Abstract
Two new molecular MRI agents have been approved for clinical use within the last 3 years, and a third agent has completed phase-2 clinical trials. A wealth of preclinical data is also emerging on the general safety of many molecular MR imaging agents. In addition, since the guidelines to avoid nephrogenic systemic fibrosis (NSF) were adopted, at most institutions no new cases of NSF have been reported. Nevertheless, in the post-NSF environment, both those developing and using molecular MR imaging agents need to be increasingly aware of safety issues. This awareness should begin with the design of the agent and, even in early preclinical studies, the demonstration of safety and efficacy should both be given high priority. In this review we discuss some of the issues relevant to the design of safe molecular MR imaging agents and highlight the excellent safety profile of those agents that have been used clinically to date.
Keywords: Molecular Imaging, MRI, Cardiovascular, Safety, Gadolinium, Iron Oxide
Introduction
Molecular MRI of the cardiovascular system is an established preclinical tool and an emerging clinical tool. The technical feasibility of molecular MRI is now beyond question and is widely accepted [1, 2]. However, this was not always the case. The birth of the field was greeted by some, who felt that molecular MRI would lack sensitivity, with a fair degree of skepticism. Over the last 10-15 years, however, those in the molecular MRI community have proved these skeptics wrong. The sensitivity, feasibility and value of molecular MRI have been demonstrated multiple times in the preclinical setting [1-8], and in the clinical setting as well [9-11]. Molecular MRI has been performed using a broad range of platforms and in a broad range of cardiovascular diseases, of which only a few can be covered in this review.
Despite this success, the potential of molecular MRI was once more called into question during the height of the NSF (nephrogenic systemic fibrosis) epidemic. It is now fair to say that the NSF storm has passed [12, 13]. In fact not a single case has been recorded at our institution since the guidelines to avoid the use of gadolium (Gd) chelates in those with renal disease were adopted [13]. Nevertheless valuable lessons have been learnt and must be applied to the ongoing development of molecular MRI. Whether one believes that the policies of the regulatory agencies are excessive or not, the landscape for molecular MRI in 2012 in clearly more complex than it was in 2002. The molecular MRI community has thus moved beyond the mere demonstration of sensitivity and biological value. Studies on the bioeffects, toxicity and long-term elimination of many molecular MRI agents are now routinely reported in the academic literature. This review aims to highlight some of the issues relevant to the design of safe agents for molecular MRI of the cardiovascular system. The reader interested in a more general overview of molecular MRI in the cardiovascular system is referred to several extensive reviews on this topic [1, 2].
Molecular MRI with Small Gd Chelates
Molecular MRI with small Gd chelates is highly appealing from a translational standpoint. The kinetics, biodistribution, toxicity and elimination of these chelates are very well understood, and many have been clinically used over a decade. While the sensitivity of these chelates is limited to the micromolar range, the selection of highly expressed biological targets overcomes this limitation. This strategy has been used to successfully image fibrin [14], collagen [5], elastin [15], martix metalloproteases (MMP) [16], and DNA [6]. All of these targets are present in large amounts and can be targeted with small chemicals or peptides conjugated to the Gd chelate.
From a toxicological standpoint it is important to determine whether the binding of the agent to its target exerts any bioeffects. This concept was very nicely demonstrated by work done with the fibrin-binding agent EP-2104. The binding of the agent to fibrin was shown not to change the stability of clots, the coagulation times of blood or the ability of fibrinolytics to dissolve clots. The agent was thus a pure diagnostic reporter and exerted no physiological effects. It is likewise likely that the elastin and collagen targeted Gd chelates have little impact on the structural integrity of elastin and collagen, but this has yet to be reported. The experience with EP-2104, however, nicely demonstrates that Gd-chelates can be designed to bind to a target of interest at a well-selected site that does not change the activity or properties of the target [14]. This is an important issue in the case of small Gd chelates since the amount of ligand binding needed for detection is significantly higher than with other MR platforms and nuclear imaging.
Only a small fraction of the injected dose of an imaging agent is taken up by the target of interest. The vast majority of the injected agent remains unbound and subject to general elimination. Here a critical distinction must be drawn between the blood half-life of an agent and its elimination half-life. If the unbound agent is taken up rapidly in the kidney or liver, and subsequently retained in these tissues, it will have a short blood half-life but long elimination half-life. It is thus critical to measure the nonspecific uptake of these imaging agents in the liver, kidneys, spleen, fat and other organs. Retention of the unbound agent in these organs is likely to be far more consequential than the possible retention of extremely small amounts of bound agent at the target.
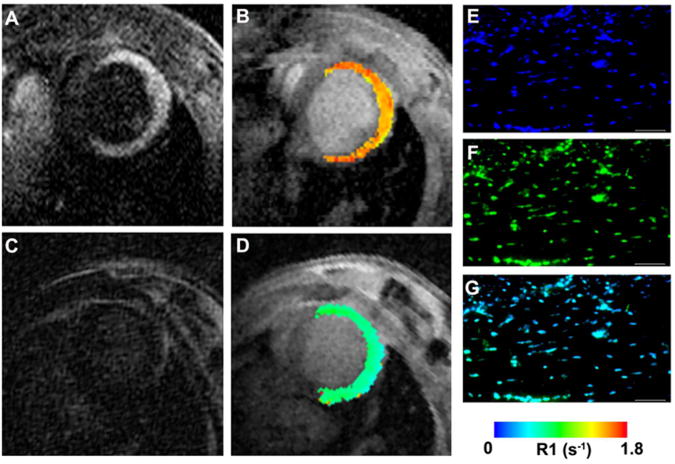
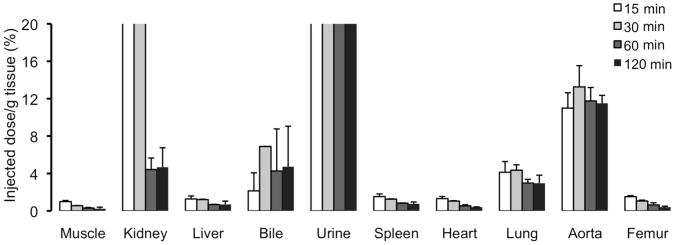
The most widely used technique to measure the elimination of Gd chelates is to measure the biodistribution of elemental Gd in the various organs using ICP-MS (inductively coupled plasma - mass spectrometry). This has been used to measure the elimination of most clinically approved gadolinium chelates, the DNA-targeted chelate Gd-TO [6], a dual MR-PET fibrin-binding chelate [17], and the elastin targeted chelate Gd-ESMA [15]. Gd-TO is a necrosis specific agent that is unable to cross the normal cell membrane. Rupture of the cell membrane, however, allows the agent to access the cell and bind to the large amounts of DNA in the nucleus (Figure 1) [6]. 24 hours after injection into mice with myocardial infarction, however, only 4% of Gd-TO remained in the body (Table 1), principally in the liver [6]. While data at additional time points are needed, the 24-hour data indicate that Gd-TO is well eliminated. Likewise, biodistribution studies showed that Gd-ESMA accumulated in the target of interest (aortic elastin) but was otherwise well and rapidly cleared from the body (Figure 2) [15].
Figure 1.
Molecular MRI of cardiomyocyte necrosis with the DNA-binding agent Gd-TO. (A, B) Infarcted mouse imaged 2 hours after the injection of Gd-TO. (C, D) Infarcted mouse imaged 2 hours after the injection of Gd-DTPA. While Gd-DTPA has washed out of the infarct, Gd-TO has bound to the exposed DNA of the necrotic cells. This manifests as an increase in signal intensity on the T1 weighted image (A) and an increase in the longitudinal relaxation rate R1 (B). (C, D) No increases in signal intensity or R1 are seen in the mouse injected with Gd-DTPA. Fluorescence microscopy of the excised heart confirms the binding of Gd-TO to DNA in the infarct. (E) DAPI, (F) Gd-TO, (G) fused image. Reproduced with permission [6].
Table 1.
Inductively coupled plasma mass spectrometry (ICP-MS) 24 hours after the injection of Gd-TO. ID = injected dose.
| Organ | %ID per g | %ID per organ |
|---|---|---|
| Heart* | 0.0102 ± 0.0037 | 0.0012 ± 0.0004 |
| Spleen | 0.115 ± 0.0339 | 0.0091 ±0.0027 |
| Stomach | 0.0916 ± 0.0268 | 0.0291 ± 0.0085 |
| Liver | 2.74 ± 0.3809 | 2.63 ± 0.3655 |
| Kidneys | 0.784 ± 0.1739 | 0.206 ± 0.0457 |
| Intestines | 0.493 ± 0.3248 | 0.774 ± 0.5101 |
| Lungs | 0.0299 ± 0.0075 | 0.0040 ± 0.0001 |
| Bone | 0.0409 ± 0.0047 | 0.0819 ± 0.0094 |
| Brain | <0.006 | <0.002 |
| Blood | <0.02 | <0.03 |
| Pancreas | <0.005 | <0.0008 |
It should be noted that half the animals studied with ICP-MS had infarcts greater than 72 hours in duration at the time of Gd-TO injection. Taken from Huang et al [6].
Figure 2.
Biodistribution of Gd-ESMA in mice determined by inductively coupled plasma mass spectrometry (ICP-MS). Within 120 minutes of injection (black bars) the agent has been largely eliminated from the body. Excretion is predominanty renal with a small component of hepatic (biliary) excretion too. Retention of the agent is seen in tissues with a high content of elastic fibers, in particular the aorta. Reproduced with permission [15].
Several additional strategies relevant to small Gd chelates merit discussion. The first involves controlled/reversible binding of the chelate to the target. This strategy is appealing in cases where the target is so highly and widely expressed that the potential exists for large amounts of the chelate to collect at the target site. A good example of this approach is the reversible binding of gadofosveset to serum albumin [18-20]. Irreversible (covalent) binding would result in massive retention of Gd in the intravascular space. Gadofosveset, however, has moderate reversible affinity for human serum albumin (Kd = 85 μM) and this results in an equilibrium of the albumin-bound and unbound fractions, with ~10-20% unbound [18-20]. When the agent dissociates from albumin it is renally excreted, causing further dissociation of the bound fraction from albumin. A related approach involves the design of agents in which the chelate detaches from the ligand over time. This allows the detached chelate to be renally excreted and the ligand to be metabolized by peptidases or other degradative enzymes.
The ability of molecular MRI to detect probe activation in vivo is an appealing strength. Iron oxide particles have been extensively used for ex vivo and in vitro diagnostic applications. In vivo, the myeloperoxidase (MPO) activatable chelate Gd-MPO has been used to image peroxidase activity in the myocardium and arterial wall [21]. Activation of the probe by MPO radicalizes the tyrosine residues on the chelate causing the agent to form dimers and oligomers with higher longitudinal relaxivity. The activated probe can also bind to tyrosine residues on the surface of proteins but these bonds are rapidly cleaved by proteases. Exposure of cells in vitro to MPO-Gd did not affect their viability. The agent has also been shown to be more resistant to transmetalation than Gd-DTPA and has been shown to be >90% eliminated within 6 hours of injection [22].
While further work is needed, a clear picture on the elimination and safety of targeted Gd chelates is emerging. Most of these agents are rapidly removed from the blood and efficiently excreted by the kidneys. Clinical experience with gadofosveset and EP-2104R has shown no acute bioeffects or signs of toxicity. Safety and elimination, however, will obviously need to be demonstrated on a case-by-case basis. Nevertheless, based on current data, it is not unreasonable to predict that with appropriate design targeted Gd-chelates will have a similar risk profile to that of the currently used untargeted chelates.
Molecular MRI with Iron Oxide
Ferumoxides and Ferumoxtran
Iron-oxide nanoparticles have been used extensively in clinical medicine and have an excellent safety record. These nanoparticles consist of a core of superparamagnetic iron-oxide, which can be coated by a variety of substances such as starch, citrate, dextran and carboxymethyl-dextran [23]. In this form, iron-oxide nanoparticles are most frequently used to image tissue macrophage infiltration and inflammation [10, 24]. Three questions must be addressed when these agents are used to image macrophages: 1) Will the coating of the nanoparticle cause an acute allergic reaction during intravenous injection, 2) will the material coating the nanoparticle prevent its breakdown and, 3) assuming complete breakdown of the nanoparticle, is there any danger of iron overload from the released iron.
The first generation of iron-oxide nanoparticles included ferumoxides (Feridex, Advanced Magnetics, Lexington MA) and Ferucarbotran (Resovist, Schering, Berlin). These agents form polydisperse aggregates in solution, which are rapidly removed from circulation by the Kupfer cells in the liver. Serial MRI of the liver revealed that the superparamagnetic crystal of Feridex is broken down into non-magnetic forms of iron within 1-2 days [25]. The incorporation of 59Fe, which has a radioactive decay half-life of over 40 days, into feridex further showed that the degraded iron is released from the liver within 3-4 days and incorporated into the general iron pool in the body, where it is either excreted or used to make hemoglobin [25]. Thousands of patients have been injected with these agents at the approved dose of 2.7 mg Fe/kg, and their safety profile is excellent.
Feridex has been used in cardiovascular imaging principally to label cells in vitro and ex vivo. In a recent first-in-human report, for instance, feridex was used to label peripheral blood monocytes ex vivo [26]. The labeled monocytes were then re-injected intravenously and shown by MRI to traffic to sites of active inflammation, induced by tuberculin skin testing. Extensive safety testing was performed in this study. Ex vivo labeling of the monocytes did not affect their viability or function in any significant way. In addition, no signs of either local or systemic toxicity were seen in the volunteers injected with the labeled monocytes [26].
Monocytes, macrophages and reticulo-endothelial cells in general have the intracellular machinery to degrade iron-oxide nanoparticles. Identical pharmacokinetics and toxicity profiles cannot be assumed when cells other than these are loaded with iron-oxide nanoparticles. The largest experience to date in this regard has involved the loading of mesenchymal stem cells with feridex. Extensive work from several groups has shown that feridex loading does not affect cell viability and the ability to differentiate into most cell types [27]. In addition, no signs of systemic toxicity were observed when the labeled cells were injected into small animal models [28].
The second generation of iron-oxide nanoparticles have thicker dextran coats than feridex and thus remain monodisperse in solution. Ferumoxtran is the most studied agent in this class and has been used in numerous clinical studies to image lymph node metastases [29], and carotid plaque inflammation [10]. Allergic reactions to ferumoxtran are rare, but to lower this adverse event rate even further, the agent is administered by slow infusion. The circulation half-life of ferumoxtran is long (24 hours in humans) but it is ultimately taken up by monocytes and the cells in the reticuloenthothelial system and metabolized much like feridex. Repeat dosing may in theory increase the risk of allergic reactions slightly and obviously raises the question of iron overload. The experience in the Atheroma study, however, is very instructive in this regard. Patients in this study were treated with either 10 or 80 mg of Atorvostatin and carotid plaque inflammation was assessed after the injection of ferumoxtran at baseline, at week 6, and 12 weeks after the initiation of therapy [10]. The administration of 3 doses of the agent in a 3-month period was well tolerated without any adverse effects.
Ferumoxytol and Targeted Agents
Ferumoxytol (Advanced Magnetics) is a third generation iron-oxide nanoparticle that is coated with carboxy-methyl dextran. The incidence of allergic reactions to feramoxytol is even lower than ferumoxtran, allowing the agent to be given as a single intravenous bolus. The kinetics of feromoxytol are similar to those of ferumonxtran but its blood half in humans is approximately 15 hours.
Ferumoxytol is FDA-approved and was recently used in patients in the setting of acute myocardial infarction [30]. Patients with recent STEMI were injected with the agent, which was used to image postinfarction myocardial inflammation (Figure 3). Serial T2* weighted imaging was performed in the heart, but also in the liver and spleen [30]. No adverse effects were seen from the administration of the agent.
Figure 3.
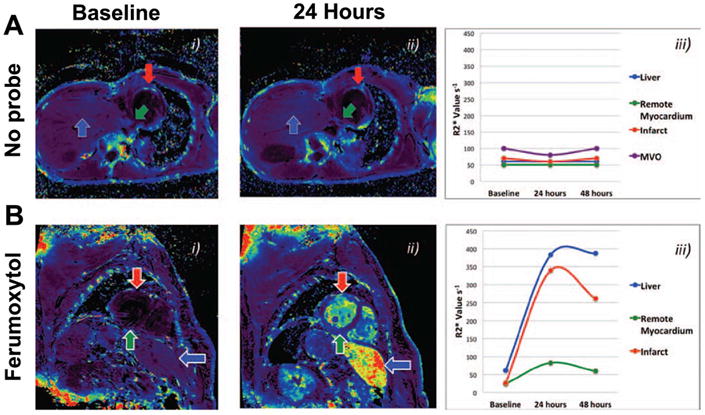
Molecular MRI of myocardial inflammation with ferumoxytol in patients with ST elevation myocardial infarction. The agent is taken up by macrophages infiltrating the infarct. (A) R2* maps in a patient with an infarct but without the injection of ferumoxytol. No change is seen in the R2* values over time in the infarct, remote zone or the liver. (B) Infarcted patient injected with ferumoxytol. R2* increases significantly in the infarct and to a lesser degree in the remote zone as well. Reproduced with permission [30].
Several other iron oxide constructs merit discussion. Both iron oxide microparticles and cross-linked iron oxide (CLIO) have been used to image VCAM-1 expression in small animal models [31, 32]. CLIO is highly analogous to ferumoxtran but the dextran chains on its surface are cross-linked and aminated. This facilitates the attachment of targeting ligands such as peptides and small proteins to the nanoparticle. Although CLIO is highly analogous to ferumoxytol and ferumoxtran, its degradation and elimination have not been studied and this agent is not being considered for clinical use. Recent work, however, has shown that the conjugation of targeting ligands to ferumoxytol is highly feasible [33].
Iron oxide microparticles have been used for in vitro diagnostics and preclinical imaging, but have not been used clinically. The safety of this class of agents in humans, therefore, remains unknown. At the other end of the size spectrum very small superparamagnetic iron oxide nanoparticles (VSOP) measure only a few nm in size. VSOPs lack a carbohydrate coat and are electrostatically stabilized in solution with a citrate coating. These agents, which can be decorated with targeting moieties, have been administered to humans with mild but no serious adverse effects [34]. Further work will be needed to establish the feasibility of using these agents in the clinical setting more broadly.
Molecular MRI with Gd and 19F Nanoparticles
Sparsely expressed molecular targets cannot be detected with small Gd chelates. To increase sensitivity to these targets large amounts of Gd must be incorporated into a nanoparticle, whether a liposome, micelle or quantum dot. The use of liposomes offers two additional possibilities – they can be loaded with large amounts of a fluorinated material for 19F MRI [35, 36], and a therapeutic drug can be incorporated into their payload for local delivery [37, 38]. Much like iron oxide nanoparticles, in the presence of tissue inflammation many of these constructs are taken up non-specifically by infiltrating macrophages. In fact, a Gd loaded liposome was recently used to image the kinetics of macrophage infiltration into the myocardium in a small animal model of myocardial infarction [8].
Gd labeled liposomes can be decorated with ligands targeting fibrin, integrins and other surface receptors [38, 39]. Likewise, immunomicelles have been used to target oxidized-LDL and the macrophage scavenger receptor [4, 7]. The pharmacokinetic and elimination issues with these constructs are more complex and will need to be extensively studied [40]. Encouraging data, however, are beginning to emerge. Histology of the liver and kidneys revealed no signs of toxicity in mice injected with a complex nanoemulsion [41]. It should also be noted that liposomal preparations of several medications, such as doxorubicin, are currently approved for clinical use and have an established safety record.
Liposomes can be loaded with fluorine-containing emulsions to enable their detection by 19F MRI [35, 36]. This strategy has the advantage of high specificity, since there is no background 19F signal in the body. This must be weighted against the lower sensitivity of these agents, which limits their ability to detect sparse molecular targets. 19F MRI of liposomal nanoparticles, however, has been used with great success for cell tracking, imaging myocardial macrophage infiltration and for the evaluation of vascular permeability [35, 36, 42]. Many of these particles are highly similar to the fluorinated agents that have been clinically tested as blood substitutes [43], and an Investigational New Drug (IND) application for the use of these liposomes as 19F MR imaging agents has been approved [42].
Theranostic Agents
The ideal diagnostic imaging agent should be completely inert and exert no bioeffects at all on the body. However, if a diagnostic imaging agent can be designed to exert a therapeutic bioeffect, then its lack of inertness become a positive feature and not a flaw. This is the concept behind the emerging field of theranostic imaging agents, which combine a diagnostic and therapeutic function. The emergence of theranostics has in many ways created a complete paradigm shift in imaging agent design. Rather than striving to develop the most inert and non-reactive agents, numerous groups are now exploiting the therapeutic opportunities of MRI-detectable nanoparticles.
Most theranostic agents at the present time consist of a liposome loaded with a therapeutic drug (fumagillin, prednisolone) and either Gd or 19F [37, 38]. These agents can be decorated with a ligand for specific targeting or rely on the non-specific uptake of liposomes by macrophages. The breakdown of the liposome in the macrophage, or slow local release of the drug by diffusion, results in high local concentrations of the released drug. This has been shown to produce a robust anti-inflammatory effect in models of atherosclerotic plaque (Figure 4) [37, 38]. The addition of Gd or 19F in the agent allows the delivery of the liposome to the plaque to be imaged and it kinetics to be monitored.
Figure 4.
Theranostic imaging with molecular MRI. (A-C) Liposomes loaded with prednisolone are taken up by the macrophages in atherosclerotic plaque. The anti-inflammatory affect of the liposomes can be demonstrated by dynamic contrast enhanced (DCE) MRI and with PET imaging of fluorodeoxyglucose (FDG) [37]. (A) Signal intensity in the plaque is significantly higher on DCE before the injection of the liposomes (A) compared to 1 week after their injection (B). (C) Likewise FDG uptake (arrows) in the plaque is significantly reduced after the injection of liposomes. (D) Molecular MRI with Gd-loaded liposomes targeting the αvβ3 integrin [38]. The liposomes in the left column also contain the anti-angiogenic agent fumagillin. Repeat injection 1 week after the initial injection shows a marked reduction in the uptake of the targeted liposomes only in those animals initially injected with the fumagillin-containg liposomes. Reproduced with permission [37, 38].
The anti-angiogenic agent fumagillin has been incorporated into liposomes targeted to the αVβ3 integrin receptor. The uptake of the agent in a tumor or atherosclerotic plaque reveals that high levels of angiogenesis are present. The local release of fumagillin at these sites exerts a strong anti-angiogenetic effect, which can be quantified by subsequent imaging with the integrin-targeted liposome (Figure 4) [38]. High local concentrations of the therapeutic agent are produced at the target of interest, allowing far smaller doses of the drug to be given than would be required by systemic administration. This mitigates, to some degree, the potential of hepatic uptake of the liposomes to produce high concentrations of the drug in the liver. Further study, of this issue, however, will be needed for all theranostic nanoparticles.
The release of a therapeutic moiety from an iron-oxide nanoparticle is more complex, but these agents are also well suited to theranostic imaging. Moreover, in a recent application, the apoptosis sensing nanoparticle AnxCLIO-Cy5.5 was used in a therapeutic capacity based on its ability to physically stabilize the cell membrane [44]. The uptake of AnxCLIO-Cy5.5 by apoptotic cells in vitro did not activate pathways deleterious to cell health on gene array analysis. No differences were seen between the viability, ATP-content and reducing capacity of apoptotic cells exposed to the probe and those that were not. In addition, the exposure of apoptotic cells to the nanoparticle had a stabilizing effect on the cell membrane and attenuated its rupture. Most importantly, the administration of AnxCLIO-Cy5.5 to mice with ischemia-reperfusion injury resulted in a reduction in infarct size [44]. This experience highlights the point that a theranostic agent can exert a protective effect via drug release, a physical/mechanical effect or via the modulation of cell signaling. Significant activity and progress can be expected in this burgeoning field over the next few years.
Conclusions
The molecular MRI armamentarium is continuing to grow. Likewise, the awareness in the molecular MRI community to gather biodistribution and elimination data on a new construct at the earliest stages of design and testing has also grown. This should be considered standard and be given high priority even in early preclinical studies. Encouragingly, the biodistribution and elimination data collected on numerous molecular MRI agents currently under development appears highly promising.
The financial cost of bringing a new imaging agent to market is poorly understood by many. It is true that preclinical toxicity studies with molecular MRI agents are more complex and expensive than those with radiolabeled probes. Likewise, it is true that the acquisition of an institutional IND to perform first in man studies at a single center is far easier to obtain for a radiolabeled agent than a molecular MRI agent. However, by far the major cost of bringing a new drug (including diagnostic drugs) to the point of FDA approval is the phase-3 study. This is no more expensive to perform with a molecular MRI agent than a radiolabeled one. Moreover, most molecular MR agents can be synthesized on a large scale and have excellent stability with respect to storage. Unlike radiolabeled probes, which must be synthesized daily, once the manufacturing of the MR probe is complete no more synthesis is required. The absence of ionizing radiation is another appealing feature of molecular MRI, particularly in younger patients. Recent clinical and preclinical experience with molecular MRI agents has shown no concerning signs of toxicity. The NSF storm has passed and the issue of Gd elimination is now well understood. While ongoing vigilance is required, the outlook for molecular MRI of the cardiovascular system is as bright as ever.
Acknowledgments
The authors are supported in part by the following NIH grants: R01 HL093038 (DES) and EB009062 (PC).
Footnotes
Disclosure: D. E. Sosnovik: receives research support from, and is a consultant for, Siemens Medical Solutions; P. Caravan: equity in Factor 1A, LLC, the company which holds the patent rights to EP-2104R, and equity in Collagen Medical, LLC, a company developing a collagen-targeted MR probe.
References
- 1.Nahrendorf M, Sosnovik DE, French BA, et al. Multimodality cardiovascular molecular imaging, Part II. Circ Cardiovasc Imaging. 2009;2(1):56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosnovik DE, Nahrendorf M, Weissleder R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 2007;115(15):2076–2086. doi: 10.1161/CIRCULATIONAHA.106.658930. [DOI] [PubMed] [Google Scholar]
- 3.Sosnovik DE, Schellenberger EA, Nahrendorf M, et al. Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med. 2005;54(3):718–724. doi: 10.1002/mrm.20617. [DOI] [PubMed] [Google Scholar]
- 4.Briley-Saebo KC, Shaw PX, Mulder WJ, et al. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation. 2008;117(25):3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helm PA, Caravan P, French BA, et al. Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology. 2008;247(3):788–796. doi: 10.1148/radiol.2473070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Huang S, Chen HH, Yuan H, et al. Molecular MRI of acute necrosis with a novel DNA-binding gadolinium chelate: kinetics of cell death and clearance in infarcted myocardium. Circ Cardiovasc Imaging. 2011;4(6):729–737. doi: 10.1161/CIRCIMAGING.111.966374. Detailed biodistribution data showing that a DNA-targeted Gd chelate is well cleared. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipinski MJ, Frias JC, Amirbekian V, et al. Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc Imaging. 2009;2(5):637–647. doi: 10.1016/j.jcmg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naresh NK, Xu Y, Klibanov AL, et al. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology. 2012;264(2):428–435. doi: 10.1148/radiol.12111863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spuentrup E, Botnar RM, Wiethoff AJ, et al. MR imaging of thrombi using EP-2104R, a fibrin-specific contrast agent: initial results in patients. Eur Radiol. 2008;18(9):1995–2005. doi: 10.1007/s00330-008-0965-2. [DOI] [PubMed] [Google Scholar]
- 10.Tang TY, Howarth SP, Miller SR, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53(22):2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Vymazal J, Spuentrup E, Cardenas-Molina G, et al. Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a phase II clinical study of feasibility. Invest Radiol. 2009;44(11):697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 12.Reiter T, Ritter O, Prince MR, et al. Minimizing risk of nephrogenic systemic fibrosis in cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:31. doi: 10.1186/1532-429X-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines. Radiology. 2011;260(1):105–111. doi: 10.1148/radiol.11102340. Important study showing that by following broadly accepted guidelines the occurrence of NSF can be completely eliminated. [DOI] [PubMed] [Google Scholar]
- 14.Kolodziej AF, Nair SA, Graham P, et al. Fibrin specific peptides derived by phage display: characterization of peptides and conjugates for imaging. Bioconjug Chem. 2012;23(3):548–556. doi: 10.1021/bc200613e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Makowski MR, Wiethoff AJ, Blume U, et al. Assessment of atherosclerotic plaque burden with an elastin-specific magnetic resonance contrast agent. Nat Med. 2011;17(3):383–388. doi: 10.1038/nm.2310. Detailed biodistribution data showing that an elastin-targeted Gd chelate is well cleared. [DOI] [PubMed] [Google Scholar]
- 16.Amirbekian V, Aguinaldo JG, Amirbekian S, et al. Atherosclerosis and matrix metalloproteinases: experimental molecular MR imaging in vivo. Radiology. 2009;251(2):429–438. doi: 10.1148/radiol.2511080539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Uppal R, Catana C, Ay I, et al. Bimodal thrombus imaging: simultaneous PET/MR imaging with a fibrin-targeted dual PET/MR probe--feasibility study in rat model. Radiology. 2011;258(3):812–820. doi: 10.1148/radiol.10100881. Properties and kinetics of a dual MR-PET fibrin binding probe are described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caravan P, Cloutier NJ, Greenfield MT, et al. The interaction of MS-325 with human serum albumin and its effect on proton relaxation rates. J Am Chem Soc. 2002;124(12):3152–3162. doi: 10.1021/ja017168k. [DOI] [PubMed] [Google Scholar]
- 19.Caravan P, Comuzzi C, Crooks W, et al. Thermodynamic stability and kinetic inertness of MS-325, a new blood pool agent for magnetic resonance imaging. Inorg Chem. 2001;40(9):2170–2176. doi: 10.1021/ic001117r. [DOI] [PubMed] [Google Scholar]
- 20.Eldredge HB, Spiller M, Chasse JM, et al. Species dependence on plasma protein binding and relaxivity of the gadolinium-based MRI contrast agent MS-325. Invest Radiol. 2006;41(3):229–243. doi: 10.1097/01.rli.0000199293.86956.48. [DOI] [PubMed] [Google Scholar]
- 21.Nahrendorf M, Sosnovik D, Chen JW, et al. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation. 2008;117(9):1153–1160. doi: 10.1161/CIRCULATIONAHA.107.756510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez E, Nilges M, Weissleder R, Chen JW. Activatable magnetic resonance imaging agents for myeloperoxidase sensing: mechanism of activation, stability, and toxicity. J Am Chem Soc. 2010;132(1):168–177. doi: 10.1021/ja905274f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sosnovik DE, Nahrendorf M, Weissleder R. Magnetic nanoparticles for MR imaging: agents, techniques and cardiovascular applications. Basic Res Cardiol. 2008;103(2):122–130. doi: 10.1007/s00395-008-0710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sosnovik DE, Nahrendorf M, Deliolanis N, et al. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation. 2007;115(11):1384–1391. doi: 10.1161/CIRCULATIONAHA.106.663351. [DOI] [PubMed] [Google Scholar]
- 25.Weissleder R, Stark DD, Engelstad BL, et al. Superparamagnetic iron oxide: pharmacokinetics and toxicity. AJR Am J Roentgenol. 1989;152(1):167–173. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 26*.Richards JM, Shaw CA, Lang NN, et al. In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: feasibility and safety in humans. Circ Cardiovasc Imaging. 2012;5(4):509–517. doi: 10.1161/CIRCIMAGING.112.972596. Clinical study involving the injection of monocytes labeled with iron oxide nanoparticles. [DOI] [PubMed] [Google Scholar]
- 27.Arbab AS, Yocum GT, Rad AM, et al. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18(8):553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 28.Yocum GT, Wilson LB, Ashari P, et al. Effect of human stem cells labeled with ferumoxides-poly-L-lysine on hematologic and biochemical measurements in rats. Radiology. 2005;235(2):547–552. doi: 10.1148/radiol.2352040383. [DOI] [PubMed] [Google Scholar]
- 29.Harisinghani MG, Barentsz J, Hahn PF, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 30*.Alam SR, Shah AS, Richards J, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging. 2012;5(5):559–565. doi: 10.1161/CIRCIMAGING.112.974907. Clinical study in which ferumoxytol was used to image myocardial inflammation in patients with STEMI. [DOI] [PubMed] [Google Scholar]
- 31.McAteer MA, Schneider JE, Ali ZA, et al. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb Vasc Biol. 2008;28(1):77–83. doi: 10.1161/ATVBAHA.107.145466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nahrendorf M, Jaffer FA, Kelly KA, et al. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114(14):1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Alcantara D, Josephson L. A magnetofluorescent nanoparticle for ex-vivo cell labeling by covalently linking the drugs protamine and Feraheme. J Nanosci Nanotechnol. 2011;11(4):3058–3064. doi: 10.1166/jnn.2011.4164. [DOI] [PubMed] [Google Scholar]
- 34.Wagner M, Wagner S, Schnorr J, et al. Coronary MR angiography using citrate-coated very small superparamagnetic iron oxide particles as blood-pool contrast agent: initial experience in humans. J Magn Reson Imaging. 2011;34(4):816–823. doi: 10.1002/jmri.22683. [DOI] [PubMed] [Google Scholar]
- 35.Partlow KC, Chen J, Brant JA, et al. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. Faseb J. 2007;21(8):1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 36.Flogel U, Ding Z, Hardung H, et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation. 2008;118(2):140–148. doi: 10.1161/CIRCULATIONAHA.107.737890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Lobatto ME, Fayad ZA, Silvera S, et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm. 2010;7(6):2020–2029. doi: 10.1021/mp100309y. Study demonstrating the anti-inflammatory effects of steroid loaded liposomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winter PM, Neubauer AM, Caruthers SD, et al. Endothelial alpha(v)beta3 integrin-targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26(9):2103–2109. doi: 10.1161/01.ATV.0000235724.11299.76. [DOI] [PubMed] [Google Scholar]
- 39.Flacke S, Fischer S, Scott MJ, et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104(11):1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 40.Unger E, Cardenas D, Zerella A, et al. Biodistribution and clearance of liposomal gadolinium-DTPA. Invest Radiol. 1990;25(6):638–644. doi: 10.1097/00004424-199006000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Gianella A, Jarzyna PA, Mani V, et al. Multifunctional nanoemulsion platform for imaging guided therapy evaluated in experimental cancer. ACS Nano. 2011;5(6):4422–4433. doi: 10.1021/nn103336a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Zhang L, Myerson J, et al. Quantifying the evolution of vascular barrier disruption in advanced atherosclerosis with semipermeant nanoparticle contrast agents. PLoS One. 2011;6(10):e26385. doi: 10.1371/journal.pone.0026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flaim SF. Pharmacokinetics and side effects of perfluorocarbon-based blood substitutes. Artif Cells Blood Substit Immobil Biotechnol. 1994;22(4):1043–1054. doi: 10.3109/10731199409138801. [DOI] [PubMed] [Google Scholar]
- 44.Chen HH, Feng Y, Zhang M, et al. Protective effect of the apoptosis-sensing nanoparticle AnxCLIO-Cy5.5. Nanomedicine. 20128(3):291–298. doi: 10.1016/j.nano.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]