Abstract
A highly efficient chemical ligation was developed for quantitative conjugation of PNA with DNA (PNA or peptide) using the copper-catalyzed azide-alkyne cycloaddition reaction. While PNAs with an alkyne at the C-terminus and an azide at the N-terminus have been used, an efficient click-click reaction occurs. The PNA click ligation is sequence-specific and capable of single nucleotide discrimination.
Keywords: Peptide Nucleic Acids, Chemical Ligation, Click Chemistry, Single Nucleotide Discrimination, DNA detection
Introduction
Peptide nucleic acids (PNAs) are nuclease resistant DNA mimics in which the sugar-phosphate backbone has been replaced by a pseudo-peptide backbone (Figure 1).[1,2] PNA binding to complementary DNA or RNA shows higher stability, greater specificity, and a faster rate than DNA binding.[3-5] Due to these properties, PNAs are currently of considerable interest as potential reagents for antisense/antigene therapy, molecular diagnostics, biosensors, molecular biology, and nanotechnology.[6-8] However, the biological applications of PNAs are limited by their poor water solubility, tendency to self-aggregation, and low cell penetration ability. One of the approaches for improving physico-chemical and biological properties is to synthesize PNA-DNA [9] or PNA-peptide conjugates.[10] As the standard methods for the synthesis of DNA (or RNA) and PNA are incompatible, complex modifications of PNA monomers and synthetic protocols were required for the construction of PNA conjugates. Furthermore, during chain elongation aggregation of the growing oligomer chain can be caused by either intra- or intermolecular interactions. This can lead to low coupling efficiencies. Thus, postsynthetic conjugation (e.g. ligation) must be considered as a route to PNA conjugates or longer PNAs. Recently, native chemical ligation and EDC-mediated ligation have been developed for conjugating PNA with DNA, PNA, or peptide in the Seitz’s and Orgel laboratories.[11-13] PNA ligation has also been used for single nucleotide polymorphisms detection in ligation-based chemical approaches.[12,13] However, there is considerable scope for developing more efficient methods for PNA conjugation for its outstanding applications.
Figure 1.
Template-directed click-click-ligation of DNA/PNAs: (a) schematic structure and (b) chemical structure at ligation point.
Ideally, the chemistry for postsynthetic modification would be clean, fast, high-yielding, and operate under mild conditions. The copperI – catalyzed Huisgen cycloadditions of azides and alkynes, the most prominent example of click chemistry,[14] appears to fulfill all the necessary requirements. It functions efficiently in aqueous media and has been proved to be a powerful method for the postsynthetic DNA modification.[15] It has previously been used for the preparation of surface-immobilized DNA,[16] DNA-protein conjugates,[17] cyclic peptide structures,[18] DNA cross-linking and DNA labeling.[19, 20] Recently, the click reaction has been used for template-directed DNA ligation, covalent intramolecular DNA circularization and catenation in the Brown’s laboratory.[21]
Here we report a convenient, versatile, and highly efficient method for sequence-specific conjugation of PNA with DNA using template-directed click ligation, which is capable of single nucleotide discrimination (Figure 1). This method is also useful for the P N A-PNA and PNA-peptide ligation. When a PNA was modified with an azide at N-terminus and an alkyne at the C-terminus, an efficient click-click reaction occurs. This enables the preparation of extended PNA sequences. The ligation reaction is characterized by fast, clean, mild reaction conditions, and by its tolerance of a broad range of functional groups.
Results and Discussion
Azide-containing oligodeoxynucleotides (ODN) were prepared by labeling 5′-bromo-modified ODN with NaN3 in DMF in the presence of NaI.[22] PNAs containing an alkyne at the N-terminus were synthesized using Fmoc-L-propargylglycine (Supporting information). DNA template-directed ligations were performed using a 3′-[32P]-labeled DNA-substrate (ODN-1 or 2), a DNA template (ODN-3 or 4), and a PNA substrate (PNA-5 or 6) (Table 1). All ligation reactions were carried out in 100 mM NaCl and 10 mM potassium phosphate buffer to ensure complete formation of DNA/PNA duplex with template. Duplex stability of PNA-5•ODN-3 (Tm = 62.5 ± 1.5 °C) and PNA-6•ODN-3 (Tm = 63.2 ± 1.3 °C) was determined by UV-melting analysis. Each ligation reaction was studied with 50 nM of 3′-[32P]-labeled DNA substrate and different concentrations of PNA substrate in the presence or absence of DNA template.
Table 1.
Oligonucleotide sequences used for this study.
| code | Sequences |
|---|---|
| ODN-1 | 5′-dN3(CH2)6 GA TTG CGG TAG TGA TGG A |
| ODN-2 | 5′-dN3(CH2)6GCA CGC GTC G |
| ODN-3 | 5′-dTCCATCACTACCGCAATCAGGCCAGATC |
| ODN-4 | 5′-dCGA CGC GTG C AG GCC AGA TCA GGC CAG ATC AGG CCA GAT CAG GCC AGA TC |
| PNA-5 | H-(Lys)GATCTGGCCT(Propargylglycine) |
| PNA-6 | N3CH2CO(Lys)GATCTGGCCT(Propargylglycine) |
| [a] 7 | ODN-1-L-PNA-5 |
| Peptide-8 | N3CH2CO(Lys)6 |
| [a] 9 | PNA-5-L-peptide-8 |
| [a] 10 | ODN-2-(L-PNA-6)4 |
| PNA-15 | H-(Lys)GGT CAG AG (Propargylglycine) |
| 16a-d | 5′-dGGC GGC ATG ACTCXGACC (a: X = T; b: X = A; c: X = C; d: X = G) |
| ODN-17 | 5′-dCGA CGC GTG CAG GCC AGA TC |
L: Triazole linker
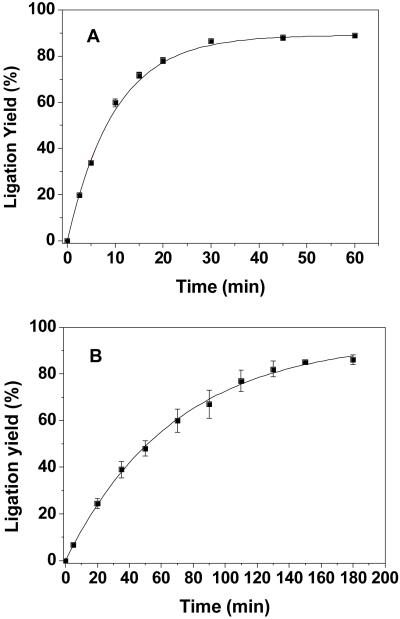
The ligation conditions were optimized using substrates ODN-1, PNA-5, and the template ODN-3. The effect of CuI-catalyst/ligand, template, and concentration of PNA on the ligation reaction was investigated. The ligation product was not observed without the CuI-catalyst (Figure 2, lane 1). In the presence of CuI-catalyst, 50% ligation yield was obtained. Unfortunately considerable degradation of DNAs and DNA-PNA ligation products occurred. Indeed, we observed 72% DNA degradation within 30 min and 98% within 1 h (Figure 2, lanes 2 and 3) (Supporting information). This problem has been encountered previously in other laboratories.[21, 23] However, addition of the water-soluble tris-triazolylamine CuI-binding ligand[21, 24] greatly reduced degradation and increased the ligation yield (Figure 2, lanes 4 and 5). When 10-fold excess of ligand relative to CuI was employed almost no decomposition was observed and a near quantitative conversion to the DNA-PNA conjugate 7 was observed by denaturing PAGE as well as by HPLC (Supporting information). The ligation reaction did not depend on PNA concentration and was highly efficient using equimolar ratios of DNA and PNA substrates (50 nM). The ligation reaction is completed within 1 h at room temperature. The rate of conjugate 7 formation at 25 °C in the presence of template (ODN-3) followed first order kinetics (kICL = 1.7 ± 0.5 × 10−3 s−1; t1/2 = 7 min) (Figure 3A). DNA-PNA conjugate 7 was purified by gel electrophoresis, and characterized by ESI-Mass ([M + H+] calcd 8780.9, found, 8780.0) (Supporting information).
Figure 2.
Phosphorimage autoradiogram of denaturing PAGE analysis of the click ligation reaction with ODN-1 and PNA-5 under different conditions (lanes 1,3,5-7: the reactions were carried out at r.t. for 60 min; lanes 2,4: the reactions were carried out at r.t. for 30 min; Lanes 1-5: [PNA] = [DNA substrate] = 50 nM; Lane 6: [PNA] = 20 × [DNA substrate] = 1.0 μM; Lane 7: [PNA] = 100 × [DNA substrate] = 5.0 μM).
Figure 3.
Rate of ligation product formation from ODN-1 and PNA-5 (A) in the presence of template ODN-3 ([ODN-3] = [ODN-1] = [PNA-5] = 50 nM) or (B) in the absence of template ([ODN-1] = 50 nM, [PNA-5] = 5.0 μM).
In the absence of template no significant ligation (< 2%) was observed when the PNA concentration was below 1.0 μM ([DNA] = 50.0 nM) (Figure 2, lane 6). However, the increase of PNA concentration enhanced the ligation yield. At high PNA concentration (> 5.0 μM) 80% of DNA formed a PNA conjugate. The rate of click ligation between ODN-1 and PNA-5 without template is approximately one order of magnitude slower than in the presence of template (kICL = 2.44 ± 0.3 × 10−4 s−1; t1/2 = 47 min) (Figure 3B). Apart from the formation of DNA-PNA conjugates, a byproduct (DNA*) was observed during click ligation reaction (Figure 2, lanes 4-7). This compound is most likely caused by the side reaction between DNA and ligand as it was not observed in the absence of ligand (Figure 2, lane 2 and 3). The non-template-directed click ligation was applied for the conjugation of PNA and peptide. The reaction of PNA-5 and peptide-8 in the presence of ligand/CuSO4/Na-ascorbate yielded the PNA-peptide conjugate 9 that was characterized by ESI-mass (Supporting information).
The template-dependence of the DNA-PNA click ligation indicated that the efficiency of click reaction depended on the duplex formation. Further investigation showed that this approach was capable of detecting DNA single-nucleotide polymorphism. To determine the sequence selectivity, the ligation reaction of PNA-11 and ODN-12 was carried out with DNA targets 13a-d (Scheme 1). The template contained sequences from the region around codon 248 in exon 7 of the p53 gene that is often mutated in human cancer.[25] Hybridization of PNA-11 and ODN-12 to the fully complementary target 13a followed by click ligation led to a near quantitative formation of ligation product 14 (92%) within 1 h (Figure 4, lane 2). However, in the presence of the mutant targets 13b-d, all of which contain a mismatched base at a central position in the PNA complementary region, only traces of the ligation product (< 2%) were detected by denaturing PAGE (Figure 4, lane 3, 4, and 5). UV-melting analysis of PNA:DNA duplexes showed that the PNA:mismatched DNA hybrids were much less stable (Tm value < 19 °C when X = T, A, C versus 49 °C when X = G, the matched base). We did observe a low level of product formation with the mismatched templates. However since the same amount of ligation was observed in the absence of target (Figure 4, lane 1), it appears most likely that it was due to a template-independent background reaction. In order to assess the generality of the sequence specificity, the click ligation was performed with a different PNA probe (PNA-15), the DNA templates 16a-d, and ODN-12. Similar results were observed. An efficient ligation (90%) was obtained in the presence of a fully complementary target 16a and less than 2% of the ligation product was formed in the presence of the mutant targets 16b-d (Supporting information). The sequence selective reaction argues that a PNA-based click ligation system is a promising method for detecting DNA sequences capable of single nucleotide discrimination. The yields provided by the click reaction were almost quantitative. The reaction is characterized by fast, clean, mild reaction conditions, and by its high selectivity.
Scheme 1.
Single-nucleotide-specific click ligation of PNA 11 and ODN 12. DNA targets 13a-d differ in one central position (highlighted in bold, 13a: X = G; 13b: X = T; 13c: X = A; 13d: X = C). The PNA base opposite to the mutation site is also in bold. Concentration of ligation probes 11, 12, and target DNA 13a-d: 50 nM.
Figure 4.

Phosphorimage autoradiogram of denaturing PAGE analysis of the single-nucleotide-specific click ligation of PNA 11 and DNA 12 in the presence of DNA templates 13a-d (lane 1: no template; lane 2: 13a; lane 3: 13b; lane 4: 13c; lane 5: 13d) (The ligation reactions were carried out at r.t. for 60 min with 50 nM of PNA and DNA substrate).
Having established the optimum conditions for PNA-DNA click-ligation reaction, the approach was applied to the preparation of extended PNAs using the click-click ligation. The click-click reaction was performed with a 50-mer (ODN-4) template, a DNA substrate (ODN-2), and four equivalents of PNA-6. The template is partly complementary to the 10-mer, ODN-2. The remaining part contains four repeated oligonucleotide sequences (the underlined sequence) that are fully complementary to PNA-6, also a 10-mer. In order to determine the efficiency of the click-click ligation using denaturing PAGE, the 3′-32p-labeled DNA template was used in this experiment. The PNA-6 contains an azide at N-terminus and an alkyne at the C-terminus. After hybridization and the addition of CuI-catalyst/ligand, we observed around 70% of full length ligated product 10 by denaturing PAGE (Figure 5). This indicated a highly efficient click-click ligation. By choosing different template and PNA substrates one can make any length of DNA-PNA and PNA-PNA conjugates. Apart from the main product, a few percent (< 5%) of byproducts were observed, which we attribute to the DNA conjugating to different numbers of PNA-6 (1, 2, 3, and 4 × PNA-6).
Figure 5.
Phosphorimage autoradiogram of denaturing PAGE analysis of the click-click ligation of ODN-2 and PNA-6 in the presence of template ODN-4 (lane 1: ODN-2/ PNA-6/ODN-17 (1:1:1); lane 2: ODN-2/ PNA-6/ODN-4 (1:4:1) (The ligation reactions were carried out at r.t. for 60 min with 50 nM of PNA and DNA substrate).
Conclusions
We have demonstrated an efficient, versatile PNA ligation method using click chemistry, which enables quantitative conjugation of PNA with DNA, PNA, or peptide under mild conditions. The PNA click ligation is template-dependent and is capable of discriminating between templates differing by a single nucleotide. Thus PNA-probes could be used for single nucleotide specific detection of DNA, in hybridization and PCR based protocols. This approach could also be useful for the construction of PNA nanomaterials.
Supplementary Material
Acknowledgments
We are grateful for the financial support of this research by the University of Wisconsin-Milwaukee (UWM start-up fund). This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. We thank Prof. K. K. Surerus for generously sharing her lab space/equipments.
Footnotes
Supporting Information: Experimental details, ESI-MS of oligonucleotides, and phosphorimage autoradiograms.
Supporting information for this article is available on the WWW under http://www.eurjoc.org/ or from the author.
References
- [1].Nielsen PE, Egholm M, Berg RH, Buchardt O. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- [2].Demidov VV, Potaman VN, Frank-Kamenetskii MD, Egholm M, Buchard O, Sönnichsen SH, Nielsen PE. Biochem. Pharmacol. 1994;48:1310–1313. doi: 10.1016/0006-2952(94)90171-6. [DOI] [PubMed] [Google Scholar]
- [3].Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA, Berg RH, Kim SK, Norden B, Nielsen PE. Nature. 1993;365:566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- [4].Egholm M, Nielsen PE, Burchardt O, Berg RH. J. Am. Chem. Soc. 1992;114:9677–9678. [Google Scholar]
- [5].Iyer M, Norton JC, Corey DR. J. Biol. Chem. 1995;270:14712–14717. doi: 10.1074/jbc.270.24.14712. [DOI] [PubMed] [Google Scholar]
- [6].Koh W. In: Peptide Nucleic Acids-Protocol and Applications Nielsen. Nielsen PE, editor. Horizon Bioscience; Norfolk: 2004. [Google Scholar]
- [7].Ali MM, Li Y. Angew. Chem. Int. Ed. 2009;48:3512–3515. doi: 10.1002/anie.200805966. [DOI] [PubMed] [Google Scholar]
- [8].Socher E, Bethge L, Knoll A, Jungnick N, Herrmann A, Seitz O. Angew. Chem. Int. Ed. 2008;47:9555–9559. doi: 10.1002/anie.200803549. [DOI] [PubMed] [Google Scholar]
- [9].a) Uhlmann E, Will DW, Breipohl G, Langner D, Ryte A. Angew. Chem. Int. Ed. 1996;35:2632–2635. [Google Scholar]; b) Finn PJ, Gibson NJ, Fallon R, Hamilton A, Brown T. Nucleic Acid. Res. 1996;24:3357–3363. doi: 10.1093/nar/24.17.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mier W, Eritja R, Mohammed A, Haberkorn U, Eisenhut M. Angew. Chem. Int. Ed. 2003;42:1968–1971. doi: 10.1002/anie.200219978. [DOI] [PubMed] [Google Scholar]
- [11].Koppitz M, Nielsen PE, Orgel LE. J. Am. Chem. Soc. 1998;120:4563–4569. doi: 10.1021/ja974190y. [DOI] [PubMed] [Google Scholar]
- [12].a) Ficht S, Mattes A, Seitz O. J. Am. Chem. Soc. 2004;126:9970–9981. doi: 10.1021/ja048845o. [DOI] [PubMed] [Google Scholar]; b) Ficht S, Dose C, Seitz O. ChemBioChem. 2005;6:2098–2103. doi: 10.1002/cbic.200500229. [DOI] [PubMed] [Google Scholar]
- [13].Mattes A, Seitz O. Angew. Chem. Int. Ed. 2001;40:3178–3181. doi: 10.1002/1521-3773(20010903)40:17<3178::AID-ANIE3178>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- [14].a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; b) Tornøe CW, Christensen C, Meldal M. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- [15].(a) Gramlich PME, Wirges CT, Manetto A, Carell T. Angew. Chem. Int. Ed. 2008;47:8350–8358. doi: 10.1002/anie.200802077. [DOI] [PubMed] [Google Scholar]; (b) Gramlich PME, Warncke S, Gierlich J, Carell T. Angew. Chem. Int. Ed. 2008;47:3442–3444. doi: 10.1002/anie.200705664. [DOI] [PubMed] [Google Scholar]
- [16].Seo TS, Bai X, Ruparel H, Li Z, Turro NJ, Ju J. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5488–5493. doi: 10.1073/pnas.0401138101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Duckworth BP, Chen Y, Wollack JW, Sham Y, Mueller JD, Taton TA, Distefano MD. Angew. Chem. Int. Ed. 2007;46:8819–8822. doi: 10.1002/anie.200701942. [DOI] [PubMed] [Google Scholar]
- [18].Punna S, Kuzelka J, Wang Q, Finn MG. Angew. Chem. Int. Ed. 2005;44:2215–2220. doi: 10.1002/anie.200461656. [DOI] [PubMed] [Google Scholar]
- [19].Kocalka P, EI-Sagheer AH, Brown T. ChemBioChem. 2008;9:1280–1285. doi: 10.1002/cbic.200800006. [DOI] [PubMed] [Google Scholar]
- [20].Sirivolu VR, Chittepu P, Seela F. ChemBioChem. 2008;9:2305–2316. doi: 10.1002/cbic.200800313. [DOI] [PubMed] [Google Scholar]
- [21].Kumar R, Ei-Saheer A, Tumpane J, Lincoln P, Wilhelmsson LM, Brown T. J. Am. Chem. Soc. 2007;129:6859–6864. doi: 10.1021/ja070273v. [DOI] [PubMed] [Google Scholar]
- [22].Lietard J, Meyer A, Vasseur JJ, Morvan F. Tetrahedron Lett. 2007;48:8795–8798. [Google Scholar]
- [23].Kanan MW, Rozenman MM, Sakurai K, Snyder TM, Liu DR. Nature. 2004;431:545–549. doi: 10.1038/nature02920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chan TR, Hilgraf R, Sharpless KB, Fokin V. Org. Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- [25]. The following website catalogues p53 mutations: http://p53.free.fr.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.