Abstract
Aims
To determine incidence rates of severe hypoglycaemia and compare incidence rates by insulin regimen in a diverse sample of youth with type 1 diabetes from two sites.
Methods
In this observational study, 255 youth (51% female) aged 9–15 years receiving varied insulin regimens provided data prospectively for a median of 1.2 years. Reported episodes of severe hypoglycaemia, defined as episodes requiring help from another person for oral treatment or episodes resulting in seizure/coma, and current insulin regimens were collected systematically. Incidence rates were calculated and compared according to insulin regimen in bivariate and multivariate analyses.
Results
At first encounter, participants had a median age of 12.2 years (range 9.0–15.0), median diabetes duration of 4.4 years (range 1.0–13.0) and mean A1C of 67±12 mmol/mol (8.3±1.1%). The incidence rate was 37.6/100-patient-years for all severe hypoglycaemia and 9.6/100-patient-years for seizure/coma. The incidence rate for severe hypoglycaemia was 31.8/100-patient-years on continuous subcutaneous insulin infusion (CSII), 34.4/100-patient-years on basal-bolus injections (B-B) and 46.1/100-patient-years on NPH (NPH vs. CSII: p=.04). The incidence rate for seizure/coma was 4.5/100-patient-years on CSII, 11.1/100-patient-years on B-B, and 14.4/100-patient-years on NPH (NPH vs. CSII: p=.004). In the multivariate analysis, the rate of seizure/coma was significantly higher for those on NPH vs. CSII (rate ratio 2.9, p=.03).
Conclusions
Rates of severe hypoglycaemia in youth with type 1 diabetes remain high. CSII was associated with lower rates of all severe hypoglycaemia and seizure/coma in comparison to NPH.
Keywords: Hypoglycaemia, type 1 diabetes, paediatrics, insulin therapy
Introduction
Balancing the risks of hypoglycaemia with the goal of optimal glycemic control challenges patients, families, and providers almost every day. Experiencing hypoglycaemia may increase fear of hypoglycaemia and discourage tight glycemic control [1]. In addition, repeated or severe hypoglycaemia may lead to subtle problems in learning and memory in children [2]. Finally, severe hypoglycaemia may occasionally cause significant morbidity or even death [3]. However, hyperglycaemia may contribute to cognitive dysfunction in youth with Type 1 Diabetes (Type 1 DM) as well [4].
The Diabetes Control and Complications Trial (DCCT), conducted in the 1980s and early 1990s, described very high rates of severe hypoglycaemia in youth treated with intensive insulin therapy [5]. Since the DCCT, there have been significant advances in the treatment of Type 1 DM including the availability of insulin analogs, varied insulin delivery devices, and continuous glucose monitoring [6]. Despite these advances, concerns regarding hypoglycaemia continue to be an obstacle to achieving normal glycemia in youth with Type 1 DM. Studies of the contemporary rates of severe hypoglycaemia have contributed substantially to our understanding of modern diabetes management but have had important limitations as well. Larger studies are frequently cross-sectional in design and thus may be subject to recall bias [7,8]. Incidence rates of severe hypoglycaemia reported from single sites have also varied substantially [9–12].
We sought to describe contemporary incidence rates of severe hypoglycaemia in youth with Type 1 DM from two large centres using uniformly-collected, longitudinal data. We then sought to describe the rates of severe hypoglycaemia according to particular insulin regimens, as choice of insulin regimen may offer a means to decrease rates of severe hypoglycaemia without compromising glycemic control.
Patients and Methods
Study Population
Youth (n=284) receiving diabetes care at 1 of 2 paediatric centres, Joslin Diabetes Center in Boston, MA or Texas Children’s Hospital, Houston, TX, were recruited to participate in a prospective family-focused diabetes study. Eligibility criteria included age 9–15 years, Type 1 DM diagnosed according to standard criteria [13], most recent A1C <119 mmol/mol (<13.0%), and no other major chronic medical or psychiatric disease. For these analyses, we eliminated participants who had only a single visit (n=4). In order to exclude those in the honeymoon period, we excluded youth with a diabetes duration <1 year throughout the study (n=22), or an initial A1C <42 mmol/mol (6.0%) ( n=1). In order for a visit to be included in these analyses, the participant had to be using a regimen of continuous subcutaneous insulin infusion (CSII), a basal insulin analog (detemir or glargine), or NPH at that visit. We eliminated participants who were not using one of these regimens at any visits (n=2). Thus, 255 participants were included in these analyses.
Across the two sites, data were collected in two overlapping waves. Care did not change between the two waves. The first wave occurred from 2004–2006 and the second wave occurred from 2005–2009. We included all patients in wave 1, which served as the pilot study for the main trial, in the analysis. For wave 2, we included 6 months of baseline data for the entire cohort and 2 years of follow-up data only for those receiving routine follow-up care without any intervention. Institutional Review Board approval was obtained at each institution.
Definition of Severe Hypoglycaemic Events
Severe hypoglycaemia was defined according to the DCCT definition [14], including both hypoglycaemia requiring assistance from another person for oral treatment and hypoglycaemia with seizure/coma (altered consciousness) as determined by report of seizure or coma, requirement for parenteral therapy (i.e., glucagon or intravenous dextrose), or use of emergency services (i.e., 911 or emergency room). We determined incidence rates of severe hypoglycaemia (all events) as well as the rates of the subset of seizure/coma events.
Data from Family Interview and Medical Chart Review
Study personnel at the two sites received uniform training in data collection using structured family interviews and medical record reviews. Interview and record review captured data on treatment regimen and occurrence of severe hypoglycaemic events at quarterly visits. While most participants received either exclusive CSII, basal-bolus injection therapy (B-B), or NPH therapy, some patients received a combination of these simultaneously. We categorized combination B-B and CSII regimens as CSII regimens (0.4% of all observation time). We categorized regimens including NPH with any other combination of insulin types to be an NPH regimen (9.4% of all observation time). If participants’ regimens changed over the course of the study, they could contribute observation time to more than one regimen group.
Blood glucose monitoring frequency was derived from glucose meter download for the majority of visits, or by self-report when meter download was not available (5.4% of visits). Body mass index z score calculations followed US Center for Disease Control and Prevention standards. Blood samples were sent to a central laboratory for A1C assay (reference range 20–42 mmol/mol (4–6%); Tosoh Medics, South San Francisco, CA).
Analysis
Statistical analysis employed SAS Version 9.2 (Cary, NC). We report means and standard deviations for normally distributed data, medians and ranges for data that were not normally distributed, and percents for categorical data. Two-sample t-tests were used for parametric analysis, Wilcoxon rank sum tests were used for nonparametric analysis, and Chi squared or Fisher’s Exact tests were used for categorical data. Incidence rate (IR) was calculated as number of episodes per 100 patient-years. We calculated IR of severe hypoglycaemia for the entire sample and according to insulin regimen. The first study visit served as the initial regimen exposure. Regimen exposure for events subsequent to the first study visit reflects the insulin regimen of the visit preceding the hypoglycaemic event. Thus, a single individual could contribute events or patient-years in more than one regimen category if she/he switched insulin regimens during the course of follow-up.
Multivariate Poisson modelling predicting all severe hypoglycaemic events and events of seizure/coma employed generalized estimating equations (proc genmod) to allow for time-varying covariates: age, diabetes duration, regimen, and A1C. We additionally adjusted for sex and site. In order to adjust for varying intervals between visits, we used an offset equal to the natural log of follow-up time [12]. We limited the number of visits per participant to <10 visits each in order to improve the stability of the model as there were fewer subjects with 10 visits or more which did not allow estimation of the correlation matrix for those visits. An autoregressive matrix structure was used. Two-tailed p values of <0.05 were considered significant.
Results
Overall, 255 participants contributed 1,324 encounters during 386.1 patient-years of follow-up. Participants were followed for a median of 1.2 years (range 0.2–3.4 years) and had a median of 4 (range 1–12) encounters. At the beginning of the follow-up period, participants had a median age of 12.2 years (range 9.0–15.0), with median diabetes duration of 4.4 years (range 1.0–13.0) and mean A1C of 67±12 mmol/mol (8.3±1.1%). Participants received a mean of 0.9±0.3 units of insulin/kg/day and checked blood glucose levels 4–5 times/day.
There were significant differences between participants according to their regimen at baseline. Participants on CSII received fewer units of insulin/kg/day, checked blood glucose more frequently, were less likely to be non-white and had a lower A1C than participants on B-B or NPH (see Table 1). There were no significant differences between B-B and NPH.
Table 1.
Baseline Clinical Characteristics for All Participants and by Regimen
| All Participants (N=255) | NPH (n=112) | B-B (n=50) | CSII (n=93) | |
|---|---|---|---|---|
| Age (years) | 12.2 (9.0–15.0) | 12.4 (9.2–15.0) | 13.1 (9.0–15.0) | 11.8 (9.1–15.0) |
| Diabetes duration (years) | 4.4 (1.0–13.0) | 3.3 (1.0–13.0) | 4.4 (1.0–12.6) | 5.0 (1.0–12.0) |
| Sex (% female) | 51.4% | 55.4% | 44.0% | 50.5% |
| Race/ethnicity (% non-white) | 25.2% | 40.2% | 25.0% | 7.7% |
| zBMI (SDS) | 0.6±0.8 | 0.7±0.8 | 0.6±0.7 | 0.6±0.8 |
| Insulin dose (U/kg/day) | 0.9±0.3 | 1.0±0.3 | 1.0±0.3 | 0.9±0.2 |
| Blood glucose monitoring frequency (x/day) | 4.8±2.0 | 4.1±1.9 | 4.6±1.4 | 5.7±2.2 |
| A1C (mmol/mol) A1C (%) |
67±12 8.3±1.1 |
69±13 8.5±1.2 |
69±10 8.5±0.9 |
63±11 7.9±1.0 |
Values are in provided as mean±SD, median (range) (for diabetes duration and age), or percentage.
The distribution of participants on NPH, B-B, and CSII was 44%, 20%, and 36%, respectively, at the first visit; at the last visit, the regimens had shifted to 31%, 25%, and 44%, respectively. Over the study course, 59 participants changed their insulin regimen at least once; there were 8 changes to NPH, 39 changes to B-B, and 28 changes to CSII.
Incidence Rates of Severe Hypoglycaemia
All Severe Hypoglycaemia
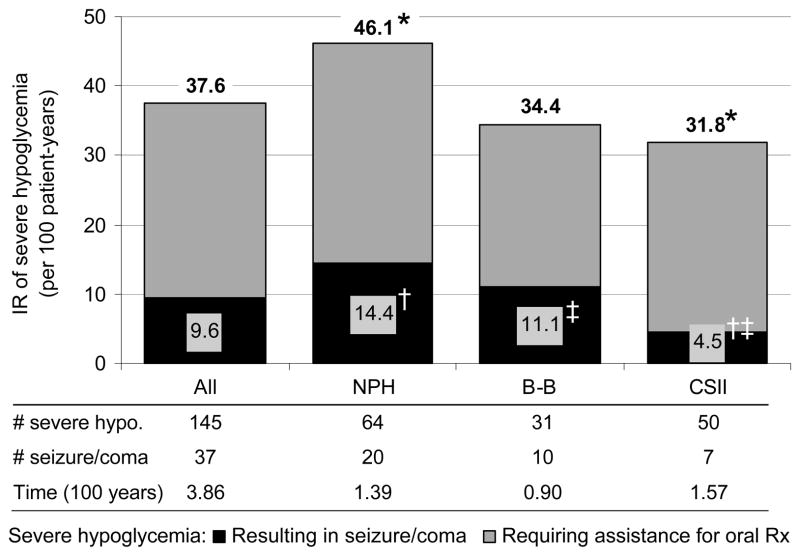
During the study period, 145 episodes of severe hypoglycaemia occurred in 64 participants: 31 participants had only one episode, 14 participants had 2 episodes, 8 participants had 3 episodes, and 11 participants had ≥4 episodes. The overall IR of severe hypoglycaemia was 37.6/100-patient-years. The IR of severe hypoglycaemia was similar in waves 1 and 2 (IRs of 37.9 and 36.1, respectively). Those on NPH experienced significantly more severe hypoglycaemia than those on CSII (NPH: 46.1, CSII: 31.8/100-patient-years; NPH vs. CSII, p=.04).
Severe Hypoglycaemia Resulting in Seizure/Coma
During the study period, 37 episodes of seizure/coma occurred in 22 participants, yielding an IR of 9.6/100-patient-years. The IR of seizure/coma was significantly lower for those on CSII (4.5/100-patient-years) when compared to NPH (14.4/100-patient-years, p=.004) and borderline significantly less than B-B (11.1/100-patient-years, p=.05). There was no significant difference between B-B and NPH for seizure/coma. Results are shown in Figure 1.
Figure 1.
Incidence rate of severe hypoglycemia by insulin regimen. Gray = severe hypoglycemia requiring assistance of another person for oral treatment. Black = severe hypoglycemia resulting in seizure or coma. Incidence rates for all severe hypoglycemia are shown above each bar. All severe hypoglycemia: *NPH vs. CSII, p=.04. Severe hypoglycemia resulting in seizure/coma: †CSII vs. NPH, p=.004; ‡CSII vs. B-B, p=.05.
Incidence Rates of Severe Hypoglycaemia by Age, Sex, A1C and Diabetes Duration
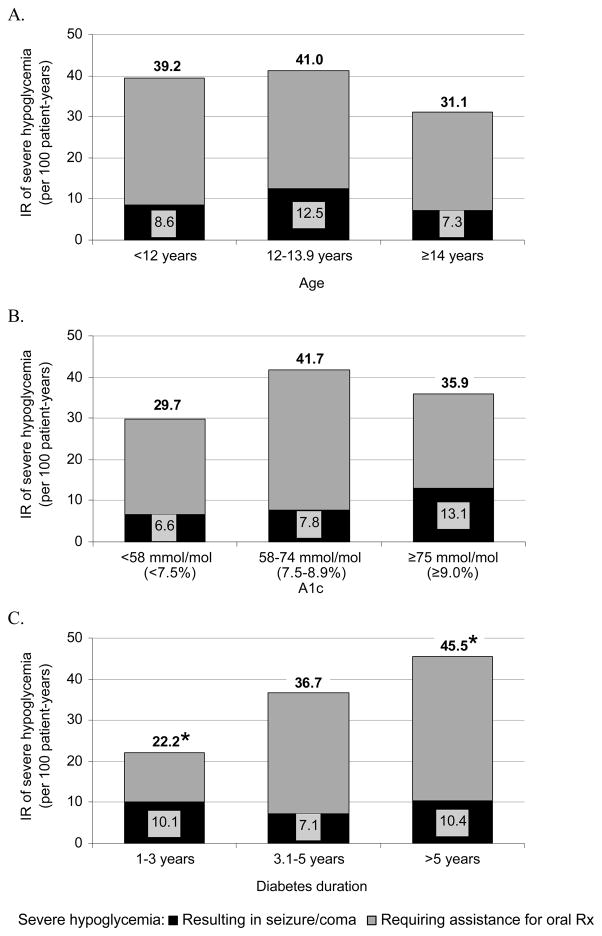
IRs of all severe hypoglycaemia and severe hypoglycaemia resulting in seizure/coma did not differ significantly by age (Figure 2A), sex or A1C (Figure 2B). Youth with the shortest diabetes duration (≤3 years) had significantly lower IR of severe hypoglycaemia than youth with duration >5 years (Figure 2C) but rates of seizure/coma did not differ by diabetes duration.
Figure 2.
Incidence rate of severe hypoglycemia by (A) age, (B) A1C, and (C) diabetes duration. Gray = severe hypoglycemia requiring assistance of another person for oral treatment. Black = severe hypoglycemia resulting in seizure or coma. Incidence rates for all severe hypoglycemia are shown above each bar. Figure 2A and 2B: No significant differences. Figure 2C: All severe hypoglycemia: *1–3 vs. >5 years, p=.002; Severe hypoglycemia resulting in seizure/coma: no significant differences.
Adjusted Odds of Severe Hypoglycaemia by Regimen
In multivariate models predicting all severe hypoglycaemia and severe hypoglycaemia resulting in seizure/coma, insulin regimen was a significant predictor of rate of seizure/coma. After adjusting for age, sex, diabetes duration, A1C, and site, participants on NPH had 2.9 (95% CI: 1.1–7.6) times the rate of seizure/coma than those on CSII. There were no significant differences in the rate of severe hypoglycaemia between regimens. There were no significant differences in the rates of seizure/coma between B-B and NPH or CSII.
Discussion
Despite burgeoning clinical experience with intensive insulin therapy and the development and refinement of new therapeutic tools, severe hypoglycaemia remains common in youth with Type 1 DM. In this prospective study of nonrandomized youth at two clinical sites, NPH-based insulin regimen significantly predicted higher rates of severe hypoglycaemia resulting in seizure/coma. NPH had a strong association with increased frequency of seizure/coma in both unadjusted and adjusted analyses when compared to CSII. There does appear to be a trend for severe hypoglycaemic events on B-B to be intermediate between NPH and CSII, but significant differences were not demonstrated. In bivariate analyses, longer duration of Type 1 DM was associated with higher IR of severe hypoglycaemia while age, A1C and sex were not.
The incidence rates of severe hypoglycaemia in our study represent at least a 50% reduction from the IRs observed for the intensively treated adolescents in the DCCT (all severe hypoglycaemia: 85.7/100-patient-years; severe hypoglycaemia resulting in seizure coma: 26.7/100-patient-years) [15].
The rates we report for severe hypoglycaemia appear higher than those reported recently in some studies while the rates of seizure/coma are more similar. In a large, paediatric cohort study conducted from 1996–2000, the IR of seizure/coma was 19/100-patient-years [12]. Another prospective, observational study noted a rise in rates of seizure/coma from 7.8/100-patient-years to 16.6 events/100-patient-years from 1992 to 2002[9]. In multinational, cross-sectional studies of paediatric patients using CSII, the IR for seizure/coma varied from 7–12/100 patient-years [16,17]. In the Juvenile Diabetes Research Association Continuous Glucose Monitoring crossover extension trial, among subjects ages 8–14 years, the IR of seizure/coma was 26.4/100-patient-years without CGM and decreased to 13.3/100-patient-years after initiation of CGM[18]. It is notable, however, than no events of seizure/coma were noted in either the control or intervention group during the more intensive, earlier phase of the trial[19]. Many of these rates are commensurate with our seizure/coma rate of 9.6/100-patient-years.
When the more inclusive definition of severe hypoglycaemia is used, the rates we report appear higher than contemporary clinical trials which frequently have strict inclusion criteria. Differences in the eligible participants among the various studies may underlie much of the IR variation. A recent trial of CGM use to reduce the time spent with hypoglycaemia did not report any severe hypoglycaemic events over 26.5 patient-years of observation time in 53 paediatric patients. However, participants needed to have an A1c <58 mmol/mol (<7.5%) and to already use intensive insulin therapy [20]. In a 1 year randomized, controlled trial of sensor augmented pump therapy versus multiple daily injections (MDI) in 485 adults and children, the rate of severe hypoglycaemia was 13/100-patient-years in both the pump and MDI groups. However, one of the exclusion criteria was ≥2 episodes of severe hypoglycaemia in the year before enrolment [21] and, since past hypoglycaemia has been shown to be highly predictive of future hypoglycaemia [22], this likely affected the IR. The IR of severe hypoglycaemia of 24.4/100-patient-years among the 58 children aged 8–14 years (on intensive insulin therapy without the use of CGM) in the JDRF trial do appear lower than the rates we present here[19]. We would suggest that this may be attributable to their having 84% of participants using CSII, and to differences between our study population and those who successfully completed the run-in period with adequate home blood glucose monitoring and compliance with CGM in the JDRF CGM study [19].
Randomized trials have examined the association of insulin regimen with severe hypoglycaemia. A meta-analysis of adult and paediatric randomized controlled trials comparing B-B therapies (with either glargine or detemir) to NPH-based therapies in Type 1 DM showed decreased risk of severe hypoglycaemia (defined as requiring help from another person) with B-B therapies) [23]. Another recent meta-analysis of 15 adult and paediatric randomized trials since 2002 did not show a significant difference in odds of severe hypoglycaemia requiring help from another person with CSII versus multiple daily injections [24], although the point estimate favoured CSII (OR 0.48, 95% CI 0.23–1.00).
Avoidance of hypoglycaemia is especially important in the management of youth with Type 1 DM. Fear of hypoglycaemia contributes to parental stress and burden [25] and parents may feel this stress even more acutely than adults with Type 1 DM [26]. Additionally, studies suggest an association between severe hypoglycaemia and neuro-cognitive impairment [2], although this finding is not consistent across all studies.
Comparing rates of severe hypoglycaemia in youth with Type 1 DM is complicated by varied definitions of severe hypoglycaemia. The DCCT [14] defined severe hypoglycaemia as events requiring the help of another person with enteral or parenteral therapy and this has become a standard definition. However, some other studies [9,12] have defined severe hypoglycaemia as hypoglycaemia resulting in seizure or coma or requiring the administration of glucagon or intravenous dextrose. While the validity of the less restrictive definition of severe hypoglycaemia in children could be distorted by the developmental inability of children to self-treat their hypoglycaemia, such concerns are less likely to apply here because our study involved only youth ages 9 and older. We present results according to both commonly used definitions in this study.
The study design allowed for carefully collected data at visits consistent in frequency with routine care and with A1C assessed at a central laboratory. For 84% of all visits, youth were using intensive insulin therapy as defined by 3 or more injections per day or CSII. For a substantial proportion of the observation time, youth were on NPH-based regimens as part of either conventional or intensive therapy. The SEARCH study group recently described 53% of paediatric patients who were neither on CSII nor glargine including 27% on 1–2 injections daily [28], suggesting that NPH remains a large part of the therapeutic landscape.
There are limitations inherent in the observational design of this study. Because patients were not randomized to treatment regimen, there are likely to be both measured and unmeasured differences between the three groups. There is known to be an increased risk of hypoglycaemia with younger age [29], history of psychiatric disorders [12], underinsurance [12], longer diabetes duration [12], and lower A1C [12,14]. Those with major psychiatric disorders were excluded from this study and we were able to adjust for age, diabetes duration, and A1C in our adjusted analyses. However, there are likely patient variables (e.g., fear of injections, adherence), provider factors (e.g., experience and preference), and environmental influences (e.g., insurance coverage, absence of school nurse) that lead providers and families to choose a particular insulin regimen and not all of these factors could be accounted for in our analysis. An additional unmeasured factor is that initiating CSII is likely to be accompanied by more frequent diabetes educational visits which may also lead to decreased rates of severe hypoglycaemia. Data were collected in two waves but within our study the overall incidence rates of severe hypoglycaemia were similar between waves.
While regimen and severe hypoglycaemic events were assessed consistently at every encounter, another limitation of the design is the potential for regimens to have changed between encounters. We used the regimen reported at the preceding encounter (except at baseline) so that severe hypoglycaemic episodes were not misattributed to a new regimen that was changed in response to a severe hypoglycaemic event. In addition, the time between visits was short (median time, 3.3 months), diminishing the likelihood of regimen misclassification. Finally, our study may have been underpowered to detect differences in the IR of hypoglycaemia between B-B and CSII.
New therapeutic tools are impacting outcomes in youth with Type 1 DM. Continuous glucose monitoring may prove to be a valuable tool in reducing rates of severe hypoglycaemia. A recent study of adult and paediatric subjects with Type 1 DM demonstrated that CGM reduced the amount of time/day with an interstitial glucose <3.5 mmol/l by about 50%, although it was not powered to detect differences in severe hypoglycaemic episodes [20].
Severe hypoglycaemia remains a limiting factor in achieving optimal glycemic control [30]. Minimizing severe hypoglycaemia remains an important but elusive therapeutic goal. Our data suggest that severe hypoglycaemia continues to be a challenge for medical providers and paediatric patients with Type 1 DM. Avoiding the use of NPH will provide one step towards reduction of rates of severe hypoglycaemia in young adolescents with Type 1 DM. Further research is needed to establish whether severe hypoglycaemia is reduced in CSII when compared to B-B regimens and to determine the long-term benefits of CGM in reducing this acute complication in youth with Type 1 DM.
Table 2.
Adjusted Relative Rates of Severe Hypoglycaemia
| All Severe Hypoglycaemia | |||
|---|---|---|---|
| Rate Ratio | CI | P value | |
| NPH vs. CSII | 1.7 | 0.9–3.1 | 0.1 |
| B-B vs. CSII | 1.2 | 0.6–2.4 | 0.6 |
| NPH vs. B-B | 1.4 | 0.7–2.8 | 0.3 |
| Seizure/Coma | |||
| Rate Ratio | CI | P value | |
| NPH vs. CSII | 2.9 | 1.1–7.6 | 0.03 |
| B-B vs. CSII | 2.2 | 0.7–7.3 | 0.2 |
| NPH vs. B-B | 1.3 | 0.5–3.7 | 0.6 |
Acknowledgments
We want to thank the families who participated in this project and the study staff at the Joslin Diabetes Center in Boston, MA, and Texas Children’s Hospital in Houston, TX. This research was supported by contracts N01-HD-4-3364 (Joslin Diabetes Center, Boston) and N01-HD-4-3362 (Texas Children’s Hospital, Houston). Portions of this manuscript were presented at the 70th Scientific Sessions of the American Diabetes Association (2010). MLK analyzed the data, wrote the manuscript, and contributed to discussion. LKV, BJA, and LML researched the data, reviewed/edited the manuscript, and contributed to discussion.
Abbreviations
- B-B
basal-bolus injection therapy
- CGM
continuous glucose monitoring
- CSII
continuous subcutaneous insulin infusion
- DCCT
Diabetes Control and Complications Trial
- IR
incidence rate
- Type 1 DM
Type 1 Diabetes
Footnotes
The definitive version is available at Blackwell-synergy.com.
Declaration of Competing Interests: Nothing to declare for all authors.
References
- 1.Haugstvedt A, Wentzel-Larsen T, Graue M, Sovik O, Rokne B. Fear of hypoglycaemia in mothers and fathers of children with Type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study. Diabet Med. 2010;27 (1):72–78. doi: 10.1111/j.1464-5491.2009.02867.x. [DOI] [PubMed] [Google Scholar]
- 2.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24(9):1541–1546. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- 3.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch Dis Child. 1999;81(4):318–323. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aye T, Reiss AL, Kesler S, Hoang S, Drobny J, Park Y, et al. The feasibility of detecting neuropsychologic and neuroanatomic effects of type 1 diabetes in young children. Diabetes Care. 2011;34(7):1458–1462. doi: 10.2337/dc10-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.Wood JR, Laffel LMB. Technology and intensive management in youth with type 1 diabetes: State of the art. Curr Diab Rep. 2007;7(2):104–113. doi: 10.1007/s11892-007-0019-1. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen HB, Hougaard P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. The Hvidore Study Group on Childhood Diabetes. Diabetes Care. 1997;20(5):714–720. doi: 10.2337/diacare.20.5.714. [DOI] [PubMed] [Google Scholar]
- 8.Rosilio M, Cotton JB, Wieliczko MC, Gendrault B, Carel JC, Couvaras O, et al. Factors associated with glycemic control. A cross-sectional nationwide study in 2,579 French children with type 1 diabetes. The French Pediatric Diabetes Group. Diabetes Care. 1998;21 (7):1146–1153. doi: 10.2337/diacare.21.7.1146. [DOI] [PubMed] [Google Scholar]
- 9.Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care. 2004;27(10):2293–2298. doi: 10.2337/diacare.27.10.2293. [DOI] [PubMed] [Google Scholar]
- 10.Braun D, Konrad D, Lang-Muritano M, Schoenle E. Improved glycemic control and lower frequency of severe hypoglycemia with insulin detemir; long-term experience in 105 children and adolescents with type 1 diabetes. Pediatr Diabetes. 2008;9(4 Pt 2):382–387. doi: 10.1111/j.1399-5448.2008.00371.x. [DOI] [PubMed] [Google Scholar]
- 11.Svoren BM, Volkening LK, Butler DA, Moreland EC, Anderson BJ, Laffel LMB. Temporal trends in the treatment of pediatric type 1 diabetes and impact on acute outcomes. J Pediatr. 2007;150(3):279–285. doi: 10.1016/j.jpeds.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287(19):2511–2518. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–48. [PubMed] [Google Scholar]
- 14.The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med. 1991;90(4):450–459. [PubMed] [Google Scholar]
- 15.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125(2):177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 16.Danne T, Battelino T, Kordonouri O, Hanas R, Klinkert C, Ludvigsson J, et al. A cross-sectional international survey of continuous subcutaneous insulin infusion in 377 children and adolescents with type 1 diabetes mellitus from 10 countries. Pediatr Diabetes. 2005;6 (4):193–198. doi: 10.1111/j.1399-543X.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 17.Danne T, Battelino T, Jarosz-Chobot P, Kordonouri O, Pankowska E, Ludvigsson J, et al. Establishing glycaemic control with continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes: experience of the PedPump Study in 17 countries. Diabetologia. 2008;51(9):1594–1601. doi: 10.1007/s00125-008-1072-2. [DOI] [PubMed] [Google Scholar]
- 18.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33(1):17–22. doi: 10.2337/dc09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 20.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34(4):795–800. doi: 10.2337/dc10-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 22.Fiallo-Scharer R, Cheng J, Beck RW, Buckingham BA, Chase HP, Kollman C, et al. Factors predictive of severe hypoglycemia in type 1 diabetes: analysis from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized control trial dataset. Diabetes Care. 2011;34(3):586–590. doi: 10.2337/dc10-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues vs. NPH human insulin in type 1 diabetes. A meta-analysis. Diabetes Obes Metab. 2009;11(4):372–378. doi: 10.1111/j.1463-1326.2008.00976.x. [DOI] [PubMed] [Google Scholar]
- 24.Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: Hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab. 2009;94(3):729–740. doi: 10.1210/jc.2008-1415. [DOI] [PubMed] [Google Scholar]
- 25.Streisand R, Swift E, Wickmark T, Chen R, Holmes CS. Pediatric parenting stress among parents of children with type 1 diabetes: the role of self-efficacy, responsibility, and fear. J Pediatr Psychol. 2005;30(6):513–521. doi: 10.1093/jpepsy/jsi076. [DOI] [PubMed] [Google Scholar]
- 26.Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 1998;11 (Suppl 1):189–194. doi: 10.1515/jpem.1998.11.s1.189. [DOI] [PubMed] [Google Scholar]
- 27.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28(3):726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 28.Paris CA, Imperatore G, Klingensmith G, Petitti D, Rodriguez B, Anderson AM, et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155(2):183–189. doi: 10.1016/j.jpeds.2009.01.063. [DOI] [PubMed] [Google Scholar]
- 29.Levine BS, Anderson BJ, Butler DA, Brackett J, Laffel L. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139:197–203. doi: 10.1067/mpd.2001.116283. [DOI] [PubMed] [Google Scholar]
- 30.Cryer PE. Banting Lecture. Hypoglycemia: the limiting factor in the management of IDDM. Diabetes. 1994;43(11):1378–1389. doi: 10.2337/diab.43.11.1378. [DOI] [PubMed] [Google Scholar]