effusion and testing. While all patients in our sample were recently injured, the effects of the experimentally induced effusion are tested minutes to hours after the onset of effusion and even large experimentally induced effusions resolve in a few hours.13,15,16,24 In those highly controlled settings, a direct cause-and-effect relationship exists for knee joint effusion: the injection of saline immediately decreases the excitability of the alpha motor neuron, which decreases the force-producing capability of the quadriceps.24 The nervous system may adapt to the effusion over time, diminishing arthrogenic muscle inhibition caused by effusion. The decreasing arthrogenic muscle inhibition with increased time from injury noted in this sample may be explained by this mechanism.
The activity level between injury and testing can also contribute to the CAR measured at the time of testing. In the injection studies, the subjects’ activity level was controlled between the introduction of the effusion and testing.13,15,16,24 In this sample, the activity level between injury and testing was not controlled and could have varied greatly. Although none of the patients in this analysis had formal physical therapy between injury and testing, it is possible that they engaged in higher-level activities that did not cause instability and contributed to the adaptation of the nervous system. During MVIC testing with burst superimposition, low-level activation failure may be overcome within the 3 attempted MVIC trials of a single session, which can be attributed to the forceful contractions.23 High-intensity activities may also increase the amount of effusion in the knee joint if the activities are an irritant to the knee joint, allowing arthrogenic muscle inhibition to persist. Relative inactivity can decrease the amount of effusion by not irritating the joint; however, it may not allow adaptation to the arthrogenic muscle inhibition present after injury. Therefore, the activity level of the patient between injury and evaluation may affect effusion and quadriceps activity and thus affect their relationship.
Other inflammatory pathways, such as pain and chemical markers of inflammation causing inhibition, may be mitigated during the interim between injury and testing, while effusion may persist, resulting in normal activation even in the presence of effusion. Pain can result in failure of voluntary activation, typically acting through a protective mechanism, although there is some controversy about its contributions to arthrogenic muscle inhibition.10,18,31 Pain can be ruled out in this sample, as pain scores during evaluation averaged less than 1/10. The few burst superimposition tests that caused knee joint pain were documented, considered unreliable for activation measurement, and not included in this analysis, according to our clinical protocol.
Inflammation increases the activity of receptors in the joint, increasing arthrogenic muscle inhibition.18 Inflammation is likely present to some degree, as effusion is one of the cardinal signs of inflammation. However, if patients had been taking nonsteroidal anti-inflammatory drugs, it is possible that the residual effusion was the last marker of inflammation and those chemical inflammatory markers were not present in the knee joint in substantial levels.
The hypothesis that the quadriceps index would be strongly correlated with CAR was not supported, although there was a moderate correlation (r = 0.323, P<.001). The lack of a strong correlation is likely due to the presence of bilateral CAR less than 95% after injury. In this sample, a quarter of patients with a unilateral ACL injury demonstrated a CAR of less than 95% bilaterally, similar to the previously reported 21%.3 Artificially induced effusion does not cause contra-lateral arthrogenic muscle inhibition; however, the effect of injury on contra-lateral arthrogenic muscle inhibition is not explicitly known. The average uninvolved limb quadriceps CAR of this group was below the 95% cutoff, demonstrating a difference compared to the previously studied healthy control subjects.20 With standard isometric strength testing, a bilateral CAR less than 95% will result in an artificially inflated quadriceps index, due to poor performance of both quadriceps.18,30 These inflated quadriceps indexes explain some of the discrepancy; when those with an uninvolved-quadriceps CAR of less than 95% were removed from the sample, the correlation between the involved-limb CAR and quadriceps index increased from 0.323 to 0.518. This relationship is still only moderate but underscores the inability of strength testing to estimate activation failure due to confounding factors. Clinicians must be aware of the threats to the validity of strength measurements, as impaired quadriceps activation of the uninvolved limb may mask strength and activation deficits of the involved quadriceps.
The final hypothesis that a CAR of less than 95% would be predictable based on the combination of effusion grade, quadriceps index, and time from injury was also not supported by these data. Although the regression was statistically significant, the combined model was not strong enough to be used for predictive purposes. Due to middling relationships among the variables and the confounding nature of bilateral inhibition, the predictive ability of the regression was weak. The authors chose a CAR of 95% as a conservative cutoff (assuming that CAR values below the cutoff are a deficit) to identify those patients who need additional treatment to target quadriceps deficits and to ensure that these deficits are treated aggressively to restore quadriceps function.
Because many clinical facilities are unable to assess quadriceps CAR and no reliable predictors for a CAR of less than 95% exist, it is prudent to assume a CAR of less than 95% after ACL injury. To assume that arthrogenic muscle inhibition exists and to implement interventions to overcome it would greatly benefit patients postinjury. This is especially important for patients who demonstrate a low quadriceps index (less than 90%), as preoperative quadriceps strength is predictive of improved self-reported outcomes after reconstruction.6 Considering the relationship between preoperative quadriceps strength and future function, treating all patients with aggressive strengthening and interventions aimed at decreasing arthrogenic muscle inhibition prior to reconstruction should be considered as a uniform treatment.
To augment typical volitional strengthening, interventions to decrease arthrogenic muscle inhibition that have been successful include cryotherapy, transcutaneous electrical nerve stimulation, and neuromuscular electrical stimulation (NMES).10,12,18 Nonsteroidal anti-inflammatory drugs and injected corticosteroids also have a potential benefit.18 Each of these interventions, with the exception of NMES, aims to decrease the inflammation or efferent nerve impulses from the knee joint, which reduces arthrogenic muscle inhibition. NMES aims to strengthen the motor units under volitional control as well as those that are not, increasing strength and preventing atrophy.31 As an adjunct strengthening modality, NMES activates all neurons that are within the field of the stimulus, potentially activating neurons that were not activated during volitional exercise, maintaining the strength of the muscle fibers that they innervate, and providing a level of neuromuscular re-education. The combination of NMES and high-intensity, volitional quadriceps strengthening improves strength in ACL-deficient and reconstructed patients greater than volitional exercise alone.12,17,22
Targeting assumed arthrogenic muscle inhibition with high-intensity exercise, NMES, and inflammation-reducing modalities before surgery may greatly benefit the patient in the long term. These guidelines will prevent the undertreatment of quadriceps deficits before patients proceed to reconstruction. Failure to consider and treat potential quadriceps activation failure after injury may lead to poor functional outcomes in both operative and nonoperative management. Alteration of treatment strategies to include high-intensity progressive resistive exercises and NMES has been shown to improve quadriceps strength and has the potential to improve activation if deficits exist.5,9,12,21,22
Limitations
The measure of effusion used in this study is commonly used in clinical practice. However, it has not been validated against other measures of effusion such as aspiration. Other measurement techniques yield a CAR for uninjured subjects closer to 95%, with a standard deviation of 4%. However, the normative values used in this study came from the same laboratory with identical methodology.
CONCLUSION
Quadriceps activation of less than 95% is prevalent after ACL injury, with up to 43% of involved limbs experiencing some degree of arthrogenic muscle inhibition. A CAR of less than 95% may contribute to the poor performance of the quadriceps after injury and impede strength gains in volitional exercise. However, effusion after an injury does not consistently produce activation failure. Due to the presence of bilateral activation failure, quadriceps strength indexes and CAR do not correlate strongly and may invalidate strength measurements. As no clinical predictors of CAR less than 95% have been identified at this time, clinicians should consider activation failure to be present in all patients following an ACL injury. Further research is necessary, but NMES and other interventions are promising interventions to help restore normal activation. Effusion, activation failure, and quadriceps strength deficits all have negative implications for functional outcomes and should be resolved before reconstruction or completion of nonoperative management.
KEY POINTS.
FINDINGS
Standard clinical variables are unable to predict the presence of quadriceps activation failure. Forty-three percent of patients after ACL injury had a CAR of less than 95%.
IMPLICATIONS
Given the inability to identify those with a CAR below 95% using knee effusion and the quadriceps index, aggressive rehabilitation of quadriceps strength deficits is indicated in all patients with an ACL tear. Quadriceps strength measurements must be interpreted with caution after injury.
CAUTION
Patients presented at varying times from injury, and activity level was not controlled between injury and evaluation.
Acknowledgments
This work was completed when Andrew D. Lynch was a doctoral student at the University of Delaware, Department of Physical Therapy. This study was approved by the Human Subjects Review Board of the University of Delaware. This work was funded through grants from the National Institutes of Health (R01 HD07490-11, R01 HD37985, and R01 AR048212).
The authors would like to thank Martha Callahan, Megan Doran, SPT, and Brittany Patterson, PT for their help with data collection and organization.
References
- 1.Ageberg E, Thomee R, Neeter C, Silbernagel KG, Roos EM. Muscle strength and functional performance in patients with anterior cruciate ligament injury treated with training and surgical reconstruction or training only: a two to five-year followup. Arthritis Rheum. 2008;59:1773–1779. doi: 10.1002/art.24066. http://dx.doi.org/10.1002/art.24066. [DOI] [PubMed] [Google Scholar]
- 2.Chen CH, Chuang TY, Wang KC, Chen WJ, Shih CH. Arthroscopic anterior cruciate ligament reconstruction with quadriceps tendon autograft: clinical outcome in 4–7 years. Knee Surg Sports Traumatol Arthrosc. 2006;14:1077–1085. doi: 10.1007/s00167-006-0111-0. http://dx.doi.org/10.1007/s00167-006-0111-0. [DOI] [PubMed] [Google Scholar]
- 3.Chmielewski TL, Stackhouse S, Axe MJ, Snyder-Mackler L. A prospective analysis of incidence and severity of quadriceps inhibition in a consecutive sample of 100 patients with complete acute anterior cruciate ligament rupture. J Orthop Res. 2004;22:925–930. doi: 10.1016/j.orthres.2004.01.007. http://dx.doi.org/10.1016/j.orthres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 5.Delitto A, Snyder-Mackler L. Two theories of muscle strength augmentation using percutaneous electrical stimulation. Phys Ther. 1990;70:158–164. doi: 10.1093/ptj/70.3.158. [DOI] [PubMed] [Google Scholar]
- 6.Eitzen I, Holm I, Risberg MA. Preoperative quadriceps strength is a significant predictor of knee function two years after anterior cruciate ligament reconstruction. Br J Sports Med. 2009;43:371–376. doi: 10.1136/bjsm.2008.057059. http://dx.doi.org/10.1136/bjsm.2008.057059. [DOI] [PubMed] [Google Scholar]
- 7.Fahrer H, Rentsch HU, Gerber NJ, Beyeler C, Hess CW, Grunig B. Knee effusion and reflex inhibition of the quadriceps. A bar to effective retraining. J Bone Joint Surg Br. 1988;70:635–638. doi: 10.1302/0301-620X.70B4.3403614. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald GK, Axe MJ, Snyder-Mackler L. Proposed practice guidelines for nonoperative anterior cruciate ligament rehabilitation of physically active individuals. J Orthop Sports Phys Ther. 2000;30:194–203. doi: 10.2519/jospt.2000.30.4.194. [DOI] [PubMed] [Google Scholar]
- 9.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010;45:87–97. doi: 10.4085/1062-6050-45.1.87. http://dx.doi.org/10.4085/1062-6050-45.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hopkins J, Ingersoll CD, Edwards J, Klootwyk TE. Cryotherapy and transcutaneous electric neuromuscular stimulation decrease arthrogenic muscle inhibition of the vastus medialis after knee joint effusion. J Athl Train. 2002;37:25–31. [PMC free article] [PubMed] [Google Scholar]
- 11.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: part 1, outcomes. Am J Sports Med. 2008;36:40–47. doi: 10.1177/0363546507308190. http://dx.doi.org/10.1177/0363546507308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KM, Croy T, Hertel J, Saliba S. Effects of neuromuscular electrical stimulation after anterior cruciate ligament reconstruction on quadriceps strength, function, and patient-oriented outcomes: a systematic review. J Orthop Sports Phys Ther. 2010;40:383–391. doi: 10.2519/jospt.2010.3184. http://dx.doi.org/10.2519/jospt.2010.3184. [DOI] [PubMed] [Google Scholar]
- 13.Palmieri RM, Ingersoll CD, Edwards JE, et al. Arthrogenic muscle inhibition is not present in the limb contralateral to a simulated knee joint effusion. Am J Phys Med Rehabil. 2003;82:910–916. doi: 10.1097/01.PHM.0000098045.04883.02. http://dx.doi.org/10.1097/01.PHM.0000098045.04883.02. [DOI] [PubMed] [Google Scholar]
- 14.Palmieri RM, Ingersoll CD, Hoffman MA, et al. Arthrogenic muscle response to a simulated ankle joint effusion. Br J Sports Med. 2004;38:26–30. doi: 10.1136/bjsm.2002.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmieri RM, Weltman A, Edwards JE, et al. Pre-synaptic modulation of quadriceps arthrogenic muscle inhibition. Knee Surg Sports Traumatol Arthrosc. 2005;13:370–376. doi: 10.1007/s00167-004-0547-z. http://dx.doi.org/10.1007/s00167-004-0547-z. [DOI] [PubMed] [Google Scholar]
- 16.Palmieri-Smith RM, Kreinbrink J, Ashton-Miller JA, Wojtys EM. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35:1269–1275. doi: 10.1177/0363546506296417. http://dx.doi.org/10.1177/0363546506296417. [DOI] [PubMed] [Google Scholar]
- 17.Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61:174–183. doi: 10.1002/art.24167. http://dx.doi.org/10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- 18.Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010;40:250–266. doi: 10.1016/j.semarthrit.2009.10.001. http://dx.doi.org/10.1016/j.semarthrit.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Rutherford OM, Jones DA, Newham DJ. Clinical and experimental application of the percutaneous twitch superimposition technique for the study of human muscle activation. J Neurol Neurosurg Psychiatry. 1986;49:1288–1291. doi: 10.1136/jnnp.49.11.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shelbourne KD, Klotz C. What I have learned about the ACL: utilizing a progressive rehabilitation scheme to achieve total knee symmetry after anterior cruciate ligament reconstruction. J Orthop Sci. 2006;11:318–325. doi: 10.1007/s00776-006-1007-z. http://dx.doi.org/10.1007/s00776-006-1007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder-Mackler L, Delitto A, Bailey SL, Stralka SW. Strength of the quadriceps femoris muscle and functional recovery after reconstruction of the anterior cruciate ligament. A prospective, randomized clinical trial of electrical stimulation. J Bone Joint Surg Am. 1995;77:1166–1173. doi: 10.2106/00004623-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Snyder-Mackler L, Delitto A, Stralka SW, Bailey SL. Use of electrical stimulation to enhance recovery of quadriceps femoris muscle force production in patients following anterior cruciate ligament reconstruction. Phys Ther. 1994;74:901–907. doi: 10.1093/ptj/74.10.901. [DOI] [PubMed] [Google Scholar]
- 23.Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR., 3rd Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76:555–560. doi: 10.2106/00004623-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Spencer JD, Hayes KC, Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65:171–177. [PubMed] [Google Scholar]
- 25.Stackhouse SK, Dean JC, Lee SC, Binder-MacLeod SA. Measurement of central activation failure of the quadriceps femoris in healthy adults. Muscle Nerve. 2000;23:1706–1712. doi: 10.1002/1097-4598(200011)23:11<1706::aid-mus6>3.0.co;2-b. http://dx.doi.org/10.1002/1097-4598(200011)23:11<1706::AID-MUS6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 26.Stackhouse SK, Stevens JE, Lee SC, Pearce KM, Snyder-Mackler L, Binder-Macleod SA. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81:1102–1109. [PubMed] [Google Scholar]
- 27.Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21:775–779. doi: 10.1016/S0736-0266(03)00052-4. http://dx.doi.org/10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 28.Sturgill LP, Snyder-Mackler L, Manal TJ, Axe MJ. Interrater reliability of a clinical scale to assess knee joint effusion. J Orthop Sports Phys Ther. 2009;39:845–849. doi: 10.2519/jospt.2009.3143. http://dx.doi.org/10.2519/jospt.2009.3143. [DOI] [PubMed] [Google Scholar]
- 29.Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris: a prospective twitch interpolation study. J Bone Joint Surg Br. 2001;83:1104–1110. doi: 10.1302/0301-620x.83b8.11618. [DOI] [PubMed] [Google Scholar]
- 30.Urbach D, Nebelung W, Weiler HT, Awiszus F. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc. 1999;31:1691–1696. doi: 10.1097/00005768-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Young A. Current issues in arthrogenous inhibition. Ann Rheum Dis. 1993;52:829–834. doi: 10.1136/ard.52.11.829. [DOI] [PMC free article] [PubMed] [Google Scholar]