Abstract
Nuclear receptors are integrators of hormonal and nutritional signals, mediating changes to metabolic pathways within the body. Given that modulation of lipid and glucose metabolism has been linked to diseases including type 2 diabetes, obesity and atherosclerosis, a greater understanding of pathways that regulate metabolism in physiology and disease is crucial. The liver X receptors (LXRs) and the farnesoid X receptors (FXRs) are activated by oxysterols and bile acids, respectively. Mounting evidence indicates that these nuclear receptors have essential roles, not only in the regulation of cholesterol and bile acid metabolism but also in the integration of sterol, fatty acid and glucose metabolism.
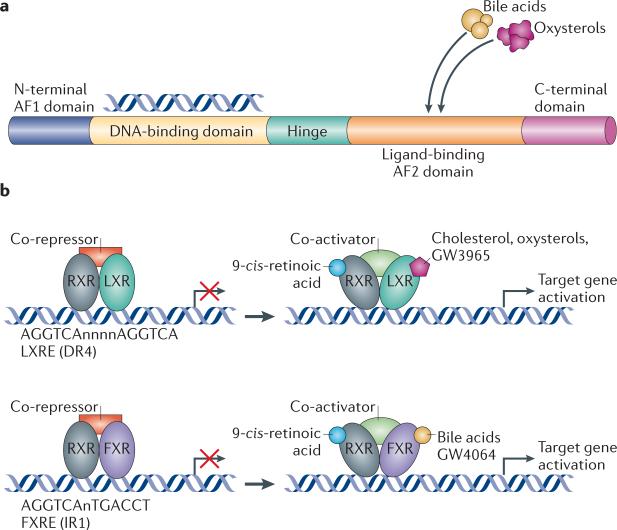
Nuclear receptors translate hormonal, metabolic and nutritional signals into alterations in gene expression. Nuclear receptors consist of a DNA-binding domain (DBD) and a ligand-binding domain (LBD) (FIG. 1a). They activate or repress gene expression by binding to regulatory regions of target genes and act in concert with co-activators and co-repressors1. Many nuclear receptors induce the transcription of target genes following ligand binding, as this results in a conformational change to the receptor that causes the release of co-repressors. Ligands include common metabolites such as fatty acids, oxysterols and bile acids. Thus, nuclear receptors respond to changes in the metabolic environment by inducing target gene expression. It is this integration of environmental stimuli with specific transcriptional responses that makes nuclear receptors crucial to whole-body physiology. A number of nuclear receptors were initially cate gorized as ‘orphan’ receptors because their natural ligands were unknown. Over the past 15 years, new ligands have been matched with their orphan parents, which has led to the discovery of new homeostatic pathways. Several receptors have turned out to be metabo lite receptors that regulate gene expression in response to diverse molecules such as fatty acids, oxysterols, bile acids, haem and xenobiotics2–4.
Figure 1. Mechanism of action of LXR and FXR.
a | The basic structure of a nuclear receptor, highlighting the DNA-binding and ligand-binding domains. b | Liver X receptor (LXR) forms an obligate heterodimer with retinoid X receptor (RXR) that binds to a DR4 (direct repeat spaced by four nucleotides) LXRE (LXR response element) in the regulatory regions of target genes, thereby repressing gene expression. Following ligand binding to LXR or RXR, the heterodimer changes conformation, which leads to the release of co-repressors and the recruitment of co-activators. This results in the transcription of target genes. Similarly, farnesoid X receptor (FXR) forms a heterodimer with RXR and binds to the FXR response element (FXRE), which is typically an inverse repeat spaced by one nucleotide (IR1), in its target genes to induce gene expression. AF domain, activation function domain; C-terminal, carboxy-terminal; N-terminal, amino-terminal.
Perturbations in lipid and glucose metabolism are linked to some of the most prevalent diseases of Western society, including type 2 diabetes, obesity and cardiovascular disease. Given that several different nuclear receptors modulate each of these pathways, it is important to gain a complete understanding of these receptors in the setting of both health and disease. As ligand-activated transcription factors, nuclear receptors are potentially attractive pharmaceutical targets.
This Review focuses on the nuclear receptors liver X receptor (LXR) and farnesoid X receptor (FXR). We provide a brief background of these receptors and discuss their effects on metabolic pathways including bile acid, lipid and carbohydrate metabolism. The role of LXRs as modulators of inflammation and immunity has been reviewed elsewhere5,6. We end with a section on disease, discussing how LXR and FXR signalling pathways intersect with common metabolic conditions.
Liver X receptors
The LXRs are so named because LXRα, the first isotype to be cloned, is highly expressed in hepatocytes7,8. There are two LXRs, termed LXRα (also known as NR1H3) and LXRβ (also known as NR1H2), that share a high degree of homology. LXRα is expressed in tissues with a high metabolic activity, including liver, adipose and macrophages, whereas LXRβ is ubiquitously expressed9. LXRs are ligand-activated nuclear receptors that act as cholesterol sensors. Both LXRs are activated by cholesterol derivatives, including oxysterols and 24(S),25-epoxycholesterol3. The LXR pathway can also be activated by synthetic agonists such as GW3965 and T0901317. LXRs form an obligate heterodimer with the retinoid X receptor (RXR) and thus can also be activated by RXR ligands8, such as 9-cis retinoic acid. The current model for LXR activation postulates that the LXR–RXR heterodimer binds to LXR response elements (LXREs) in LXR target genes in association with co-repressors such as silencing mediator for retinoic acid and thyroid hormone receptor (SMRT) and nuclear receptor co-repressor (NCoR) (FIG. 1b). Following ligand binding, co-repressors are released and co-activators are recruited, resulting in gene transcription. The LXRE contains the sequence AGGTCA in replicate separated by four nucleotides (DR4), although variations of this sequence have been reported in the promoters of bona fide targets. LXRs have also been shown to repress gene expression, particularly the expression of inflammatory genes in macrophages10. This Review focuses on the function of LXRs as transcriptional activators (TABLE 1). Their role as transcriptional repressors has been reviewed elsewhere5,6.
Table 1.
Direct gene targets of LXR
| Process | Gene name | Role |
|---|---|---|
| Reverse cholesterol transport | ABCA1 | Efflux |
| ABCG1 | Efflux, transport | |
| ARL7 | Cholesterol transport | |
| Cholesterol transport and modulation | PLTP | Modulation of lipoprotein composition |
| CETP | Modulation of lipoprotein composition | |
| LPL | Triglyceride hydrolysis | |
| APOC1 | CETP inhibition | |
| APOC2 | Unknown | |
| APOE | Transport | |
| Cholesterol uptake | IDOL | Lipoprotein receptor degradation |
| Absorption and excretion | ABCA1 | Efflux |
| ABCG5 | Efflux | |
| ABCG8 | Efflux | |
| Fatty acid and triglyceride regulation | SREBP1C | Fatty acid and triglyceride synthesis |
| SCD1 | Unsaturated fatty acid synthesis | |
| FASN | Fatty acid synthesis | |
| APOC2 | Activation of LPL activity | |
| ANGPTL3 | Inhibits LPL activity | |
| Bile acid metabolism | CYP7A1 | Cholesterol to bile acid conversion |
| Glucose metabolism | GLUT4 | Insulin-stimulated glucose uptake |
| Immune and inflammatory responses | AIM | Inhibition of oxidized LDL-induced apoptosis |
| ARG2 | Anti-inflammatory effects | |
| VEGFA | Angiogenesis and neovascularization | |
| MERTK | Phagocytosis | |
| Adipocyte biology | APOD | Lipid transport |
| SPOT14 | Fatty acid synthesis and lipogenesis |
ABC, ATP-binding cassette transporter; AIM, apoptosis inhibitor of macrophages; ANGPTL3, angiopoietin-like 3; APO, apolipoprotein; ARG2, arginase 2; ARL7, ADP-ribosylation factor-like 7, CETP, cholesterol ester-transfer protein; CYP7A1, cytochrome P450 7A1; FASN, fatty acid synthase; GLUT4, glucose transporter type 4; IDOL, inducible degrader of LDL receptor; LDL, low-density lipoprotein; LPL, lipoprotein lipase; LXR, liver X receptor; MERTK, Mer receptor tyrosine kinase; PLTP, phospholipid transfer protein; SCD1, steroyl CoA desaturase 1; SREBP1C, sterol-regulatory element-binding protein 1C; VEGFA, vascular endothelial growth factor A.
LXR and reverse cholesterol transport
The phenotypes of mice lacking both LXRα and LXRβ are relatively mild when they are maintained on a standard chow diet containing minimal cholesterol. However, a number of pathologies develop with age, including the accumulation of cholesterol in tissue macrophages and autoimmunity. Furthermore, LXR-deficient mice show profound phenotypes when they are challenged with a high-cholesterol diet or with additional genetic modifications that compromise lipoprotein homeostasis. Analysis of these mice and characterization of LXR target genes have outlined crucial roles for LXRs in cholesterol homeostasis (BOX 1; TABLE 1).
One of the best-characterized effects of LXR is to promote reverse cholesterol transport (RCT), the process of cholesterol delivery from the periphery to the liver for excretion (FIG. 2). The first step in RCT is the transfer of cholesterol to lipid-poor molecules in the plasma such as apolipoprotein AI (APOAI) and pre-β high-density lipoprotein (HDL) via ATP-binding cassette transporter A1 (ABCA1) and other transporters. Mutation of ABCA1 in humans causes a condition known as Tangier disease, underscoring the importance of ABCA1 in RCT11–13. Individuals suffering from this condition have markedly increased cholesterol deposition in peripheral tissues, virtually absent plasma HDL levels and die from vascular disease at an early age. ABCA1 was one of the earliest identified LXR target genes and is one of the most highly regulated LXR targets14. LXR agonists robustly induce ABCA1 expression in an LXR-dependent manner not only in macrophages but also in many tissues of the periphery such as the intestine15. ABCA1 is required for the ability of LXR agonists to stimulate cholesterol efflux to APOAI acceptors14.
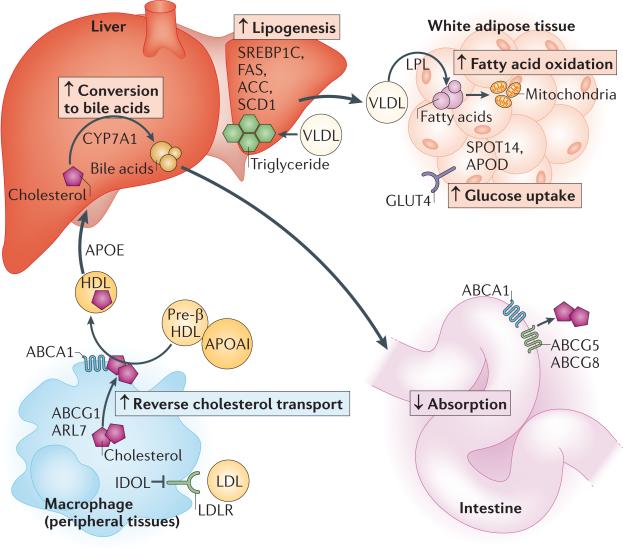
Figure 2. Coordinated effects of LXR on metabolism.
Liver X receptor (LXR) mediates effects on multiple metabolic pathways in a tissue-specific manner. In peripheral cells such as macrophages, LXR induces expression of IDOL (inducible degrader of the low-density lipoprotein receptor (LDLR)), which promotes the proteasome-mediated degradation of LDLR and thus results in reduced LDL uptake into the cell. In peripheral cells, LXR also increases ARL7 (ADP-ribosylation factor-like 7), ABCA1 (ATP-binding cassette transporter A1) and ABCG1 expression, promoting the movement of cholesterol to the plasma membrane and cholesterol efflux and transfer to lipid-poor molecules such as apolipoprotein AI (APOAI) and pre-β high-density lipoprotein (HDL), thus increasing plasma HDL levels. APOE promotes the return of HDL to the liver. Furthermore, in the liver, LXR promotes cholesterol conversion to bile acids by cytochrome P450 7A1 (CYP7A1). It also promotes fatty acid synthesis via induction of sterol-regulatory element-binding protein 1C (SREBP1C) and its targets, fatty acid synthase (FAS), acetyl CoA carboxylase (ACC) and steroyl CoA desaturase 1 (SCD1). Secretion of triglyceride-rich very low-density lipoproteins (VLDLs) by the liver transports lipids to peripheral tissues, including adipose tissue, where the action of lipoprotein lipase (LPL) liberates fatty acids from VLDL. In adipose tissue, LXR regulates the expression of lipid-binding and metabolic proteins such as APOD and SPOT14 and may promote the breakdown of fatty acids through their β-oxidation (which occurs in mitochondria). LXR also promotes glucose uptake via induction of glucose transporter type 4 (GLUT4). Finally, in the intestine, LXR inhibits cholesterol absorption by inducing the expression of the ABC transporters ABCG5 and ABCG8 and possibly ABCA1.
Coordinate with their control of ABCA1, LXRs also regulate other target genes whose products cooperate with ABCA1 to accomplish cholesterol efflux. A particularly important target is the transporter ABCG1 (REF. 16) (FIG. 2). Although ABCG1 was originally postulated to be located at the plasma membrane along with ABCA1, recent studies indicate that ABCG1 functions primarily as an intracellular transporter17. ABCG1 promotes cholesterol efflux to HDL, and it is likely that ABCA1 and ABCG1 act in concert to promote cholesterol removal in physiological contexts via HDL18,19. However, the molecular mechanisms by which ABCG1 and ABCA1 promote cholesterol transport across intracellular or plasma membranes remain poorly understood. LXR-dependent induction of the intracellular trafficking protein ADP-ribosylation factor-like 7 (ARL7; also known as ARL4C) has also been proposed to facilitate cholesterol delivery to the plasma membrane for efflux20 (FIG. 2).
LXRs induce expression of a cluster of apolipo protein genes (APOE, APOC1, APOC2 and APOC4) via activation of a multienhancer region21,22. These secreted apolipoproteins regulate lipid transport and catabolism. Single-nucleotide polymorphisms (SNPs) within this gene cluster have been associated with altered lipid profiles and risk of coronary heart disease23. Apoe–/– mice exhibit a marked elevation in very low-density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL) particles and are one of the most common atherosclerosis models24.
In addition to controlling expression of lipid transport proteins, LXRs induce lipid remodelling genes including phospholipid transfer protein (PLTP)25, human cholesterol ester transfer protein (CETP)26 and lipoprotein lipase (LPL)27, which hydrolyses triglycerides (FIG. 2). The coordinate regulation of all of these genes by LXR agonists is believed to facilitate efficient RCT from the periphery to the liver.
LXRs in hepatic metabolism
Cholesterol cannot be catabolized by animal cells and therefore must be removed from the body by excretion as described above or by conversion to bile acids. Cytochrome P450 7A1 (CYP7A1) is the rate-limiting enzyme for the conversion of cholesterol to bile acids (BOX 2; FIG. 2), and CYP7A1 was the first LXR target gene identified28. Mice lacking LXRα develop marked accumulation of cholesterol in the liver when fed a high-cholesterol diet28. Interestingly, CYP7A1 is not an LXR target in humans but is regulated secondarily via the activation of FXR and small heterodimer partner (SHP; also known as NR0B2).
In addition to modulating cholesterol metabolism, another major function of LXR in the liver is the promotion of de novo lipogenesis — the biosynthesis of fatty acids. LXR stimulates lipogenesis through the induction of sterol-regulatory element-binding protein 1C (SREBP1C), acetyl CoA carboxylase (ACC), steroyl CoA desaturase 1 (SCD1) and fatty acid synthase (FAS) expression28–30 (FIG. 2). Excess free cholesterol is toxic; therefore, when cholesterol levels are high, the promotion of fatty acid synthesis has a role in cholesterol homeostasis, with fatty acids being used as substrates for cholesterol esterification (BOX 1), as cholesterol ester is less toxic than free cholesterol. Mice treated with a synthetic LXR agonist demonstrate marked hypertriglyceridaemia, a condition mainly attributed to LXRα expression in hepatocytes28. Indeed, high triglyceride levels have been one of the key limitations in the success of LXR agonists as pharmacologic therapy. LXRβ-specific agonists would be anticipated to lack this side effect, but the identification of such compounds with favourable pharmacological properties has thus far proved difficult.
LXRs, cholesterol absorption and excretion
It has been increasingly appreciated that intestinal absorption plays an important part in the regulation of cholesterol levels within the body. LXRs modulate this pathway via the induction of the transporters ABCG5 and ABCG8 (REF. 31) (FIG. 2), which heterodimerize into a complex that mediates the apical efflux of cholesterol from enterocytes (which are intestinal absorptive cells). Loss-of-function mutations in ABCG5 or ABCG8 are responsible for sitosterolaemia, a disease characterized by hypercholesterolaemia, heightened intestinal absorption and reduced biliary excretion of dietary sterols32. Treatment of mice with an LXR agonist results in increased faecal sterol excretion and increased expression of ABCG5 and ABCG8 in the intestine33, effects that are not observed in LXR-deficient mice.
Intestinal secretion of cholesterol via enterocytes has been reported to contribute towards cholesterol removal and is stimulated by bile acids and phospholipids34. Cholesterol secretion is increased twofold following LXR activation and is dependent on ABCG5 expression35. The expression of Niemann–Pick C1-like 1 (NPC1L1), a protein that is crucial for intestinal cholesterol absorption, has been shown to be attenuated by LXR activation36. However, as LXR is not a ligand-dependent direct repressor, the decrease in NPC1L1 expression is likely to be indirect, and the mediators remain to be clarified.
LXRs and cholesterol uptake
Collectively, work from several laboratories suggests that the LXR and SREBP transcriptional pathways work in a coordinated and reciprocal fashion to maintain cellular and systemic cholesterol homeostasis. SREBPs are activated in response to low cellular cholesterol levels, whereas LXRs are activated by elevated cholesterol levels. A major target of the SREBP2 pathway is the low-density lipoprotein (LDL) receptor (LDLR) that mediates clearance of LDL particles from the circulation. LDL uptake is most prominent in the liver but occurs to some extent in most cells, including macrophages (FIG. 2). We recently demonstrated that LXR negatively regulates the LDLR pathway, providing a second mechanism for feedback regulation of cholesterol uptake by sterols. LXR controls the transcription of inducible degrader of LDLR (IDOL), an E3 ligase that targets LDLR for ubiquitylation and lysosomal degradation37 (FIG. 2). Thus, activation of LXR by sterols or synthetic agonists attenuates LDL uptake by cells. Subsequent studies have identified ubiquitin-conjugating enzyme E2D (UBE2D) as the E2 ligase that cooperates with IDOL to degrade LDLR and have defined the unique recognition sequence, which is bound directly by the IDOL FERM domain to mediate target degradation38,39. Interestingly, only LDLR and its family members VLDLR and APOER2 (also known as LRP8) contain this recognition sequence and thus these receptors are the only known targets for the LXR–IDOL degradation pathway39,40. Together, these studies suggest that LXR can mediate effects in various metabolic tissues where IDOL target proteins are expressed, including adipose tissue, heart, skeletal muscle and brain.
LXRs in glucose and fatty acid metabolism
Given the close relationship between lipid and glucose homeostasis, it is not surprising that LXR signalling has been found to affect glucose metabolism. Studies suggest that LXR expression and some LXR target genes can be upregulated by insulin41,42, although it remains to be elucidated whether this regulation occurs via direct or indirect mechanisms. A provocative and controversial study has suggested that glucose itself may act as a ligand for LXRs; however, the physiological significance of this observation remains to be determined43. Interestingly, LXR-deficient mice are resistant to weight gain in the presence of cholesterol in the diet, exhibiting increased hepatic cholesterol content, reduced plasma triglyceride levels and improved glucose tolerance44. This phenotype was associated with the induction of oxidative genes in the liver and the induction of genes promoting energy utilization in adipose tissue and skeletal muscle. Thus, in the presence of excess hepatic cholesterol, LXR appears to promote both cholesterol removal from the liver and increased triclyceride storage in peripheral tissues, highlighting the interconnected, tissue-specific effects of LXR (FIG. 2).
The function of LXRs in adipose tissue has been less well studied than in other metabolic tissues. Both LXRα and LXRβ are expressed at high levels in white adipose tissue (WAT) and brown adipose tissue (BAT). The balance of the evidence suggests that the LXR pathway does not affect adipocyte differentiation per se but is likely to be a modulator of adipocyte function45,46. Gene expression analysis in adipose tissue has identified a number of LXR-responsive genes, including those involved in cholesterol, fatty acid and glucose metabolism45,47 (FIG. 2). LXR activation increases expression of the insulin-stimulated glucose transporter GLUT4 and promotes glucose uptake in WAT and BAT48–52. Moreover, GLUT4 is a direct LXR target in both humans and mice52,49. Increased basal glucose uptake and glycogen synthesis, associated with increased GLUT1 expression, have also been observed in 3T3-L1 cells50. In adipocytes, LXR regulates the expression of lipid-binding proteins and metabolic proteins such as APOD and SPOT14 (also known as THRSP).
Activation of LXR has been reported to increase fatty acid β-oxidation and decrease glucose oxidation in WAT53. Other studies have shown increases in plasma free fatty acids and glycerol in vivo after treatment with T0901317, suggesting an effect on lipolysis50. On a high-carbohydrate diet, LXRα and LXRβ-deficient mice exhibited a shift to larger adipocytes compared to wild-type mice and increased de novo lipogenesis in adipose tissue54. In addition, LXRα- and LXRβ-deficient mice also exhibited enhanced energy expenditure and increased uncoupling protein 1 (UCP1) expression in BAT51. Consistent with this finding, wild-type mice treated with GW3965 demonstrated attenuated energy expenditure and reduced UCP1 expression in BAT. Collectively, work to date suggests that LXRs affect carbohydrate and lipid metabolism in adipose tissue, promoting glucose uptake and enhancing lipolysis and β-oxidation.
LXRs and the pancreas
Early studies indicate that activation of the LXR pathways can directly modulate glucose metabolism via effects on islet cells of the pancreas. Indeed, studies in a pancreatic β-cell line (MIN6) and in isolated human islet cells demonstrated that LXR agonists increase both basal and stimulated insulin secretion55,56. Consistent with the observation that LXRβ is the primary isotype expressed in β-cells, LXRβ-deficient but not LXRα-deficient mice demonstrated impaired glucose-stimulated insulin secretion in association with an attenuated expression of ABCA1, ABCG1 and SREBP1C and increased lipid drop accumulation in pancreatic β-cells46,55.
Farnesoid X receptor
FXR was originally named on the basis of the observation that farnesol is a ligand for this receptor, albeit at supraphysiological levels57. In subsequent years, it has become clear that FXR is more appropriately classified as a nuclear bile acid receptor (BOX 2), as bile acids such as chenodeoxycholic acid (CDCA) and cholic acid have been found to be the endogenous ligands4,58. FXR is expressed mainly in the liver, intestine, kidney and adrenal glands57. Low expression levels have been reported in the heart, adipose and vasculature, although the functional significance of the presence of the receptor is less clear in these tissues. Similarly to LXR, FXR forms a heterodimer with RXR to modulate expression of target genes (TABLE 2), and the FXR–RXR heterodimer can also respond to ligands for RXR (FIG. 1b). FXR–RXR binds to inverted repeat elements separated by one nucleotide (IR1), although other variations have been observed59,60. In addition to inducing gene expression directly, FXR mediates the repression of a number of genes indirectly through the regulation of SHP61,62(as discussed below) (TABLE 2).
Table 2.
Direct gene targets of FXR and induced by FXR via SHP
| Process | Gene name | Role |
|---|---|---|
| Genes modulated by FXR | ||
| Bile acid metabolism | SHP | Gene repression (limits bile acid accumulation) |
| Fgf15 and FGF19 | Negative feedback of bile acid synthesis | |
| BACS | Bile acid conjugation | |
| BAAT | Bile acid conjugation | |
| MDR2 (also known as ABCC2) | Bile acid secretion | |
| MDR3 (also known as ABCB4) | Transports phospholipids into bile | |
| BSEP (also known as ABCB11) | Bile acid secretion | |
| IBABP | Bile acid transport | |
| OSTA and OSTB | Bile acid release | |
| CYP3A4 | Bile acid detoxification | |
| SULT2A1 | Bile acid sulfation | |
| UGT2B4 | Bile acid glucuronidation | |
| Lipid metabolism | PLTP | Modulation of lipoprotein composition |
| APOC2 | Activation of LPL activity | |
| APOE | Transport | |
| LIPC | Triglyceride hydrolysis | |
| APOAI | Cholesterol transport | |
| Triglyceride metabolism | VLDLR | VLDL uptake |
| C3 | Fatty acid uptake and triglyceride synthesis | |
| FASN | Fatty acid synthesis | |
| AKR1B7 | Reduced hepatic gluconeogenesis | |
| Glucose metabolism | Fgf15 and FGF19 | Inhibits hepatic gluconeogenesis |
| GLUT4 | Insulin-stimulated glucose uptake | |
| PPARA | Fatty acid transport and oxidation | |
| Genes modulated by FXR via SHP | ||
| Bile acid metabolism | SHP | Negative feedback |
| LRH1 | Promotes bile acid gene expression | |
| HNF4A | Promotes bile acid gene expression | |
| CYP7A1 via LRH1 | Cholesterol conversion to bile acid | |
| CYP8B1 via HNF4A | Bile acid hydroxylation | |
| ASBT (also known as SLC10A2 or IBAT) | Bile acid transporter | |
| NTCP | Hepatic bile acid uptake | |
| Lipid metabolism | SRB1 via LRH1 | HDL uptake |
| Triglyceride metabolism | SREBP1C | Fatty acid and triglyceride synthesis |
| Glucose metabolism | PEPCK | Gluconeogenesis |
| G6PC | Glucose modulation | |
| FBP1 | Gluconeogenesis | |
AKR1B7, aldo-keto reductase 1B7; APO, apolipoprotein; ASBT, apical sodium-dependent bile salt transporter; BAAT, bile acid CoA-amino acid N-acetyltransferase; BACS, bile acid CoA synthase; BSEP, bile salt export pump; C3, complement component 3; CYP, cytochrome P450; FBP1, fructose-1,6-bisphosphatase 1; FASN, fatty acid synthase; FGF, fibroblast growth factor; FXR, farnesoid X receptor; G6PC, glucose-6-phosphatase; GLUT4, glucose transporter type 4; HDL, high-density lipoprotein; HNF4A, hepatocyte nuclear factor 4α; IBABP, ileal bile acid binding protein; LIPC, hepatic lipase; LRH1, liver receptor homologue 1; MDR, multidrug resistance protein; NTCP, sodium taurocholate cotransporting polypeptide; OST, organic solute transporter; PEPCK, phosphoenolpyruvate carboxykinase; PLTP, phospholipid transport protein; PPARA, peroxisome proliferator-activated receptor-α; SHP, small heterodimer partner; SRB1, scavenger receptor B1; SREBP1C, sterol-regulatory element-binding protein 1C; SULT2A1, sulphotransferase 2A1; UGT2B4, UDP-glucuronosyltransferase 2B4; VLDL, very low-density lipoprotein; VLDLR, VLDL receptor.
There are two FXR genes in mice, termed Fxr (also known as Nr1h4) and Fxrb (also known as Nr1h5). FXRβ is not a bile acid receptor and therefore will not be discussed further here. FXR exists in four isoforms that differ in their amino terminus and hinge region owing to alternative splicing and distinct promoters63 (FIG. 1a). One complicating factor in the study of FXR biology is that bile acids are also ligands for pregnane X receptor (PXR), constitutive androstane receptor (CAR; also known as NR1I3), vitamin D receptor (VDR) and the G protein-coupled receptor TGR5 (also known as GPBAR1). The fact that bile acids bind to several receptors highlights the importance of investigating the effects of bile acids in not only wild-type mice but also in FXR-deficient mice to determine the specificity of the effects. Indeed, FXR-deficient mice have greatly aided in studying the role of FXR in metabolism64.
FXR and bile acid metabolism
Broadly, FXR can be viewed as acting in a complementary and often reciprocal fashion to LXR in the control of lipid metabolism (FIG. 3). As outlined below, FXR inhibits whereas LXR promotes bile acid production in mice, and the two receptors both modulate glucose and lipid metabolism in a tissue-selective manner. High levels of bile acids are toxic to cells, and thus FXR has a crucial role in maintaining control of bile acid levels. Bile acids facilitate the efficient digestion and absorption of fats and cholesterol after a meal. In addition, formation of bile acids is a major pathway for cholesterol elimination from the body. Most bile acids are recycled via the enterohepatic circulation, passing from the intestine back to the liver, thereby reducing the requirement for de novo bile acid synthesis. FXR influences bile acid flux via various feedforward and feedback loops, modulating bile acid synthesis, modification, absorption and uptake. The absence of FXR in mice is associated with a number of severe pathologies, indicating that bile acid signalling through FXR is crucial for bile acid flux and metabolic homeostasis.
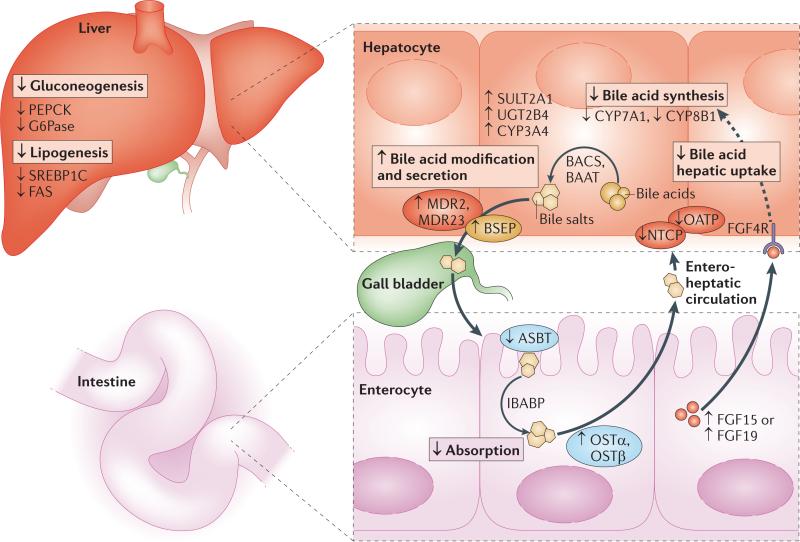
Figure 3. Coordinated effects of FXR on metabolism.
Farnesoid X receptor (FXR) mediates effects on multiple metabolic pathways in a tissue-specific manner. In the liver, FXR reduces conversion of cholesterol to bile acids by downregulating the expression of enzymes involved in bile acid synthesis, such as cytochrome P450 7A1 (CYP7A1) and CYP8B1. FXR also reduces bile acid toxicity in the liver by increasing other bile acid-modifying enzymes including sulphotransferase 2A1 (SULT2A1), UDP-glucuronosyltransferase 2B4 (UGT2B4) and CYP3A4. Bile acids are conjugated to either glycine or taurine before secretion into the bile; FXR enhances bile acid conjugation by increasing the expression of bile acid CoA synthase (BACS) and bile acid CoA–amino acid N-acetyltransferase (BAAT), and FXR promotes the transport of bile acids to the gall bladder via bile salt export pump (BSEP), multidrug resistance protein 2 (MDR2) and MDR3 (membrane transport proteins are depicted as ovals). Within the intestine, FXR reduces bile acid absorption via downregulation of the apical sodium-dependent bile acid transporter (ASBT), promotes bile acid movement across the enterocyte via ileal bile acid binding-protein (IBABP) and promotes recycling of bile acids to the liver via organic solute transporter-α (OSTα) and OSTβ. In addition, FXR reduces hepatic uptake of bile acids by reducing the expression of organic anion transporting polypeptide (OATP) and sodium taurocholate cotransporting polypeptide (NTCP). FXR also promotes the release of fibroblast growth factor 15 (FGF15) in mice or FGF19 in humans from the intestine. FGF15 or FGF19 travel to the liver, acting on FGF4 receptor (FGF4R) to reduce CYP7A1 expression and thus repress bile acid synthesis. In the liver, FXR also acts on glucose metabolism by reducing gluconeogenesis via the downregulation of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), two key enzymes in the glucose synthesis pathway. Futhermore, FXR reduces lipogenesis via inhibition of sterol-regulatory element-binding protein 1C (SREBP1C) and fatty acid synthase (FAS).
In the initial stages of bile acid production (BOX 2), FXR engages a feedback loop that inhibits bile acid synthesis via SHP induction. SHP interacts with liver receptor homologue 1 (LRH1; also known as NR5A2) to form a heterodimer, resulting in repression of CYP7A1 and SHP itself, thus feedback limiting this axis61,62. FXR mediates a similar inhibition of CYP8B1, which controls the ratio of cholic acid to CDCA, via SHP-mediated repression of hepatocyte nuclear factor 4α (HNF4α)65. Further studies have demonstrated that FXR also down-regulates CYP7A1 via induction of a mouse fibroblast growth factor 15–JUN N-terminal kinase (FGF15–JNK) cascade (or FGF19–JNK cascade in humans)66,67. Mouse FGF15 (or human FGF19) is secreted from the intestine and returns to the liver via the enterohepatic circulation to act on FGF receptor 4 (FGFR4) in the liver, providing a complementary mechanism for feedback inhibition of bile acid synthesis (FIG. 3).
Bile acids are modified to form bile salts before release into the gall bladder. This amidation involves two enzymes, bile acid CoA synthase (BACS) and bile acid CoA–amino acid N-acetyltransferase (BAAT), and results in the conjugation of bile acid with taurine or glycine. Both of these enzymes are positively regulated by FXR60. Bile acids are secreted into the gall bladder by bile salt export pump (BSEP; also known as ABCB11) and multidrug resistance protein 2 (MDR2; also known as ABCC2), which are also both targets of FXR. ABCB4, which translocates phosphatidylcholine into bile, is also an FXR target. The importance of these enzymes is highlighted by the fact that loss-of-function mutations in their genes lead to cholestasis68,69. Moreover, treatment with an FXR agonist reduces gall stone formation in mice70.
Bile salts facilitate the absorption of dietary fat and fat-soluble vitamins in the small intestine. FXR inhibits the absorption of bile salts via modulation of several transport proteins. FXR-dependent downregulation of the apical sodium-dependent bile salt transporter (ASBT; also known as SLC10A2 or IBAT) has been shown to be mediated via SHP-dependent inhibition of LRH1 in mice71. FXR then promotes movement of bile salts from the apical to the basolateral membrane of enterocytes via the upregulation of ileal bile acid-binding protein (IBABP; also known as gastrotropin)72. Bile salts are released into the portal circulation for return to the liver via FXR-induced expression of the organic solute transporters OSTα and OSTβ73. Having reached the liver, bile salts are taken up by sodium taurocholate cotransporting polypeptide (NTCP; also known as SLC10A1) and organic anion transporting polypeptide (OATP; also known as SLCO1A2), both of which are negatively regulated by FXR, limiting hepatic bile salt levels. Finally, FXR induces the expression of various genes that modify bile acids to protect the liver from toxicity. FXR-regulated genes include CYP3A4 and CYP3A11, which hydroxylate bile acids, as well as sulphotransferase 2A1 (SULT2A1) and UDP-glucuronosyltransferase 2B4 (UGT2B4), which sulphate and glucuronidate bile acids, respectively59,74. In addition, FXR has been shown to protect against drug-induced hepatotoxicity75.
FXR, lipid and triglyceride metabolism
Deletion of FXR from mice uncovered the importance of this receptor for triglyceride metabolism. FXR-deficient mice exhibit marked hypercholesterolaemia, hypertriglyceridaemia and increased intestinal cholesterol absorption64,76. This phenotype has been attributed to the ability of FXR to control the expression of several genes involved in lipoprotein metabolism. Triglyceride-rich lipoproteins transport hydrophobic lipids around the body. Triglycerides and cholesterol esters reside on the inside of the particles owing to their hydrophobic nature, whereas hydrophilic phospholipids remain on the surface, allowing the particles to circulate in the blood. VLDLs are produced in the liver, whereas chylomicrons are formed from dietary lipids in the intestine. As these lipoproteins circulate, they can interact with enzymes and proteins in the blood or at the cell surface, modifying the particles themselves. VLDLs and chylomicrons interact with LPLs via APOCII, resulting in the release of triclycerides from the particle and hydrolysis of the triclycerides into fatty acids. FXR positively regulates Apoc2 expression via two sites within the hepatic control region of the promoter, thus contributing to lower triclyceride levels77. Feeding wild-type mice cholic acid increases APOCII expression and reduces triglycerides; however, these effects were not observed in Fxr-null mice77. APOCIII works in a converse manner to APOCII, as it inhibits LPL. Negative FXR-mediated regulation of Apoc3 may also contribute to decreased plasma triglyceride levels78. Finally, induction of Vldlr, Pltp and Apoe expression has also been associated with FXR activation and could further contribute to a reduction in triclyceride levels79,80.
Activation of FXR has been shown to modulate lipogenic pathways. FXR reduces SREBP1C expression, which seems to occur via the induction of SHP, as it is not observed in Shp-null mice81. A recent study identified an IR1 site in the Fas promoter, suggesting that this gene is also a direct target of FXR82. In human cells, but not in mouse cells, FXR activation induces expression of peroxisome proliferator-activated receptor-α (PPARα) and its target genes, which is a pathway involved in fatty acid transport and oxidation83. Finally, FXR is induced by PPARγ co-activator 1α (PGC1α) both directly and indirectly via PPARγ and HNF4α84. PGC1α is known to regulate a group of genes associated with energy metabolism. Thus, FXRs seem to decrease triglyceride levels via multiple mechanisms, including reducing lipogenesis and promoting increased uptake, catabolism and oxidation of triglycerides and fatty acids. Furthermore, the above-mentioned effects on lipoproteins may also contribute to the hypocholesterolaemic effects of FXR agonists by promoting lipoprotein catabolism and clearance.
FXR and glucose metabolism
Another unexpected finding to emerge in recent years was that FXR exerts a substantial influence on hepatic carbohydrate metabolism. When energy demands are low, glucose can be converted to, and stored as, glycogen in a process known as glycogenesis. Alternatively, glucose can be converted to acetyl CoA, which can be used to generate fatty acids. When energy demands are high, stored glycogen is broken down to glucose. Glucose is catabolized to produce ATP through the process of glycolysis. Glucose can also be produced via the conversion of non-carbohydrate substrates, such as lactate and glycerol, through a process known as gluconeogenesis. Several studies have suggested that FXR activation lowers plasma glucose levels and downregulates the gluconeogenic programme, although conflicting results have been reported. A recent study demonstrated that treatment of isolated hepatocytes with an FXR agonist attenuated pyruvate kinase (Pklr), Acc and Spot14 expression85. Furthermore, the FXR agonist GW4064 repressed hepatic gluconeogenic genes such as glucose-6-phosphatase (G6pc) and increased hepatic glycogen synthesis and glycogen content in diabetic mice86. These changes were associated with decreased plasma glucose, triglyceride and cholesterol levels and were not observed in Fxr-null mice. Moreover, decreased plasma glucose levels with a concomitant attenuation in gluconeogenic genes was observed when FXR was activated by cholic acid in wild-type but not in Fxr-null mice87.
In general agreement with the effects of pharmacologic FXR activation, Fxr-null mice exhibit mild glucose intolerance and insulin insensitivity86. Other studies observed similar defects and demonstrated that fasted Fxr-null mice exhibit an age-dependent increase in plasma glucose levels87. Fasted Fxr-knockout mice also exhibit increased plasma triglyceride, free fatty acid and cholesterol levels. Interestingly, these changes resulted in hepatic lipid accumulation and defective insulin signalling despite a compensatory upregulation in fatty acid transport in skeletal muscle. Studies have also demonstrated that Fxr-knockout mice have an enhanced response to refeeding, resulting in a rapid increase of glycolytic and lipogenic gene expression and earlier suppression of gluoneogenic genes. This response is associated with decreased plasma glucose and insulin levels and reduced hepatic glycogen content85.
It has recently been reported that hepatic overexpression of a novel FXR target, aldo-keto reductase 1B7 (Akr1b7), a gene previously linked to detoxification, lowered plasma glucose levels and reduced expression of both gluconeogenic enzymes and lipogenic enzymes86,88. Thus, it is possible that AKR1B7 is responsible for mediating at least some of the effects observed with FXR activation. Finally, recent studies on mouse FGF15 and human FGF19, which are defined FXR targets, have demonstrated that these growth factors inhibit hepatic gluconeogenesis in response to feeding via inhibition of cAMP regulatory element-binding protein (CREB) and downregulation of PGC1α89. Furthermore, FGF19 mediates insulin-independent effects on hepatic glycogen synthesis in the absence of effects on lipogenesis90. Therefore, it seems likely that some of the above-mentioned effects of FXR agonists may be mediated via FGF15 in mice and FGF19 in humans.
Nuclear receptors and metabolic disease
In Western society there is an increasing prevalence of type 2 diabetes and cardiovascular disease that presents a great burden in terms of both morbidity and cost. Before developing overt diabetes and cardiovascular disease, many individuals experience the metabolic syndrome, which is a collection of disturbances including insulin resistance, obesity and dyslipidaemia. One potential pharma ceutical benefit of targeting nuclear receptor pathways such as LXR and FXR is that they act on multiple pathways involved in the metabolic syndrome. The effects of pharmacologic manipulation of the LXR and FXR pathways in obesity, diabetes and cardiovascular disease are discussed below.
LXR and FXR in obesity and diabetes
With the advent of genome-wide association studies, several groups have reported potential connections between human LXR genes and obesity and diabetes. To date, however, the data are somewhat conflicting, and mechanistic links between specific genetic changes and human physiology remain to be established. As key metabolic tissues express both LXRα and LXRβ and the vast majority of the known LXR target genes respond to both receptors, it seems unlikely that even a complete loss of function in only one of the four LXR alleles would cause a dramatic phenotype. A recent study in individuals with type 2 diabetes identified a SNP in LXRβ, with the minor allele being associated with reduced pancreatic β-cell function. As pancreatic β-cells express LXRβ but not LXRα, it is understandable how an LXRβ polymorphism might have a functional effect in this cell type91. Some studies have suggested that SNPs in both LXRα and LXRβ are linked to obesity phenotypes92,93, but others have not observed these associations94.
In murine models of obesity and diabetes, pharmacologic LXR activation has both beneficial and detrimental effects on pathways related to glucose metabolism and insulin sensitivity. It has been demonstrated that 1 week of treatment with the LXR agonist GW3965 was associated with a substantial improvement in glucose tolerance in mice fed a high-fat diet52. These effects were correlated with reduced hepatic glucose output and enhanced glucose uptake in adipose tissue52. Similar effects were observed in leptin-deficient ob/ob mice, with no change in hepatic glucose output but increased GLUT4 expression in fat95. GW3965 administration to rats on a high-fat diet improved insulin suppression of free fatty acids, increased glucose infusion rate and reduced insulin suppression of glucose production; however, glucose uptake was not affected in this model96. Thus, short-term treatment with an LXR agonist seems to increase peripheral glucose disposal and improve glycaemic control despite hepatic steatosis.
Studies in adipose tissue have shown that LXR protects against tumour necrosis factor-α (TNFα)-induced insulin resistance, improving insulin signalling at the level of insulin receptor substrate 1 (IRS1) in human adipocytes97. Similar effects were observed in mouse primary brown adipocytes and were associated with improved translocation of GLUT4 to the plasma membrane48. Longer-term studies with LXR agonists and LXR-deficient mice will be required to more precisely define the tissue-specific contributions of LXR signalling to lipid and glucose homeostasis.
Modulation of bile acid metabolism improves glycaemic parameters in various animal models of obesity and in humans with type 2 diabetes, and this would suggest a potential role for FXR modulation in the treatment of these conditions98–100. Activation of FXR has been shown to reduce plasma glucose levels and improve insulin sensitivity in several models of obesity and diabetes86,101,102. In db/db mice, these changes were associated with repression of hepatic gluconeogenic enzymes and increased hepatic glycogen synthesis and glycogen content. By contrast, others have demonstrated that administration of GW4064 to diet-induced obese mice was associated with increased weight gain, glucose intolerance and decreased bile acid pool size. These effects were reversed by administration of bile acid in a dose-dependent manner103. It is likely that some of the insulin-sensitizing effects of FXR activation are independent of bile acid metabolism.
Complementing studies with synthetic agonists, investigators have also examined the effect of Fxr deletion in models of obesity and diabetes104. There is conflicting evidence as to whether Fxr-knockout mice are insulin resistant or are insulin sensitized, and results have differed depending on the specific model used. In ob/obmice, Fxr deletion was associated with reduced weight gain and improved insulin sensitivity. Despite a doubling of hepatic triglyceride content, only a small increase in the expression of lipogenic genes was observed along with decreased expression of genes involved in β-oxidation. Increased bile acid levels were observed in Fxr-knockout ob/ob mice, and normalization via bile acid sequestration did not restore glycaemic parameters to those of Fxr+/+ ob/ob mice, suggesting that changes in bile acid metabolism do not account for the FXR-associated change in glycaemia. Furthermore, in mice fed a high-fat diet, Fxr deletion protected from weight gain, hyperglycaemia, hyperinsulinaemia and glucose intolerance despite higher plasma triglyceride levels104. Interestingly, liver-specific deletion of Fxr in mice fed a high-fat diet did not protect from obesity or insulin resistance, suggesting a role for extra-hepatic FXR104.
LXR and FXR in atherosclerosis
Agonists of both LXR and FXR exert anti-atherosclerotic effects in mouse models. LXR activation with GW3965 strongly attenuates atherosclerosis in the two most common models — the Apoe-null mouse and the Ldlr-null mouse105. Several subsequent studies have confirmed these findings using not only GW3965 but also other LXR agonists, and others demonstrated plaque regression with LXR agonist treatment106,107. It has also been shown that treatment with an LXR agonist alters the cellular composition of athero-sclerotic plaques, with reductions in inflammation and adhesion molecules and changes in fibrous cap thickness observed105,107. In several reports, a beneficial effect of LXR activation on lesion development was observed in the absence of changes in plasma lipid levels. These observations are consistent with the idea that LXR activation promotes lipid movement from peripheral cells back to the liver for excretion through the RCT pathway. In addition, it has been suggested that flux through the RCT pathway can occur without marked changes in static plasma lipid levels105,106,108.
Loss-of-function studies have also clearly demonstrated that LXR expression is required for whole-body cholesterol homeostasis. When maintained on a normal chow diet, which contains very little cholesterol, mice lacking both LXRs develop foam-cell-rich atherosclerotic lesions109. When challenged with hypercholesterolaemia, mice lacking LXRs exhibit massive peripheral cholesterol accumulation and markedly accelerated atherosclerosis110. Bone marrow transplant studies have revealed the importance of macrophage LXR expression in lesion development. Transfer of LXRα- and LXRβ-deficient marrow into Ldlr-null recipients strongly promotes atherosclerosis111. Conversely, macrophage-specific overexpression of LXRα attenuates atherosclerosis112. Mice deficient in bone marrow LXR expression are only partially refractory to the beneficial effects of LXR agonist treatment106, suggesting that tissues other than bone marrow are likely to contribute to their anti-atherogenic effects113.
Given the role of LXRα in promoting hepatic lipogenesis and hypertriglyceridaemia, drug development strategies have focused on LXRβ-selective agonists. An important question then is, whether activation of LXRβ alone is sufficient to inhibit atherogenesis. Our laboratory demonstrated that activation of LXRβ in Lxra and Apoe double-knockout mice did indeed attenuate atherosclerosis, even in the absence of increased triglyceride levels110. Subsequent studies in Ldlr-deficient mice have revealed that whole-body deletion of Lxra strongly promotes atherogenesis but Lxrb deletion does not113. The basis for this difference is not yet clear but is likely to involve a preferential role for LXRα in hepatic or intestinal cholesterol metabolism.
The FXR pathway has been less well studied in the context of atherosclerosis. Nevertheless, interesting connections have been uncovered. In particular, effects of FXR on atherosclerosis in mice appear to be gender-specific. Despite the fact that Fxr-null mice exhibit a pro-atherogenic lipid profile (such as increased VLDL and LDL levels), these mice do not develop atherosclerosis on a Western diet64,114. Analysis of Fxr-deficient, Apoe-null and Ldlr-null mice has yielded mixed results. Male Fxr- and Ldlr-deficient mice were reported to have reduced plasma LDL and HDL levels and reduced atherosclerosis compared to Ldlr-deficient mice114. By contrast, female Fxr- and Ldlr-deficient mice demonstrated increased plasma cholesterol but no change in lesion area. Fxr- and Apoe-deficent mice fed a Western diet showed elevated plasma cholesterol and a twofold increase in lesions compared to Apoe-null mice115. However, female Fxr- and Apoe-deficient mice showed increased total cholesterol but reduced lesion area116. Some of these gender-associated differences may be accounted for by variation in methods or by differences in the genetic background of the mice used.
Although limited, the data demonstrating an anti-atherogenic effect of pharmacologic FXR activation are more consistent117–119. A comprehensive study using the FXR agonist WAY-362450 was performed, demonstrating that FXR activation in Ldlr-null or Apoe-null mice was associated with decreased non-HDL cholesterol and markedly reduced lesion formation in both male and female mice118. Similar studies were performed in Shp- and Ldlr-deficient mice and Shp- and Apoe-deficient mice to investigate the contribution of the key FXR target gene Shp to this effect 118. Interestingly, reductions in lesion area and non-HDL cholesterol were only observed in male mice. In addition to showing that some of the anti-atheroslerotic effects of FXR are SHP-independent, these studies further confirm gender differences in the FXR pathway, at least in mice.
Conclusions and perspectives
LXR and FXR were initially characterized as nuclear regulators of cholesterol and bile acid homeostasis, respectively. However, metabolic pathways are highly integrated and therefore perturbations to one pathway may provoke compensatory or complementary responses from another pathway. Therefore, it is not surprising that LXR and FXR are now recognized to influence numerous aspects of physiology. In addition to controlling sterol metabolism, LXR modulates fatty acid and carbohydrate metabolism in tissues such as liver, adipose and skeletal muscle. Similarly, FXR mediates effects on these pathways in addition to its well-known regulation of bile acid homeostasis. Thus, the LXR and FXR pathways have the potential to become pharmaceutical targets for the treatment of metabolic disorders including diabetes and obesity, as well as atherosclerosis. However, further studies are required to delineate the tissue-specific effects of the LXR and FXR pathways in order to eliminate potential side effects. In addition, a more detailed understanding of the mechanisms underlying the effects of LXR and FXR agonists in various cell types may allow the development of agonists with selective effects on beneficial metabolic pathways.
High-density lipoprotein
(HDL). Lipoprotein that transports cholesterol from the periphery to the liver; known as ‘good’ cholesterol.
Very low-density lipoprotein
(VLDL). Lipoprotein that enables fats and cholesterol to move in the bloodstream. It is the primary carrier of hepatic triglyceride to the periphery. It is composed of triglycerides, apolipoproteins and cholesterol. VLDL is formed in the liver and transported to the periphery; known as ‘very bad’ cholesterol.
Intermediate-density liporotein
(IDL). Lipoprotein that is intermediate between very low-density lipoprotein and low-density lipoprotein.
Phospholipid transfer protein
(PLTP). Transfers phospholipids and cholesterol to high-density lipoproteins from very low-density lipoproteins and chylomicrons.
Cholesterol ester transfer protein
(CETP). A circulating protein secreted from the liver. CETP mediates the transfer of triglycerides from low-density lipoprotein and very low-density lipoprotein to high-density lipoprotein in exchange for cholesterol esters.
Box 1 Cholesterol metabolism.
Cholesterol is essential to our survival, being crucial for membrane structure and fluidity, transport and neural signalling. Furthermore, cholesterol is a precursor to bile acids, steroid hormones and vitamin D. Cholesterol can be obtained from the diet in addition to being synthesized, primarily in the liver. The levels of cholesterol in individual cells and the body as a whole are tightly regulated by the coordinated actions of sterol-regulatory element-binding proteins (SREBPs) and liver X receptors (LXRs). When cholesterol levels are low, the SREBP pathway is activated, resulting in increased transcription of genes involved in endogenous cholesterol production and increased cholesterol uptake into cells such as low-density lipoprotein (LDL) receptor (LDLR). Cholesterol can be esterified by acetyl CoA cholesterol acytyl transferase (ACAT) to form cholesterol esters, which can then be stored and transported. Cholesterol and cholesterol esters are transported in the body as lipoproteins, including chylomicrons, very low-density lipoproteins (VLDLs) and LDLs, which can deposit cholesterol in peripheral tissues. Increased levels of cholesterol-rich LDLs are associated with increased risk of cardiovascular disease. LDL can deliver cholesterol to the vessel wall, eventually leading to the formation of atherosclerotic ‘plaques’, which may block blood vessels. Rupture of an atherosclerotic plaque may lead to complete vessel occlusion, resulting in stroke or heart attack. Cholesterol and cholesterol esters can also be transported as high-density lipoproteins (HDLs), which are returned to the liver for recycling and excretion. Thus, in contrast to LDL, high levels of HDL, or ‘good cholesterol’, have been linked to reduced risk of cardiovascular disease. Pathways that lower circulating cholesterol levels are therefore seen as attractive targets for the treatment of cardiovascular disease. Indeed, statins, the most common class of cholesterol-lowering drugs, target one of the rate-limiting enzymes in the production of cholesterol, namely hydroxymethylglutaryl CoA reductase. In addition, the LXR pathway is a potentially attractive target for improving cholesterol metabolism, as this pathway regulates a series of processes (including reverse cholesterol transport (FIG. 2)) that promote the removal of cholesterol from the periphery to the liver.
Lipoprotein lipase
(LPL). Hydrolyses triglycerides in chylomicrons and very low-density lipoproteins to fatty acids and glycerol.
E3 ligase
Substrate recognition enzyme that allows covalent attachment of ubiquitin from E2 ligase to a Lys or Cys residue in the substrate.
Ubiquitylation
Process of attaching one or more ubiquitin molecules to a substrate, thereby targeting the substrate for degradation by the proteosome or lysosome.
E2 ligase
E2 ubiquitin conjugating enzyme that forms an intermediate with ubiquitin in the E1–E2–E3 ubiquitin cascade.
Fatty acid β-oxidation
An enzymatic pathway for metabolizing fatty acids to generate energy.
Farnesol
An intermediate in the cholesterol biosynthetic pathway.
Box 2 Bile acid metabolism.
Bile acids act as detergents, allowing the efficient digestion and absorption of lipids, cholesterol and fat-soluble vitamins after a meal. Furthermore, bile acids are the major form in which cholesterol is removed from the body. Bile acid production and circulation is tightly regulated via the farnesoid X receptor (FXR) pathway, with bile acids now recognized as the endogenous ligands for this nuclear receptor (FIG. 3). Bile acids are produced primarily in the liver via the oxidation of cholesterol. The first step in this ‘classical’ pathway is the 7α-hydroxylation of cholesterol by the rate-limiting enzyme cytochrome P450 7A1 (CYP7A1). Alternative production can occur by 27-hydroxylase in the mitochondria of extra-hepatic tissues such as endothelial cells. Both pathways lead to formation of the most common bile acids in humans, which are cholic acid and chenodeoxycholic acid, via a subsequent series of enzymatic reactions. The final modification of bile acids before release from the liver is the conjugation of bile acids with taurine or glycine to form less toxic, more hydrophilic bile salts. These are then secreted into the gall bladder, where they are stored and concentrated. Bile salts are released from the gall bladder into the intestine following postprandial stimuli. Bile acids are recycled from the intestine back to the liver via the enterohepatic circulation, thereby reducing the requirement for de novo bile acid synthesis. Bile acid sequestrants are used clinically to lower cholesterol levels. By inhibiting bile acids from returning to the liver, these sequestering agents promote de novo bile acid synthesis, leading to lower plasma cholesterol levels.
Chylomicrons
Large particles composed of triglycerides, lipoproteins and phospholipids. They are formed in the intestine from dietary components and transported to the periphery, where they are hydrolysed to liberate fatty acids.
db/db
The diabetic gene (db), transmitted as an autosomal recessive trait, encodes for a G-to-T point mutation of the leptin receptor, leading to abnormal splicing and defective signalling of the adipocyte-derived hormone leptin. db/db mice are obese and become hyperphagic soon on weaning.
Acknowledgements
This work was supported by US National Institutes of Health (NIH) grants HL066088 and HL030568.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Peter tontonoz's homepage: http://www.hhmi.org/research/investigators/tontonoz.html
References
- 1.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 2.Keller H, et al. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc. Natl Acad. Sci. USA. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [This study identified oxysterols as endogenous ligands for LXR.] [DOI] [PubMed] [Google Scholar]
- 4.Parks DJ, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [The first demonstration that bile acids are endogenous ligands for FXR.] [DOI] [PubMed] [Google Scholar]
- 5.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signalling. J. Clin. Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong C, Tontonoz P. Coordination of inflammation and metabolism by PPAR and LXR nuclear receptors. Curr. Opin. Genet. Dev. 2008;18:461–467. doi: 10.1016/j.gde.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apfel R, et al. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell. Biol. 1994;14:7025–7035. doi: 10.1128/mcb.14.10.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willy PJ, et al. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 9.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 10.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 11.Rust S, et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nature Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 12.Bodzioch M, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nature Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 13.Brooks-Wilson A, et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nature Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 14.Venkateswaran A, et al. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα. Proc. Natl Acad. Sci. USA. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [This work identified ACBA1 as a target gene for LXR and established the requirement for ABCA1 in LXR-dependent cholesterol efflux.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Repa JJ, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [This study shows that LXR regulates ABCA1 expression and cholesterol absorption.] [DOI] [PubMed] [Google Scholar]
- 16.Kennedy MA, et al. Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J. Biol. Chem. 2001;276:39438–39447. doi: 10.1074/jbc.M105863200. [DOI] [PubMed] [Google Scholar]
- 17.Tarling EJ, Edwards PA. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl Acad. Sci. USA. 2011;108:19719–19724. doi: 10.1073/pnas.1113021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl Acad. Sci. USA. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy MA, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [This paper outlined the importance of ABCG1 in whole-body sterol metabolism.] [DOI] [PubMed] [Google Scholar]
- 20.Hong C, et al. Constitutive activation of LXR in macrophages regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target. J. Lipid Res. 2011;52:531–539. doi: 10.1194/jlr.M010686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak PA, et al. Regulated expression of the apolipoprotein E/C-I/C-IV/C-II gene cluster in murine and human macrophages. A critical role for nuclear liver X receptors-α and -β. J. Biol. Chem. 2002;277:31900–31908. doi: 10.1074/jbc.M202993200. [DOI] [PubMed] [Google Scholar]
- 22.Laffitte BA, et al. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl Acad. Sci. USA. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ken-Dror G, Talmud PJ, Humphries SE, Drenos F. APOE/C1/C4/C2 gene cluster genotypes, haplotypes and lipid levels in prospective coronary heart disease risk among UK healthy men. Mol. Med. 2010;16:389–399. doi: 10.2119/molmed.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plump AS, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 25.Laffitte BA, et al. The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions. Mol. Cell. Biol. 2003;23:2182–2191. doi: 10.1128/MCB.23.6.2182-2191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Y, Tall AR. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J. Clin. Invest. 2000;105:513–520. doi: 10.1172/JCI8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Repa JJ, Gauthier K, Mangelsdorf DJ. Regulation of lipoprotein lipase by the oxysterol receptors, LXRα and LXRβ. J. Biol. Chem. 2001;276:43018–43024. doi: 10.1074/jbc.M107823200. [DOI] [PubMed] [Google Scholar]
- 28.Peet DJ, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [The first study to describe the Lxra-knockout mouse and to identify CYP7A1 as an LXR target.] [DOI] [PubMed] [Google Scholar]
- 29.Repa JJ, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz JR, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Repa JJ, et al. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors-α and -β. J. Biol. Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 32.Berge KE, et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda T, et al. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 2010;30:781–786. doi: 10.1161/ATVBAHA.109.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Velde AE, et al. Direct intestinal cholesterol secretion contributes significantly to total faecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 35.van der Veen JN, et al. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 2009;284:19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duval C, et al. Niemann–Pick C1 like 1 gene expression is downregulated by LXR activators in the intestine. Biochem. Biophys. Res. Commun. 2006;340:1259–1263. doi: 10.1016/j.bbrc.2005.12.137. [DOI] [PubMed] [Google Scholar]
- 37.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [This study identifies IDOL as a novel LXR target, establishing a direct link between LXR and LDL uptake.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, et al. The IDOL-UBE2D complex mediates sterol-dependent degradation of the LDL receptor. Genes Dev. 2011;25:1262–1274. doi: 10.1101/gad.2056211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calkin AC, et al. FERM-dependent E3 ligase recognition is a conserved mechanism for targeted degradation of lipoprotein receptors. Proc. Natl Acad. Sci. USA. 2011;108:20107–20112. doi: 10.1073/pnas.1111589108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong C, et al. The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J. Biol. Chem. 2010;285:19720–19726. doi: 10.1074/jbc.M110.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobin KA, et al. Liver X receptors as insulin-mediating factors in fatty acid and cholesterol biosynthesis. J. Biol. Chem. 2002;277:10691–10697. doi: 10.1074/jbc.M109771200. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc. Natl Acad. Sci. USA. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitro N, et al. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445:219–223. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 44.Kalaany NY, et al. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Hummasti S, et al. Liver X receptors are regulators of adipocyte gene expression but not differentiation: identification of apoD as a direct target. J. Lipid Res. 2004;45:616–625. doi: 10.1194/jlr.M300312-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Gerin I, et al. LXRβ is required for adipocyte growth, glucose homeostasis, and β-cell function. J. Biol. Chem. 2005;280:23024–23031. doi: 10.1074/jbc.M412564200. [DOI] [PubMed] [Google Scholar]
- 47.Stulnig TM, et al. Novel roles of liver X receptors exposed by gene expression profiling in liver and adipose tissue. Mol. Pharmacol. 2002;62:1299–1305. doi: 10.1124/mol.62.6.1299. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Veledo S, Nieto-Vazquez I, Rondinone CM, Lorenzo M. Liver X receptor agonists ameliorate TNFα-induced insulin resistance in murine brown adipocytes by downregulating protein tyrosine phosphatase-1B gene expression. Diabetologia. 2006;49:3038–3048. doi: 10.1007/s00125-006-0472-4. [DOI] [PubMed] [Google Scholar]
- 49.Dalen KT, Ulven SM, Bamberg K, Gustafsson JA, Nebb HI. Expression of the insulin-responsive glucose transporter GLUT4 in adipocytes is dependent on liver X receptor-α. J. Biol. Chem. 2003;278:48283–48291. doi: 10.1074/jbc.M302287200. [DOI] [PubMed] [Google Scholar]
- 50.Ross SE, et al. Microarray analyses during adipogenesis: understanding the effects of Wnt signalling on adipogenesis and the roles of liver X receptor-α in adipocyte metabolism. Mol. Cell. Biol. 2002;22:5989–5999. doi: 10.1128/MCB.22.16.5989-5999.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korach-Andre M, Archer A, Barros RP, Parini P, Gustafsson JA. Both liver-X receptor (LXR) isoforms control energy expenditure by regulating brown adipose tissue activity. Proc. Natl Acad. Sci. USA. 2011;108:403–408. doi: 10.1073/pnas.1017884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laffitte BA, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc. Natl Acad. Sci. USA. 2003;100:5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stenson BM, et al. Activation of liver X receptor regulates substrate oxidation in white adipocytes. Endocrinology. 2009;150:4104–4113. doi: 10.1210/en.2009-0676. [DOI] [PubMed] [Google Scholar]
- 54.Korach-Andre M, et al. Liver X receptors regulate de novo lipogenesis in a tissue-specific manner in C57BL/6 female mice. Am. J. Physiol. Endocrinol. Metab. 2011;301:E210–E222. doi: 10.1152/ajpendo.00541.2010. [DOI] [PubMed] [Google Scholar]
- 55.Ogihara T, et al. Liver X receptor agonists augment human islet function through activation of anaplerotic pathways and glycerolipid/free fatty acid cycling. J. Biol. Chem. 2010;285:5392–5404. doi: 10.1074/jbc.M109.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Efanov AM, Sewing S, Bokvist K, Gromada J. Liver X receptor activation stimulates insulin secretion via modulation of glucose and lipid metabolism in pancreatic β-cells. Diabetes. 2004;53(Suppl. 3):75–78. doi: 10.2337/diabetes.53.suppl_3.s75. [DOI] [PubMed] [Google Scholar]
- 57.Forman BM, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81:687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 58.Makishima M, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [This study shows that bile acids are endogenous ligands for FXR.] [DOI] [PubMed] [Google Scholar]
- 59.Song CS, et al. Dehydroepiandrosterone sulphotransferase gene induction by bile acid activated farnesoid X receptor. J. Biol. Chem. 2001;276:42549–42556. doi: 10.1074/jbc.M107557200. [DOI] [PubMed] [Google Scholar]
- 60.Pircher PC, et al. Farnesoid X receptor regulates bile acid-amino acid conjugation. J. Biol. Chem. 2003;278:27703–27711. doi: 10.1074/jbc.M302128200. [DOI] [PubMed] [Google Scholar]
- 61.Goodwin B, et al. A regulatory cascade of the nuclear receptors, FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [This study demonstrates that FXR negatively regulates genes such as CYP7A1 via induction of SHP.] [DOI] [PubMed] [Google Scholar]
- 62.Lu TT, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol. Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 63.Huber RM, et al. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene. 2002;290:35–43. doi: 10.1016/s0378-1119(02)00557-7. [DOI] [PubMed] [Google Scholar]
- 64.Sinal CJ, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [The first study characterizing the Fxr-knockout mouse, describing effects on bile acids, cholesterol and triglycerides.] [DOI] [PubMed] [Google Scholar]
- 65.Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4α in mediating bile acid repression. J. Biol. Chem. 2001;276:41690–41699. doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- 66.Holt JA, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inagaki T, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Strautnieks SS, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nature Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 69.de Vree JM, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc. Natl Acad. Sci. USA. 1998;95:282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moschetta A, Bookout AL, Mangelsdorf DJ. Prevention of cholesterol gallstone disease by FXR agonists in a mouse model. Nature Med. 2004;10:1352–1358. doi: 10.1038/nm1138. [This study demonstrates that treatment with an FXR agonist reduces disease in a model that recapitulates human cholesterol gallstone disease.] [DOI] [PubMed] [Google Scholar]
- 71.Chen F, et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J. Biol. Chem. 2003;278:19909–19916. doi: 10.1074/jbc.M207903200. [DOI] [PubMed] [Google Scholar]
- 72.Grober J, et al. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J. Biol. Chem. 1999;274:29749–29754. doi: 10.1074/jbc.274.42.29749. [DOI] [PubMed] [Google Scholar]
- 73.Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-α and -β genes. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G476–G485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- 74.Gnerre C, Blattler S, Kaufmann MR, Looser R, Meyer UA. Regulation of CYP3A4 by the bile acid receptor FXR: evidence for functional binding sites in the CYP3A4 gene. Pharmacogenetics. 2004;14:635–645. doi: 10.1097/00008571-200410000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Lee FY, et al. Activation of the farnesoid X receptor provides protection against acetaminophen-induced hepatic toxicity. Mol. Endocrinol. 2010;24:1626–1636. doi: 10.1210/me.2010-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lambert G, et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J. Biol. Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 77.Kast HR, et al. Farnesoid X-activated receptor induces apolipoprotein C-II transcription: a molecular mechanism linking plasma triglyceride levels to bile acids. Mol. Endocrinol. 2001;15:1720–1728. doi: 10.1210/mend.15.10.0712. [DOI] [PubMed] [Google Scholar]
- 78.Claudel T, et al. Farnesoid X receptor agonists suppress hepatic apolipoprotein CIII expression. Gastroenterology. 2003;125:544–555. doi: 10.1016/s0016-5085(03)00896-5. [DOI] [PubMed] [Google Scholar]
- 79.Sirvent A, et al. The farnesoid X receptor induces very low density lipoprotein receptor gene expression. FEBS Lett. 2004;566:173–177. doi: 10.1016/j.febslet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 80.Mak PA, Kast-Woelbern HR, Anisfeld AM, Edwards PA. Identification of PLTP as an LXR target gene and apoE as an FXR target gene reveals overlapping targets for the two nuclear receptors. J. Lipid Res. 2002;43:2037–2041. doi: 10.1194/jlr.c200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe M, et al. Bile acids lower triglyceride levels via a pathway involving, FXR, SHP, and SREBP-1c. J. Clin. Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsukuma KE, et al. Coordinated control of bile acids and lipogenesis through FXR-dependent regulation of fatty acid synthase. J. Lipid Res. 2006;47:2754–2761. doi: 10.1194/jlr.M600342-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Pineda Torra I, et al. Bile acids induce the expression of the human peroxisome proliferator-activated receptor-γ gene via activation of the farnesoid X receptor. Mol. Endocrinol. 2003;17:259–272. doi: 10.1210/me.2002-0120. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA. Peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duran-Sandoval D, et al. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J. Biol. Chem. 2005;280:29971–29979. doi: 10.1074/jbc.M501931200. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl Acad. Sci. USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [This study shows that activation of FXR reduces blood glucose levels and improves insulin sensitivity in diabetic mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J. Clin. Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ge X, et al. Aldo-keto reductase 1B7 is a target gene of FXR and regulates lipid and glucose homeostasis. J. Lipid Res. 2011;52:1561–1568. doi: 10.1194/jlr.M015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Potthoff MJ, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [This study describes a new mechanism by which mouse FGF15 and human FGF19 suppress gluconeogenesis. Given that FGF15 and FGF19 are FXR targets, this suggests a novel mechanism by which FXR may control carbohydrate metabolism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kir S, et al. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ketterer C, et al. Genetic variation within the NR1H2 gene encoding liver X receptor-β associates with insulin secretion in subjects at increased risk for type 2 diabetes. J. Mol. Med. (Berl.) 2011;89:75–81. doi: 10.1007/s00109-010-0687-1. [DOI] [PubMed] [Google Scholar]
- 92.Dahlman I, et al. Liver X receptor gene polymorphisms and adipose tissue expression levels in obesity. Pharmacogenet. Genomics. 2006;16:881–889. doi: 10.1097/01.fpc.0000236334.49422.48. [DOI] [PubMed] [Google Scholar]
- 93.Solaas K, et al. Suggestive evidence of associations between liver X receptor-β polymorphisms with type 2 diabetes mellitus and obesity in three cohort studies: HUNT2 (Norway), MONICA (France) and HELENA (Europe). BMC Med. Genet. 2010;11:144. doi: 10.1186/1471-2350-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Auboeuf D, et al. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-α in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319–1327. doi: 10.2337/diab.46.8.1319. [DOI] [PubMed] [Google Scholar]
- 95.Grefhorst A, et al. Differential effects of pharmacological liver X receptor activation on hepatic and peripheral insulin sensitivity in lean and ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 2005;289:E829–E838. doi: 10.1152/ajpendo.00165.2005. [DOI] [PubMed] [Google Scholar]
- 96.Commerford SR, et al. Dissection of the insulin-sensitizing effect of liver X receptor ligands. Mol. Endocrinol. 2007;21:3002–3012. doi: 10.1210/me.2007-0156. [DOI] [PubMed] [Google Scholar]
- 97.Fernandez-Veledo S, Vila-Bedmar R, Nieto-Vazquez I, Lorenzo M. c-Jun N-terminal kinase 1/2 activation by tumour necrosis factor-α induces insulin resistance in human visceral but not subcutaneous adipocytes: reversal by liver X receptor agonists. J. Clin. Endocrinol. Metab. 2009;94:3583–3593. doi: 10.1210/jc.2009-0558. [DOI] [PubMed] [Google Scholar]
- 98.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G419–G424. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 99.Li T, et al. Transgenic expression of cholesterol 7α-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology. 2010;52:678–690. doi: 10.1002/hep.23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch. Intern. Med. 2008;168:1975–1983. doi: 10.1001/archinte.168.18.1975. [DOI] [PubMed] [Google Scholar]
- 101.Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cariou B, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 103.Watanabe M, et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J. Biol. Chem. 2011;286:26913–26920. doi: 10.1074/jbc.M111.248203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Prawitt J, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861–1871. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joseph SB, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl Acad. Sci. USA. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [The first demonstration of the anti-atherosclerotic effects of LXR agonists.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Levin N, et al. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler. Thromb. Vasc. Biol. 2005;25:135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [This study describes the importance of macrophages in the atheroprotective effects of LXR agonists.] [DOI] [PubMed] [Google Scholar]
- 107.Verschuren L, de Vries-van der Weij J, Zadelaar S, Kleemann R, Kooistra T. LXR agonist suppresses atherosclerotic lesion growth and promotes lesion regression in apoE*3Leiden mice: time course and mechanisms. J. Lipid Res. 2009;50:301–311. doi: 10.1194/jlr.M800374-JLR200. [DOI] [PubMed] [Google Scholar]
- 108.Terasaka N, et al. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003;536:6–11. doi: 10.1016/s0014-5793(02)03578-0. [DOI] [PubMed] [Google Scholar]
- 109.Schuster GU, et al. Accumulation of foam cells in liver X receptor-deficient mice. Circulation. 2002;106:1147–1153. doi: 10.1161/01.cir.0000026802.79202.96. [DOI] [PubMed] [Google Scholar]
- 110.Bradley MN, et al. Ligand activation of LXRβ reverses atherosclerosis and cellular cholesterol overload in mice lacking LXRα and apoE. J. Clin. Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tangirala RK, et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl Acad. Sci. USA. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teupser D, et al. Effect of macrophage overexpression of murine liver X receptor-α (LXR-α) on atherosclerosis in LDL-receptor deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008;28:2009–2015. doi: 10.1161/ATVBAHA.108.175257. [DOI] [PubMed] [Google Scholar]
- 113.Bischoff ED, et al. Non-redundant roles for LXRα and LXRβ in atherosclerosis susceptibility in low density receptor knockout mice. J. Lipid Res. 2010;51:900–906. doi: 10.1194/jlr.M900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y, et al. FXR deficiency causes reduced atherosclerosis in Ldlr–/– mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:2316–2321. doi: 10.1161/01.ATV.0000235697.35431.05. [DOI] [PubMed] [Google Scholar]
- 115.Hanniman EA, Lambert G, McCarthy TC, Sinal CJ. Loss of functional farnesoid X receptor increases atherosclerotic lesions in apolipoprotein E-deficient mice. J. Lipid Res. 2005;46:2595–2604. doi: 10.1194/jlr.M500390-JLR200. [DOI] [PubMed] [Google Scholar]
- 116.Guo GL, et al. Effects of FXR in foam-cell formation and atherosclerosis development. Biochim. Biophys. Acta. 2006;1761:1401–1409. doi: 10.1016/j.bbalip.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Flatt B, et al. Discovery of XL335 (WAY-362450), a highly potent, selective, and orally active agonist of the farnesoid X receptor (FXR). J. Med. Chem. 2009;52:904–907. doi: 10.1021/jm8014124. [DOI] [PubMed] [Google Scholar]
- 118.Hartman HB, et al. Activation of farnesoid X receptor prevents atherosclerotic lesion formation in LDLR–/– and apoE–/– mice. J. Lipid Res. 2009;50:1090–1100. doi: 10.1194/jlr.M800619-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mencarelli A, Renga B, Distrutti E, Fiorucci S. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H272–H281. doi: 10.1152/ajpheart.01075.2008. [DOI] [PubMed] [Google Scholar]