Abstract
The objective of this study was to determine if intra-operative auditory monitoring is feasible during cochlear implantation and whether this can be used as feedback to the surgeon to improve the preservation of residual hearing. This prospective non-randomised study was set in a paediatric tertiary referral hospital. Thirty eight consecutive paediatric patients undergoing cochlear implantation who had measurable auditory thresholds pre-operatively were divided into two cohorts. The unmonitored cohort included the fi rst 22 patients and the monitored cohort included the last 16 patients. The main outcome measure(s) were pre-operative, intra-operative and more than one month post-operative average auditory thresholds at 500, 1000 and 2000 Hz measured using auditory steady-state response audiometry. The average pre-operative thresholds were 103.5 dB HL and 99.7 dB HL in the unmonitored and monitored cohorts, respectively. These were not statistically different (p > 0.3). In the monitored cohort, we measured auditory thresholds to assess cochlear function at multiple time points during the operation. Compared to baseline, thresholds were increased 0.7 dB after drilling the mastoidectomy and well, 0.2 dB after opening the cochlea and 4.6 dB after inserting the electrode array. One month post-operatively, the average thresholds were 114.0 dB HL in the unmoni-tored cohort but only 98.8 dB HL in the monitored cohort (p < 0.001). Both the use of intra-operative auditory monitoring and higher pre-operative thresholds were associated with improved preservation of residual hearing (p < = 0.001). Intra-operative auditory monitoring is a viable tool that can provide real-time feedback to the surgeon during cochlear implant surgery. These data suggest that this can lead the surgeon to modify his or her surgical technique in ways that can improve the rate of long-term hearing preservation.
Keywords: cochlear implant, cochlea, trauma, hearing loss
Introduction
The prevalence of congenital deafness has been estimated to be one in every 1000 children (Haggard and Pullan, 1989). These children have non-serviceable hearing in that hearing aids will not provide enough benefit to permit the development of normal speech and language. However, few of these children have absolutely no hearing. Many children with congenital ‘deafness’ will have some degree of residual hearing that can be detected if appropriate testing paradigms are used. Fortunately, cochlear implants have been able to help alleviate this developmental disability for many patients.
The techniques of cochlear implantation have been shown to mechanically traumatise the cochlea. Analyses of temporal bones that have undergone implantation show a wide range of damage, the most concerning of which is spiral ganglion cell loss because this may ultimately affect the ability of the implant to provide functional benefit (Adunka et al., 2006; Naldol et al., 2001; Roland, 2005; Roland and Wright, 2006; Wardrop et al., 2005). However, there is substantial evidence that residual hearing can be preserved after cochlear implantation (Cullen et al., 2004; Gantz and Turner, 2003, 2004; Gantz et al., 2005, 2006; Hodges et al., 1997; Rizer et al., 1988; Skarzynski et al., 2002; Yao et al., 2006). Cochlear implant manufacturers are continuing to focus their engineering efforts on designing electrodes that provide the best electrical stimulation possible while causing the least trauma to cochlear structures during implantation (Hochmair et al., 2003; Marrinan et al., 2004; Tykocinski et al., 2000). Preservation of residual hearing during cochlear implantation, no matter how little sensation there was at the beginning of surgery, can be taken to indicate that minimal cochlear trauma occurred as a result of the surgery. In theory, this should be associated with improved spiral ganglion cell survival and improvements in long-term functional outcomes (Cohen, 1997; Scarpidid et al., 2003).
As part of our efforts to assess and hopefully reduce cochlear trauma during paediatric cochlear implantation, we began a prospective study to measure auditory thresholds pre-operatively and post-operatively. Because the post-operative threshold measurements were made several months after surgery, our efforts were frustrated by diffi culties in correlating good and bad hearing preservation outcomes with specific details of the surgical procedure. In order to get more rapid feedback during the surgery, we developed a technique for intra-operative auditory monitoring during cochlear implantation surgery. The ultimate goal of this effort was to allow the individual surgeons to identify what specific aspects of their own surgical technique can lead to hearing loss and hopefully to improve in these areas.
Intra-operative auditory monitoring in children that are cochlear implant candidates is not simple. Behavioural audiometry has long been considered the gold standard for the determination of hearing thresholds but this technique does not provide reliable response thresholds in the very young paediatric patient or in patients with visual or developmental disabilities (Madell, 1998; Rance and Briggs, 2002). It certainly cannot be used under general anaesthesia. Auditory brainstem response audiometry using tone-burst stimulus can provide ear-specifi c, frequency-specific thresholds in the young paediatric patient for which behavioural thresholds cannot be obtained; however, the threshold estimates are limited by stimulus output limitations which precludes an accurate estimate of the shape and degree of the hearing loss when there is more than a severe sensorineural hearing loss.
Auditory steady-state response (ASSR) audiometry appears to resolve this clinical conundrum. ASSR audiometry works by measuring far-fi eld electrical potentials derived from the cerebral cortex in response to AM and FM modulated, frequency-specific auditory stimuli. Also, using technology that is approved for clinical use, this technique is able to determine frequency-specific thresholds from 250 to 8000 Hz at stimulus levels up to 127 db HL (Picton et al., 1998). This is substantially higher than the typical 105 dB HL maximum stimulus intensity level permitted by most clinical ABR audiometers. Therefore, ASSR thresholds can often be measured in ears that demonstrate no ABR responses to equipment limits (Cone-Wesson et al., 2002a; Vander Werff et al., 2002).
ASSR signals are unaffected by sedation or sleep and are detectable in infants, children and adults (Cone-Wesson et al., 2002b). Correlations between predicted thresholds using ASSR audiometry in infancy and subsequently measured behavioural thresholds are high (r > 0.95) (Rance and Richards, 2002). In normal hearing adults, there are also strong correlations between ASSR thresholds and behavioural thresholds (r = 0.85, 0.94, 0.95 and 0.95 for 500, 1000r = 0.85, 0.94, 0.95 and 0.95 for 500, 2000 and 4000 Hz, respectively) (Dimitrijevic et al., 2002). ASSR thresholds have also been found to be highly predictive of tone-burst ABR thresholds (Cone-Wesson et al., 2002c). Comparisons of click evoked ABR and tone-burst evoked ABR with ASSR show very strong correlations (r = > 0.95) (Vander Werff et al., 2002).
In this study, we sequentially measured ASSR thresholds at several points during the cochlear implantation surgery. We assessed for threshold shifts and correlated these to the potentially causative surgical factors. Additionally, we compared thresholds measured pre-operatively and more than one month postoperatively. These data from a cohort of patients who underwent intra-operative auditory monitoring were compared to a cohort of patients who only had pre- and post-operative audiometric evaluations, but who did not undergo intra-operative monitoring.
Materials and methods
Patients
Institutional review board approval was obtained and we only enrolled children whose parents consented to participate in the study. This was a prospective non-randomised study. Eligibility criteria included the presence of severe-to-profound sensorineural hearing loss bilaterally, residual thresholds measurable by ASSR in the clinic and approval for cochlear implantation by the multidisciplinary team at The Hearing Center at Texas Children’s Hospital according to established clinical criteria. Patients with no measurable hearing to equipment limits by ASSR were ineligible.
United States Food and Drug Administration (FDA) approved cochlear implant devices from all three companies (Advanced Bionics [Sylmar, California], Cochlear [Lane Cove, Australia] and Med-El [Innsbruck, Austria]) were all eligible to be included for this study. These were all full-length cochlear implants; the 10 mm short hybrid electrode was not studied (Gantz et al., 2005). The choice of device selection was made solely by the parents and this decision was made prior to offering them inclusion into this study. No parent was infl uenced to choose a certain type of device because of this study. Thus, all patients who fit the eligibility criteria were offered enrolment into the study. Every family with an eligible child agreed to participate.
ASSR audiometry
We measured the ASSR threshold using the Audera system (Grason-Stadler, Viasys, Conshohocken, PA, USA). The stimulus was an amplitude and frequency modulated tone generated by an Etymotic speaker (EAR3A, Elk Grove Village, IL, USA), directed into the ear canal. Recording electrodes were placed below the ipsilateral tragus (signal), below the contralateral tragus (control), in the scalp (ground) and in the bridge of the nose. Pre- and post-operatively we used external contact electrodes; intra-operatively we used needle electrodes. The phase of the stimulus and of the Electroencephalogram (EEG) response was calculated. An ASSR was determined to be present if there was phase coherence between the two values. The probability of phase coherence is based on a statistical analysis, and the probability level was set at a 0.03 level of confi dence.
Pre- and post-operative audiometry
Every child had auditory thresholds measured at less than three months pre-operatively and more than one month post-operatively. In one reliable patient who was 17 years old unsedated behavioural audiometry was used rather than ASSR. For all other patients, thresholds were measured with ASSR under sedation. The patients were sedated with chloral hydrate and monitored by our sedation nurse during the procedure.
We always attempted to measure thresholds at 500, 1000, 2000, 4000 and 8000 Hz utilising a 10 dB bracketing procedure. Because time is at a premium when performing audiometry on sedated children, we fi rst measured ASSR thresholds at 500, 1000 and 2000 Hz sequentially. The other frequencies were only able to be measured sporadically and thus are not presented in this report. In order to clarify data presentation and simplify the data analyses, we calculated the average threshold at 500, 1000 and 2000 Hz for each patient. Occasionally, a child would even unexpectedly awaken from sedation before the 2000 Hz threshold could be determined. In this rare situation, we instead used the average of the thresholds at 500 and 1000 Hz.
Intra-operative protocol
After induction by general inhalation anaesthesia, pre-operative antibiotics (van-comycin and cefuroxime) were given according to our standard protocol for cochlear implantation. Facial nerve monitoring was performed throughout the procedure. All intra-operative monitoring was performed using ASSR audiometry. Subdermal electrodes for ASSR monitoring were placed. The speaker that generated the acoustic stimulus was coupled to the ear canal using a short length of tubing with a foam earplug around it to seal the canal. A Tegaderm (3 M, St Paul, MN, USA) bandage was placed over the meatus to hold the tubing in place and to prevent fl uid from entering the ear canal. The pinna was refl ected anteriorly and the post-auricular area prepped and draped in a sterile fashion.
At this point, the fi rst threshold measurements were performed (baseline). These measurements were made at 1 kHz, and occasionally at 2 kHz. If we could not detect a threshold at 1 or 2 kHz, we simply performed a routine cochlear implant without any further intra-operative measurements and these patients were excluded from the study. Data plotted in the fi gures are the ASSR threshold at 1 kHz. Each ASSR threshold took about 5 min to measure.
The surgery for cochlear implantation was then begun. We fi rst drilled a mas-toidectomy with a facial recess approach to the round window. Care was taken not to remove the incudal buttress or contact the incus with the drill. We then drilled the well to countersink the implant body. The wound was thoroughly irrigated and we changed our gloves and gowns to maximise sterility. The implant was then opened and the body secured in place within the well. The ground electrode was positioned under the temporalis muscle.
Next, the opening into the cochlea was made (Figure 1). This procedure varied depending on the brand of the implant. For Cochlear and Advanced Bionics devices, we drilled a cochleostomy using a Skeeter microdrill (Xomed-Treace, Jacksonville, FL, USA) anterior-inferior to the round window (Briggs et al., 2005). A 1 mm diamond drill bit was used and care was taken to expose the cochlear endosteum, but not open it, as previously described (Gantz et al., 2005). For the Med-El device, we did not make a cochleostomy. Instead, we accessed the scala tympani directly through the round window. In order to completely visualise the circumference of the round window membrane, we used the 1 mm microdrill to remove the superior lip of the round window niche. At this point, the second threshold measurements were made (before opening cochlea).
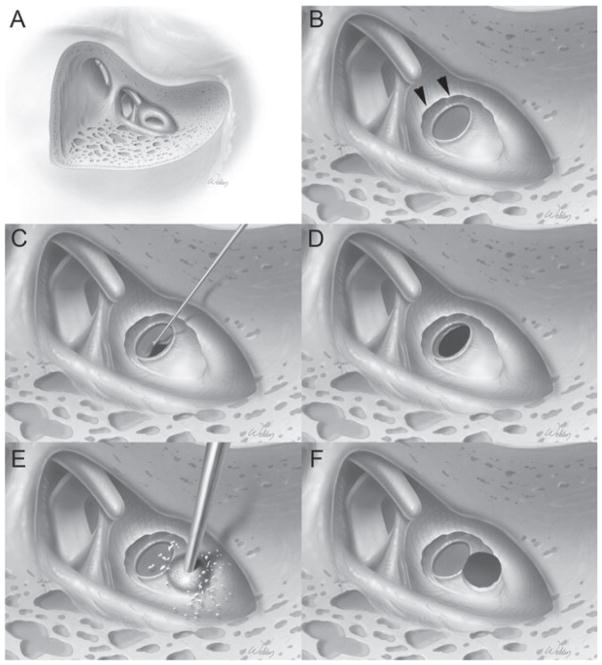
Figure 1.
Surgical technique of opening the cochlea to maximise preservation of residual acoustical hearing. This is a right ear. (A) Standard cortical mastoidectomy with facial recess approach has been performed. The round window niche and part of the round window membrane are visible. (B) The superior portion of the round window niche bone has been drilled away (arrowheads) to visualise the entire round window membrane. (C, D) Technique of opening the round window membrane for insertion of the Med-El electrode. Care is taken to start at the inferior border of the round window to reduce the risk of traumatising the basilar membrane, which is close to the superior border. (E, F) Technique of drilling a cochleostomy for the Cochlear and Advanced Bionics electrodes. The cochle-ostomy is made tangential to the anterior-inferior edge of the round window.
We then applied hyaluronic acid (Healon, 10 mg/ml, Advanced Medical Optics, Santa Ana, CA) over the planned opening to the cochlea. For the cochleostomy approach, the endosteum was gently splayed opened with a fi ne straight pick. For the round window approach, the membrane was separated from the otic capsule bone at the anterior-inferior margin fi rst, and then gently pulled superiorly. In either case, careful suctioning technique with a 22 gauge suction was performed in order to prevent aspiration of any cochlear perilymph. The third threshold measurements were then made (after opening cochlea).
Finally, the electrode array was advanced into the scala tympani as gently as possible. A fourth threshold measurement was then made (after insertion). A small piece of temporalis fascia was harvested and placed around the electrode at the site of entry into the cochlea. Care was taken so it did not enter the cochlea, nor contact the stapes or tympanic membrane.
Statistical analysis
All analysis was performed using SPSS (SPSS, Inc., version 11) in consultation with a biostatistician. The Student’s T-test was used to compare measurements between the cohorts and the Student’s paired T-test was used to compare values within the same cohort. The Analysis of Variance (ANOVA) test was used to assess for differences between multiple measurements. Univariate and multivariate linear regressions were also used to look for associations between the various independent variables (described in the Results) and the peri-operative threshold shifts.
Results
Demographics
We enrolled a total of 38 children into the study and there were two cohorts. The unmonitored cohort consisted of 22 patients who did not undergo intra-operative auditory monitoring. The monitored cohort consisted of 16 patients who did undergo auditory monitoring. All patients had pre-operative and post-operative threshold measurements. The patients in each cohort were selected sequentially because when we began this study, we did not have intra-operative monitoring capability. Thus the fi rst 22 patients were enrolled in the non-monitored cohort. However, once we were able to make ASSR threshold measurements during surgery, the next 16 patients were all monitored. All patients had complete insertions, except for one that could only be inserted about 80 per cent of its length.
The average patient age was about fi ve years and was not different between the two cohorts (Table 1). Additionally, the pre-operative average threshold was about 100 dB HL, which was also similar between the two cohorts. Of the patients enrolled in the study, we implanted 31 Med-El devices, fi ve Cochlear Corporation devices and two Advanced Bionics devices. Because of this skewed distribution, we did not attempt to assess for the impact of device brand on hearing preservation ability.
Table 1.
Patient demographics and threshold shifts. Values are mean ± SEM. The range is in parentheses
| Unmonitored (n = 22) | Monitored (n = 16) | p-value | |
|---|---|---|---|
| Age at time of implantation (years) | 5.1 ± 0.8 (0.8–17.1) | 4.6 ± 0.8 (1.3–15.1) | 0.651 |
| Pre-operative average threshold (dB HL) | 103.5 ± 2.3 (73–119) | 99.7 ± 3.3 (77–113) | 0.341 |
| Post-operative average threshold (db HL) | 114.0 ± 1.4 (100–127) | 98.8 ± 4.9 (73–118) | <0.001 |
| Threshold shift (dB) | 10.5 ± 2.3 (−5–37) | −0.8 ± 2.6 (−18–13) | 0.006 |
| Post-operative average threshold to electrical stimulation when using the cochlear implant (db HL) | 27.4 ± 1.8 (10–47) | 31.3 ± 1.3 (25–40) | 0.134 |
| Time after surgery when post-operative thresholds measured (years) | 1.6 ± 0.2 (0.4–3.2) | 0.6 ± 0.1 (0.1–1.3) | 0.002 |
Pre-operative to post-operative threshold shifts
The post-operative average threshold and the average threshold shifts were statistically larger in patients in the unmonitored cohort (Table 1). On average, there was a 0.8 dB improvement in auditory thresholds after cochlear implantation when monitoring was used and a 10.5 dB worsening if monitoring was not used. In order to verify that the cochlear implants functioned similarly between the cohorts, we also compared their thresholds when electrical (rather than acoustical) stimulation of the cochlea was performed. There was no statistically signifi cant difference between the two cohorts in their threshold of hearing when using the cochlear implant.
One potentially confounding factor is that post-operative thresholds were not routinely measured at the same time point after surgery. Indeed, there was a statistically signifi cant longer time period between surgery and the post-operative threshold measurement in patients who did not undergo monitoring (1.6 years in the unmonitored cohort versus 0.6 years in the monitored cohort). In order to assess whether this affected the results, regression analyses were then performed including all 38 patients together to assess for predictors of hearing preservation and to identify confounding variables.
We performed univariate analyses with the threshold shift as the dependent (outcome) variable (Table 2 – top). The use of intra-operative auditory monitoring and a worse pre-operative average threshold were associated with improved hearing preservation outcomes. Plots of these two associations are shown in Figure 2. Patient age at the time of surgery and the time period after surgery when the postoperative thresholds were measured were not associated with improved hearing preservation. Thus, even though the time of post-operative threshold measurement in the unmonitored cohort was longer, a longer time delay was not associated with worse hearing preservation. This indicates that the time difference was not a sig-nifi cant confounding variable.
Table 2.
Regression analyses to determine predictors of improved hearing preservation
| Beta | p-value | |
|---|---|---|
| Univariate | ||
| Intra-operative auditory monitoring | −11.3 | 0.006 |
| Age at time of implantation | 0.89 | 0.090 |
| Time after surgery when post-operative thresholds measured | 1.23 | 0.561 |
| Pre-operative average threshold | −0.40 | 0.020 |
| Multivariate | ||
| Intra-operative auditory monitoring | −13.2 | <0.001 |
| Pre-operative average threshold | −0.487 | 0.001 |
Figure 2.

Predictors of hearing preservation. (A) Scatter plot of the pre-operative average threshold versus the threshold shift (post-operative minus pre-operative threshold). A regression line is also plotted (R2 = 0.167, p = 0.02). (B) Box plot showing the average threshold shift in the unmonitored and monitored cohorts. These were statistically different (p = 0.006). The boxes contain the 25–75 per cent values and the error bars contain the ten to 90 per cent values. The line within the box is the median value.
In order to verify these fi ndings and assess for interdependent effects of these factors, we performed a multivariate linear regression analysis. Again, the threshold shift was the dependent variable and all four potentially associated variables (use of intra-operative auditory monitoring, patient age, time of post-operative threshold measurement and the pre-operative threshold average) were included in backward stepwise analyses. One by one, variables that did not demonstrate statistically signifi cant associations were removed, and the multivariate regression analysis was then repeated. When completed, only two variables continued to be predictive of hearing preservation (Table 2 – bottom). This again demonstrated that the use of intra-operative monitoring and a higher pre-operative threshold average were associated with improved hearing preservation rates. Importantly, this multivariate analysis also indicates that these two factors were independent of one another.
Intra-operative monitoring threshold shifts
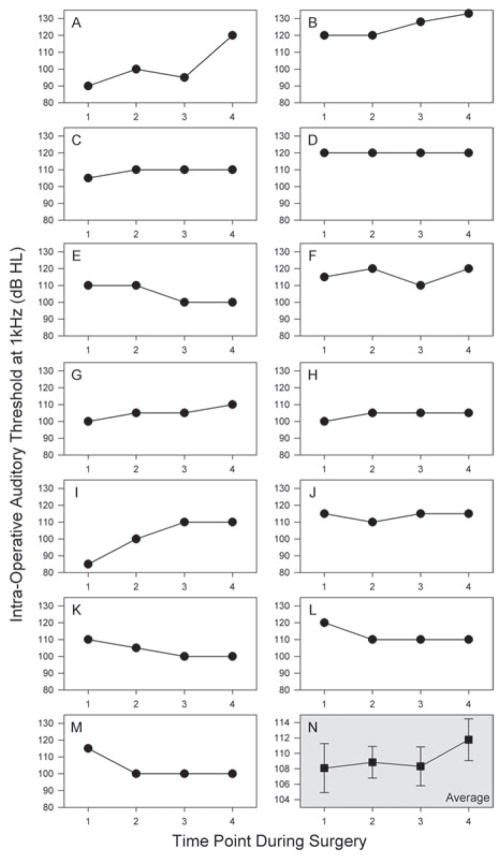
We measured ASSR thresholds just before making the skin incision (baseline), after drilling the mastoid, facial recess and well but before making the cochleos-tomy or opening the round window (before opening cochlea), after making the cochleostomy or opening the round window membrane (after opening cochlea) and after inserting the electrode (after electrode insertion). Intra-operative monitoring data at all four time points was available from 13 patients (Figure 3). The majority of the patients had minimal changes in their ASSR thresholds during the procedure. The average thresholds at each time point confi rm this (baseline: 108.1 dB HL; before opening cochlea: 108.8 dB HL; after opening cochlea: 108.3 dB HL; after insertion: 112.7 dB HL). There were no statistically signifi cant differences between any of these values (ANOVA, p = 0.6).
Figure 3.
Intra-operative thresholds. (A–M) Individual data from all 13 patients in whom thresholds could be measured at all four time points (1 – baseline, 2 – before opening cochlea, 3 – after opening cochlea and 4 – after electrode insertion) are shown. Most patients had relatively little change during the surgery, although some demonstrate threshold elevations, and some even demonstrate mild threshold improvements. (N) Within the entire cohort, there was no change in the mean thresholds during the surgery (baseline: 108.1 dB HL; before opening cochlea: 108.8 dB HL; after opening cochlea: 108.3 dB HL; after insertion: 112.7 dB HL; p = 0.6). Note the y-axis scale is reduced in this plot.
Each surgeon involved in this research project had performed in excess of 60 cochlear implants before any of the 38 patients were enrolled. Thus even though these were experienced cochlear implant surgeons, ASSR monitoring provided the opportunity to learn how subtle changes in surgical technique can affect hearing preservation. Every surgeon performs a cochlear implant slightly differently, and thus may need to refi ne their technique in different ways. One representative example in which one surgeon learned to improve his technique as a result of intra-operative monitoring is shown.
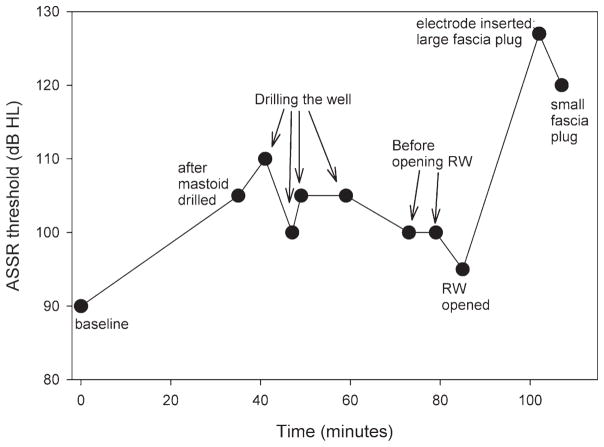
A detailed view of the auditory thresholds measured at many time points during the surgery from the patient in Figure 3A is presented in Figure 4. These data demonstrated to us how blood and irrigation fl uid in the middle ear cleft can affect ASSR thresholds. During the time that the mastoid and well were being drilled (drilling the well, before opening the round window), the middle ear fi lled with irrigation fl uid and blood clot which was unrecognised. Once we realised this, aspirated the fl uid and then opened the round window, thresholds decreased and were closer to baseline. We now always take care to suction the middle ear and hypotympanic regions before making an intra-operative ASSR measurement, and we take our measurements at the same time points during the surgery. Basically, the large impact of middle ear fl uid on threshold levels prevents us from using continuous ASSR monitoring during surgery.
Figure 4.
Intra-operative thresholds from one patient in whom hearing preservation was sub-optimal. This is the patient from Figure 3A. Thresholds were elevated while drilling the mastoid and the well because irrigation fl uid fi lled the middle ear space. When we carefully suctioned out all of the fl uid from the middle ear cavity and opened the round window (RW opened), the threshold returned to within 5 dB of baseline. Inserting the electrode array and placing a large piece of fascia around the electrode produced a very large threshold shift, which was partially reduced by exchanging the fascia for a smaller piece.
Another potential issue related to the fascia around the electrode entry site was identifi ed during this procedure. While the threshold was 95 dB after opening the round window, after inserting the electrode (Med-El device) and wrapping a piece of fascia around the entry site, the threshold increased to 127 dB. On careful inspection, it was noted that the fascia was contacting the stapes suprastructure and the tympanic membrane (Figure 5). When this was removed and a smaller piece of fascia placed so as to not contact the stapes, the threshold decreased to 120 dB. Thus, even though inserting the electrode led to a substantial threshold increase in this patient for unclear reasons, auditory thresholds were also affected by an indelicate placement of the fascia plug. We now take extreme care in placing the fascia so that the electrode entry junction is completely covered, but that the stapes and tympanic membrane are not in contact with the fascia.
Figure 5.
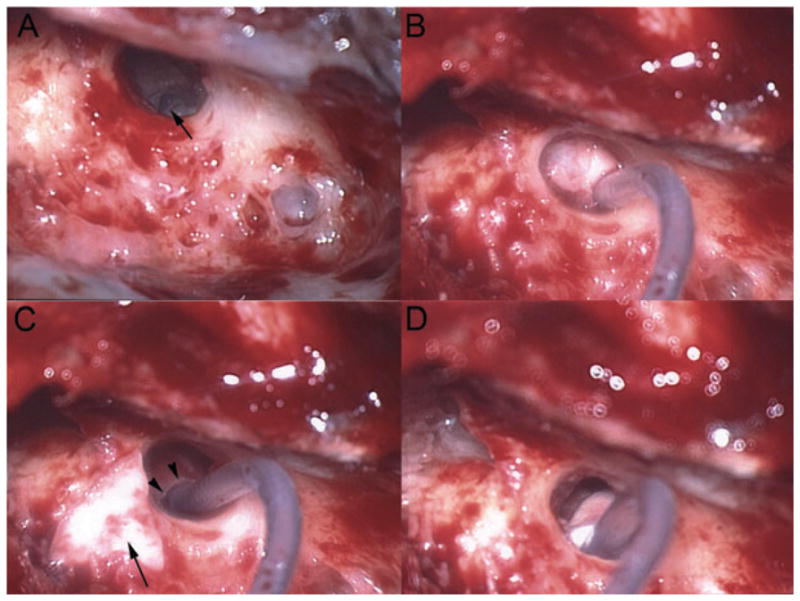
Intra-operative pictures from the patient in Figure 4. This is a right ear. (A) A view of the round window niche and part of the round window membrane (arrow) through the facial recess. (B) A Med-El electrode array was inserted through the opened round window and a large piece of temporalis fascia is seen around the electrode. The fascia also impinged upon the tympanic membrane and the stapes suprastructure. (C) The large fascia has been removed and the smaller, triangular piece of fascia to be used is shown (arrow). Incidentally, the superior portion of the round window niche bone that has been drilled away to provide full exposure of the round window membrane can be noted (arrowheads). (D) View after placing the smaller piece of fascia around the electrode entry zone so that the fascia does not create a conductive hearing loss. This fi gure is available in colour online at www.interscience.wiley.com/journal/cii.
Discussion
While obviously cochlear implant surgery is performed because a patient cannot hear, preservation of residual acoustical hearing preservation suggests maximal preservation of spiral ganglion neurons. Additionally, the concept of combined acoustical and electrical hearing can be entertained (Gantz et al., 2005, 2006). Our data indicate that intra-operative monitoring of cochlear function can be performed during cochlear implant surgery. Additionally, the degree of hearing preservation may be improved when intra-operative monitoring of hearing is performed. We believe that this occurs because providing feedback to the surgeon during the procedure can permit the development of subtle technical improvements that can aid hearing preservation attempts during cochlear implant surgery.
There are limitations to this study. This was not a randomised blinded study, and so there may be bias in the data. Also, all patients studied without intra-operative monitoring were performed fi rst, before we began using ASSR intra-operatively. The reason that this cohort has worse hearing preservation may certainly be due to the learning curve of the surgeons – indeed, this is a key point of the study. Intra-operative monitoring can provide the feedback necessary for surgeons to improve beyond their current abilities. Lastly, while the concept of hearing preservation makes logical sense, whether or not hearing preservation leads to improvements in long-term functional outcomes is unknown at this time.
The concept of intra-operative monitoring of auditory function is not new. Intra-operative ABR monitoring is considered routine when a hearing preservation approach is used for resection of acoustic neuroma or other posterior fossa lesions. Only one case report has demonstrated the use of intra-operative monitoring during cochlear implantation (Adunka et al., 2006). In this case, the cochlear microphonic was monitored in a patient with auditory neuropathy. However, the value of using the cochlear microphonic as a routine technique for intra-operative monitoring during cochlear implant surgery is minimal because this signal cannot be measured in most hearing-impaired patients using routine audiometric techniques.
Our ASSR measurements are made periodically, not continuously, during the surgery, which limits the immediate feedback provided to the surgeon. However, we believe that intra-operative auditory monitoring during cochlear implantation, while not necessarily providing benefit to the patient undergoing surgery that day, does help to improve the surgeon’s technique of hearing preservation over multiple surgeries. We see the primary benefit of intra-operative auditory monitoring for those cochlear implant surgeons currently not focusing on hearing preservation. Thus, like with the use of any type of feedback mechanism, it may stimulate changes that will produce improvements over time. It would not be surprising if the benefi ts of intra-operative auditory monitoring during cochlear implantation decline as the surgeon’s technique further develops.
Based on our experience, several important caveats should be noted when hearing preservation during cochlear implantation is desired. Threshold increases before opening the cochlea are likely due to one of three reasons: fl uid in the middle ear space, damage to the incudal buttress, or drilling on the ossicular chain with transmission of drill vibrations to the inner ear. Both of the last two technical problems can occur during chronic ear surgery and are also sub-optimal when hearing preservation during cochlear implantation is desired. During the operation, the cochlear implant surgeon needs to think specifi cally about hearing preservation, not just the most important issues (preserving the facial nerve and achieving a complete electrode insertion).
In order to preserve hearing when opening the cochlea, we believe that peri-lymphatic preservation is important. The commonly used technique of aspirating the perilymph when removing bone dust from the cochleostomy is traumatic and may lead to a lower rate of hearing preservation. While many animals have a patent cochlear aqueduct and can quickly replace aspirated perilymph, humans typically do not (Marchbanks et al., 1990). Our intra-operative monitoring results demonstrate that opening the cochlea is not necessarily associated with hearing loss. In fact, some patients even had a slight improvement, suggesting that release of the intracochlear pressure alters the bias point of the basilar membrane and hair cell stereocilia. This fi nding has been incidentally noted in animal experiments (Bobbin and Ceasar, 1987).
Exposing the round window membrane or the cochlear endosteum and then cleaning away all of the bone dust, hopefully before opening the cochlea, is not particularly diffi cult to master and in our hands certainly improves perilymphatic preservation. We now routinely use hyaluronic acid to help in this regard. It is also helpful when the endosteum is opened and there is still bone dust present. Placing a dollop of hyaluronic acid on top of the cochleostomy is benefi cial in that it tends to stick to the bone dust but not to the perilymph. A 22 gauge suction can then be used to gently aspirate the hyaluronic acid and bone dust, leaving the perilymph untouched and preserved.
Lastly, the simple act of placing temporalis fascia around the opening of the cochlea at the end of the procedure can produce conductive hearing loss if it contacts the stapes or the tympanic membrane. Thus, particular attention needs to be given at this step. The fascia should be as small as possible while still covering the electrode entry zone.
There are clearly many other issues besides those discussed above that can affect hearing preservation. For example, the site of cochleostomy placement, the angle and force of electrode insertion, the length of electrode inserted and the stiffness of the electrode array are all critical issues. This article does not attempt to assess all of these factors, and instead is simply meant to describe our technique of intra-operative monitoring and how we have used it to make subtle, yet valuable, changes in our surgical technique.
Preservation of residual hearing is of paramount importance when using a short electrode for use in patients with steeply sloping hearing loss and the average threshold increase in low frequencies (1000 Hz and below) with the use of this electrode has been reported to be 9 dB (Gantz et al., 2005). It is important to note that our patients had substantially worse hearing pre-operatively and our data indicate that better pre-operative hearing levels are associated with larger threshold elevations. Thus, it is not appropriate to compare outcomes in our patients with severe-to-profound hearing loss undergoing implantation with full-length electrodes to those with steeply sloping losses undergoing implantation with a short electrode.
Conclusions
Intra-operative auditory threshold monitoring is feasible during cochlear implantation using ASSR audiometry. Preservation of residual acoustical hearing after cochlear implantation may be improved.
Contributor Information
JOHN S OGHALAI, The Hearing Center at Texas Children’s Hospital, Houston, Texas, USA. The Bobby R. Alford Department of Otolaryngology – Head and Neck Surgery, Baylor College of Medicine, Houston, Texas, USA. Department of Neuroscience, Baylor College of Medicine, Houston, Texas, USA. Department of Bioengineering, Rice University, Houston, Texas, USA.
ROSS TONINI, The Hearing Center at Texas Children’s Hospital, Houston, Texas, USA.
JAMIE RASMUS, The Hearing Center at Texas Children’s Hospital, Houston, Texas, USA.
CLAUDIA EMERY, The Hearing Center at Texas Children’s Hospital, Houston, Texas, USA.
SPIROS MANOLIDIS, The Bobby R. Alford Department of Otolaryngology – Head and Neck Surgery, Baylor College of Medicine, Houston, Texas, USA.
JEFFREY T VRABEC, The Bobby R. Alford Department of Otolaryngology – Head and Neck Surgery, Baylor College of Medicine, Houston, Texas, USA.
JOANN HAYMOND, The Bobby R. Alford Department of Otolaryngology – Head and Neck Surgery, Baylor College of Medicine, Houston, Texas, USA.
References
- Adunka O, Roush P, Grose J, Macpherson C, Buchman CA. Monitoring of cochlear function during cochlear implantation. Laryngoscope. 2006a;116:1017–1020. doi: 10.1097/01.mlg.0000217224.94804.bb. [DOI] [PubMed] [Google Scholar]
- Adunka OF, Pillsbury HC, Kiefer J. Combining perimodiolar electrode placement and atraumatic insertion properties in cochlear implantation – fact or fantasy? Acta Otolaryngol. 2006b;126:475–482. doi: 10.1080/00016480500437393. [DOI] [PubMed] [Google Scholar]
- Bobbin RP, Ceasar G. Kynurenic acid and gamma-D-glutamylaminomethylsulfonic acid suppress the compound action potential of the auditory nerve. Hear Res. 1987;25:77–81. doi: 10.1016/0378-5955(87)90081-5. [DOI] [PubMed] [Google Scholar]
- Briggs RJ, Tykocinski M, Stidham K, Roberson JB. Cochleostomy site: implications for electrode placement and hearing preservation. Acta Otolaryngol. 2005;125:870–876. doi: 10.1080/00016480510031489. [DOI] [PubMed] [Google Scholar]
- Cohen NL. Cochlear implant soft surgery: fact or fantasy? Otolaryngol Head Neck Surg. 1997;117:214–216. doi: 10.1016/s0194-5998(97)70176-1. [DOI] [PubMed] [Google Scholar]
- Cone-Wesson B, Dowell RC, Tomlin D, Rance G, Ming WJ. The auditory steady-state response: comparisons with the auditory brainstem response. J Am Acad Audiol. 2002a;13:173–187. [PubMed] [Google Scholar]
- Cone-Wesson B, Parker J, Swiderski N, Rickards F. The auditory steady-state response: full-term and premature neonates. J Am Acad Audiol. 2002b;13:260–269. [PubMed] [Google Scholar]
- Cone-Wesson B, Rickards F, Poulis C, Parker J, Tan L, Pollard J. The auditory steady-state response: clinical observations and applications in infants and children. J Am Acad Audiol. 2002c;13:270–282. [PubMed] [Google Scholar]
- Cullen RD, Higgins C, Buss E, Clark M, Pillsbury HC, 3RD, Buchman CA. Cochlear implantation in patients with substantial residual hearing. Laryngoscope. 2004;114:2218–2223. doi: 10.1097/01.mlg.0000149462.88327.7f. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, John MS, Van Roon P, Purcell DW, Adamonis J, Ostroff J, Nedzelski JM, Picton TW. Estimating the audiogram using multiple auditory steady-state responses. J Am Acad Audiol. 2002;13:205–224. [PubMed] [Google Scholar]
- Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol. 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE. Acoustic plus Electric Speech Processing: Preliminary Results of a Multicenter Clinical Trial of the Iowa/Nucleus Hybrid Implant. Audiol Neurootol. 2006;11(Suppl 1):63–68. doi: 10.1159/000095616. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW. Combining acoustic and electrical hearing. Laryngoscope. 2003;113:1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Haggard MP, Pullan CR. Staffi ng and structure for paediatric audiology services in hospital and community units. British Journal of Audiology. 1989;23:99–116. doi: 10.3109/03005368909077828. [DOI] [PubMed] [Google Scholar]
- Hochmair I, Arnold W, Nopp P, Jolly C, Muller J, Roland P. Deep electrode insertion in cochlear implants: apical morphology, electrodes and speech perception results. Acta Otolaryn-gol. 2003;123:612–617. [PubMed] [Google Scholar]
- Hodges AV, Schloffman J, Balkany T. Conservation of residual hearing with cochlear implantation. Am J Otol. 1997;18:179–183. [PubMed] [Google Scholar]
- Madell JR. Behavioral evaluation of hearing in infants and young children. New York: Thieme; 1998. [Google Scholar]
- Marchbanks RJ, Reid A. Cochlear and cerebrospinal fl uid pressure: their inter-relationship and control mechanisms. Br J Audiol. 1990;24:179–187. doi: 10.3109/03005369009076554. [DOI] [PubMed] [Google Scholar]
- Marrinan MS, Roland JT, JR, Reitzen SD, Waltzman SB, Cohen LT, Cohen NL. Degree of modiolar coiling, electrical thresholds, and speech perception after cochlear implantation. Otol Neurotol. 2004;25:290–294. doi: 10.1097/00129492-200405000-00015. [DOI] [PubMed] [Google Scholar]
- Nadol JB, JR, Shiao JY, Burgess BJ, Ketten DR, Eddington DK, Gantz BJ, Kos I, Montandon P, Coker NJ, Roland JT, JR, Shallop JK. Histopathology of cochlear implants in humans. Ann Otol Rhinol Laryngol. 2001;110:883–891. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- Picton TW, Durieux-Smith A, Champagne SC, Whittingham J, Moran LM, Giguere C, Beauregard Y. Objective evaluation of aided thresholds using auditory steady-state responses. J Am Acad Audiol. 1998;9:315–331. [PubMed] [Google Scholar]
- Rance G, Briggs RJ. Assessment of hearing in infants with moderate to profound impairment: the Melbourne experience with auditory steady-state evoked potential testing. Ann Otol Rhinol Laryngol Suppl. 2002;189:22–28. doi: 10.1177/00034894021110s505. [DOI] [PubMed] [Google Scholar]
- Rance G, Rickards F. Prediction of hearing threshold in infants using auditory steady-state evoked potentials. J Am Acad Audiol. 2002;13:236–245. [PubMed] [Google Scholar]
- Rizer FM, Arkis PN, Lippy WH, Schuring AG. A postoperative audiometric evaluation of cochlear implant patients. Otolaryngol Head Neck Surg. 1988;98:203–206. doi: 10.1177/019459988809800304. [DOI] [PubMed] [Google Scholar]
- Roland JT., JR A model for cochlear implant electrode insertion and force evaluation: results with a new electrode design and insertion technique. Laryngoscope. 2005;115:1325–1339. doi: 10.1097/01.mlg.0000167993.05007.35. [DOI] [PubMed] [Google Scholar]
- Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11–30. doi: 10.1159/000094642. [DOI] [PubMed] [Google Scholar]
- Scarpidis U, Madnani D, Shoemaker C, Fletcher CH, Kojima K, Eshraghi AA, Staecker H, Lefebvre P, Malgrange B, Balkany TJ, Van De Water TR. Arrest of apoptosis in auditory neurons: implications for sensorineural preservation in cochlear implantation. Otol Neurotol. 2003;24:409–417. doi: 10.1097/00129492-200305000-00011. [DOI] [PubMed] [Google Scholar]
- Skarzynski H, Lorens A, D’Haese P, Walkowiak A, Piotrowska A, Sliwa L, Anderson I. Preservation of residual hearing in children and post-lingually deafened adults after cochlear implantation: an initial study. ORL J Otorhinolaryngol Relat Spec. 2002;64:247–253. doi: 10.1159/000064134. [DOI] [PubMed] [Google Scholar]
- Tykocinski M, Cohen LT, Pyman BC, Roland T, JR, Treaba C, Palamara J, Dahm MC, Shepherd RK, XUJ, Cowan RS, Cohen NL, Clark GM. Comparison of electrode position in the human cochlea using various perimodiolar electrode arrays. Am J Otol. 2000;21:205–211. doi: 10.1016/s0196-0709(00)80010-1. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Brown CJ, Gienapp BA, Schmidt Clay KM. Comparison of auditory steady-state response and auditory brainstem response thresholds in children. J Am Acad Audiol. 2002;13:227–235. quiz 283–284. [PubMed] [Google Scholar]
- Wardrop P, Whinney D, Rebscher SJ, Luxford W, Leake P. A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. II: Comparison of Spiral Clarion and HiFocus II electrodes. Hear Res. 2005;203:68–79. doi: 10.1016/j.heares.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Yao WN, Turner CW, Gantz BJ. Stability of low-frequency residual hearing in patients who are candidates for combined acoustic plus electric hearing. J Speech Lang Hear Res. 2006;49:1085–1090. doi: 10.1044/1092-4388(2006/077). [DOI] [PubMed] [Google Scholar]