Abstract
Background
The optimum strategy for stopping treatment with drugs that have different half-lives in a combination regimen to minimize the risk of selecting drug-resistant viruses remains unknown. We evaluated drug concentrations in plasma, human immunodeficiency virus (HIV) load, and development of drug resistance after a planned treatment interruption of a nonnucleoside reverse-transcriptase inhibitor (NNRTI)–containing regimen in HIV type 1–infected children.
Methods
Children with viral loads <50 copies/mL and CD4 cell percentages ≥30% (for children aged 2–6 years) or CD4 cell percentages ≥25% and CD4 cell counts ≥500 cells/μL (for children aged 7–15 years) were randomized to either a planned treatment interruption or to continuous therapy. In the planned treatment interruption arm, either (1) treatment with nevirapine or efavirenz was stopped, and treatment with the remaining drugs was continued for 7–14 days, or (2) nevirapine or efavirenz were replaced by a protease inhibitor, and all drugs were stopped after 7–14 days. Sampling for determination of plasma drug concentrations, measurement of viral load, and drug resistance testing was scheduled at day 0, day 7 (drug concentrations only), day 14, and day 28 after interruption of treatment with an NNRTI.
Results
Treatment with an NNRTI was interrupted for 35 children (20 were receiving nevirapine, and 15 were receiving efavirenz). Median time from NNRTI cessation to stopping all drugs was 9 days (range, 6–15 days) for nevirapine and 14 days (range, 6–18 days) for efavirenz. At 7 days, 1 (5%) of 19 and 4 (50%) of 8 children had detectable nevirapine and efavirenz concentrations, respectively; efavirenz remained detectable in 3 (25%) of 12 children at 14 days. At 14 days, viral load was ≥50 copies/mL in 6 of 16 children interrupting treatment with nevirapine (range, 52–7000 copies/mL) and in 2 of 12 children interrupting treatment with efavirenz (range, 120–1600 copies/mL). No new NNRTI mutations were observed.
Conclusions
In children with virological suppression who experienced interruption of treatment with an NNRTI, staggered or replacement stopping strategies for a median of 9 days for nevirapine and 14 days for efavirenz were not associated with the selection of NNRTI resistance mutations.
Current antiretroviral treatment guidelines recommend continuous HAART for the treatment of HIV infection and AIDS; however, whether planned or unplanned, therapy is sometimes interrupted. Triple-combination HAART regimens typically contain drugs with different half-lives; therefore, stopping a HAART regimen may lead to temporary functional monotherapy and, as viral load rebounds, can potentially increase the risk of selecting drug-resistant viruses [1].
Among the antiretroviral drug classes, nonnucleoside reverse transcriptase inhibitors (NNRTIs) have the longest plasma half-lives; 25–30 h for nevirapine [2] and 40–55 h for efavirenz [3]. The majority of nucleoside reverse-transcriptase inhibitors (NRTIs) and protease inhibitors have plasma half-lives of <10 h. Thus, the risk of temporary functional monotherapy when simultaneously stopping all drugs in an NNRTI-containing regimen is high, and because NNRTIs have a low genetic barrier to within-class drug resistance, this situation could potentially compromise the efficacy of future NNRTI-containing regimens. Other drugs with low genetic barriers, such as the NRTI lamivudine, may also be susceptible to the development of resistance in this situation, especially because its active moiety, the intracellular drug triphosphate, has a half-life of ~20 h [2].
In adults, several studies have examined the persistence of NNRTI plasma drug concentrations after treatment interruption. Efavirenz plasma concentrations can persist for ≥14 days after stopping therapy [3]. In contrast, at steady state, nevirapine plasma concentrations did not persist >14 days after treatment interruption [4], although after a single dose of nevirapine during labor for the prevention of mother-to-child transmission of HIV, plasma concentrations have been detected up to 21 days postpartum [5].
The impact of selecting NNRTI-resistant strains after treatment interruption on subsequent NNRTI-based therapy has been highlighted in the prevention of mother-to-child transmission of HIV setting. HIV-infected women exposed to single-dose nevirapine during labor were found to be less likely to achieve virologic suppression after 6 months of postpartum treatment with an NNRTI-based regimen, compared with nonexposed women [6]. Short-course, dual-NRTI treatment post-partum (administered for 3–7 days) after administration of single-dose nevirapine has been shown to reduce the risk of selecting NNRTI-resistant strains [7], but the optimal choice and duration of such treatment remains unknown.
To date, no pediatric data after NNRTI treatment interruption are available. Therefore, in the context of a pediatric phase II exploratory planned treatment interruption (PTI) trial, we describe plasma drug concentrations, viral rebound, and selection of HIV drug resistance mutations in children receiving an NNRTI-based HAART regimen during the first PTI cycle in the trial.
PATIENTS, MATERIALS, AND METHODS
Study population
All children were enrolled in the Paediatric European Network for Treatment of AIDS (PENTA) 11: Treatment Interruption in Children with Chronic HIV-Infection Trial. PENTA-11 is an ongoing, open, randomized, phase II exploratory trial evaluating the role of PTIs in the treatment of HIV-infected children who have responded well to antiretroviral therapy (ISRCTN36694210; http://www.controlled-trials.com). Children receiving stable HAART for at least 6 months with plasma HIV-1 RNA levels <50 copies/mL and a CD4 cell percentage ≥30% (for children aged 2–6 years) or a CD4 cell percentage ≥25% and CD4 cell count ≥500 cells/μL (for children aged 7–15 years) were randomized 1:1 to either continue antiretroviral therapy or undergo a CD4 cell–guided PTI and were followed up for at least 72 weeks. This study was approved by the ethics committees of all participating sites.
Children randomized to the PTI strategy and taking a regimen that included an NNRTI and/or lamivudine stopped therapy with either a staggered stop or a replacement stop strategy, at the discretion of the pediatrician. The original protocol (23 June 2005) recommended that, for a staggered stop strategy, treatment with the NNRTI should be stopped at randomization, and treatment with the remaining 2 drugs should be continued for 7 days. For a replacement stop strategy, the NNRTI should be switched to a single or ritonavir-boosted protease inhibitor and treatment with the 3 drugs should be continued for 7 days. For either strategy, if the regimen contained lamivudine, there was an option to switch lamivudine to an alternative NRTI with a high genetic barrier to resistance and a short half-life prior to continuing the dual-NRTI regimen. In February 2006, an interim analysis showed that all 10 children who stopped treatment with nevirapine had plasma concentrations <0.15 mg/L at day 7, but at least 2 children had detectable concentrations with use of a more sensitive assay (0.06 and 0.064 mg/L). Three of 7 children who stopped efavirenz had detectable efavirenz plasma concentrations at day 14. Therefore, the protocol was amended (on 10 March 2006) to recommend a staggered stop or replacement stop for 7–14 days for nevirapine and for at least 14 days for efavirenz. Here, we present data for all children in the PENTA-11 trial who stopped treatment with nevirapine- or efavirenz-containing regimens during their first PTI. NNRTI plasma drug concentrations and HIV-1 RNA load were measured at 0, 7 (drug concentrations only), 14, and 28 days after stopping treatment with the NNRTI, and HIV-1 population sequencing was performed in the first plasma sample with an HIV load >500 copies/mL.
Antiretroviral drug concentration assay
Nevirapine and efavirenz plasma drug concentrations were measured by validated high-performance liquid chromatography methods, with a lower limit of quantification of 0.05–0.25 mg/L for nevirapine and 0.05–0.2 mg/L for efavirenz. For this analysis, a threshold value for detection of ≥0.25 mg/L for nevirapine and ≥0.2 mg/L for efavirenz was used. All of the laboratories involved in this study participate in International Quality Control Programs for antiretroviral drug measurements [8, 9].
HIV-1 resistance assay
HIV RNA population sequencing was performed in local PENTA network laboratories. Viral nucleic acid was extracted from plasma with use of Qiagen or Roche systems, and the pol-gene PCR was amplified and sequenced using ABI capillary sequencers (Applied Biosystems). The minimum length of sequence covered all of the protease gene and positions 38–250 in the reverse transcriptase. Interpretation of mutations was undertaken using the Stanford University HIV database [10] or the AC11 National Agency for AIDS Research (ANRS) algorithm [11].
Statistical analysis
Day 0 was defined as the day on which treatment with the NNRTI was stopped; this was the day of randomization for 12 children (34%) but ranged from 1 to 16 days after randomization for the remaining children because of logistical issues. Baseline characteristics are given for the time of randomization except for laboratory measurements, which are those recorded nearest to (before or on) day 0. For the virologic evaluations after treatment interruption, viral load measurements recorded below a limit of detection of >50 copies/mL (e.g., <400 copies/mL) were assumed to be >50 copies/mL.
RESULTS
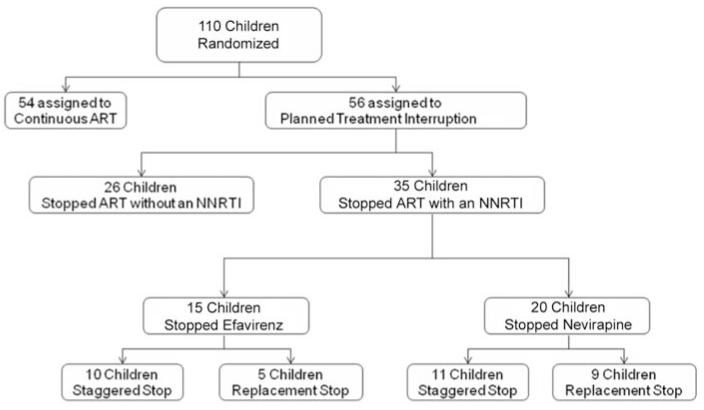
Of the 110 children enrolled in PENTA-11 between November 2004 and December 2006, 56 were randomized to the PTI arm. Of these children, 35 stopped treatment with an NNRTI-containing HAART regimen: 20 stopped treatment with nevirapine, and 15 stopped treatment with efavirenz (figure 1). Baseline characteristics of children interrupting treatment with an NNRTI are shown in table 1. Of note, at day 0, viral load was ≥50 copies/mL (range, 50–700 copies/mL) in 6 (17%) of the children despite being stable at <50 copies/mL for the previous 6 months, up to and including the screening visit. Four of these 6 children followed a staggered stop strategy, and 2 followed a replacement stop strategy.
Figure 1.
Summary of randomization assignment, HAART regimens interrupted, and stopping strategies employed during the first planned treatment interruption cycle for children enrolled in the Paediatric European Network for Treatment of AIDS 11: Treatment Interruption in Children with Chronic HIV-Infection trial. ART, antiretroviral therapy; NNRTI, nonnucleoside reverse-transcriptase inhibitor.
Table 1. Baseline demographic and clinical characteristics of children who interrupted treatment with a nevirapine (NVP)– or efavirenz (EFV)–containing HAART regimen.
| Variable | NVP group (n = 20) |
EFV group (n = 15) |
All patients (n = 35) |
|---|---|---|---|
| Sex | |||
| Male | 9 (45) | 7 (47) | 16 (46) |
| Female | 11 (55) | 8 (53) | 19 (54) |
| Ethnicity | |||
| White | 5 (25) | 3 (20) | 8 (23) |
| Black | 5 (25) | 6 (40) | 11 (31) |
| Asian | 8 (40) | 3 (20) | 11 (31) |
| Mixed | 2 (10) | 3 (20) | 5 (14) |
| Age, median years (range) | 8.3 (3.6–15.9) | 9.4 (5.0–15.0) | 8.9 (3.6–15.9) |
| HIV RNA level <50 copies/mL | 17 (85) | 11a (79) | 28a (82) |
| CD4 cell count, median cells/μL (range) | 1033b (531–2100) | 919 (487–2646) | 1026b (487–2646) |
| CD4 cell percentage, median % (range) | 38b (29–53) | 36 (24–42) | 38b (24–53) |
| Previous ART exposure | |||
| All 3 main classes | 5 (25) | 5 (33) | 10 (29) |
| NRTI plus NNRTI | 15 (75) | 10 (67) | 25 (71) |
| ART drugs received, median no. (range) | |||
| All 3 main classes | 4 (3–8) | 4 (3–8) | 4 (3–8) |
| NRTIs | 2.5 (2–4) | 3 (2–5) | 3 (2–5) |
| NNRTIs | 1 (1–2) | 1 (1–2) | 1 (1–2) |
| PIs | 0 (0–3) | 0 (0–2) | 0 (0–3) |
| Cumulative ART exposure, median years (range) | |||
| All ART drugs | 5.2 (1.7–15.3) | 6.1 (2.7–10.8) | 5.4 (1.7–15.3) |
| NRTIs | 5.2 (1.7–11.6) | 6.1 (2.7–10.8) | 5.4 (1.7–11.6) |
| NNRTIs | 4.0 (1.7–7.7) | 3.7 (1.9–7.9) | 3.7 (1.7–7.9) |
| PIs | 0 (0–8.5) | 0 (0–6.3) | 0 (0–8.5) |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Baseline characteristics are given at the time of randomization except for laboratory measurements, which are those measurements recorded nearest to or on (but not after) the day on which the NNRTI was stopped (day 0). ART, antiretroviral therapy; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor.
Data on HIV RNA level at day 0 was unavailable for 1 child; percentages are for children with available data.
CD4 cell count and CD4 cell percentage data at day 0 were unavailable for 1 child; percentages are for children with available data.
Of the NNRTI-based regimens that were interrupted, 27 (77%) of 35 included lamivudine. A staggered stop strategy was used for 11 (55%) of the children interrupting treatment with nevirapine; lamivudine was included in 7 of these regimens, and 2 children had lamivudine switched to didanosine for 1 week. All 9 children who stopped treatment with nevirapine using a replacement stop strategy were receiving lamivudine; 6 children had lamivudine replaced with didanosine and had lopinavir-ritonavir added to the regimen, and 3 children retained lamivudine and had either lopinavir-ritonavir (2 children) or nelfinavir (1 child) added to their regimen. Similarly, 10 (67%) of 15 children interrupted treatment with efavirenz using a staggered stop strategy; lamivudine was included in 8 of these regimens, but only 1 child had lamivudine switched to didanosine. Five children interrupted treatment with efavirenz with a replacement stop strategy, and all had lopinavir-ritonavir added to the regimen; lamivudine was included in 3 of these regimens, and lamivudine was switched to didanosine for 1 child.
The median time from stopping treatment with the NNRTI to stopping treatment with all drugs was 9 days (range, 6–15 days) for children receiving nevirapine and 14 days (range, 6–18 days) for children receiving efavirenz. For the staggered stop strategy, the median times were 8 days (range, 6–14 days) and 14 days (range, 6–18 days) for nevirapine and efavirenz, respectively; 14 days (range, 6–15 days) was the median time for a replacement stop strategy for both drugs.
NNRTI plasma drug concentrations after treatment interruption
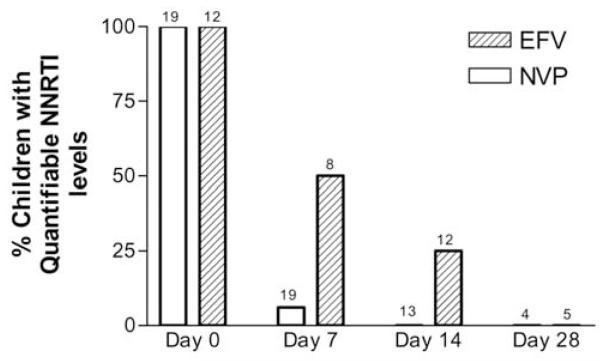
Plasma drug concentration data were available for 19 children stopping treatment with nevirapine and 14 children stopping treatment with efavirenz. The percentages of children with nevirapine and efavirenz plasma concentrations above the threshold at 0, 7, 14, and 28 days after treatment with the NNRTI was stopped are shown in figure 2.
Figure 2.
Percentage of children with quantifiable plasma nonnucleoside reverse-transcriptase inhibitor concentrations at 0, 7, 14, and 28 days after interruption of a nevirapine (NVP)– or efavirenz(EFV)–containing HAART regimen. The lower threshold of detection was 0.25 mg/L for NVP and was 0.20 mg/L for EFV. The number of children with samples available at each time point is reported above each bar.
At the time of stopping treatment with nevirapine, the median nevirapine dose was 306 mg/m2 (range, 221–410 mg/m2), and the plasma concentration was 7.0 mg/L (range, 2.2–15.1 mg/L) (3 children had plasma concentrations that were less than the target therapeutic concentration of 3.0 mg/L [14]). Of 19 children, 1 child (whose plasma concentration at day 0 was 15.05 mg/L) had a nevirapine plasma concentration >0.25 mg/L (1.31 mg/L) 7 days after stopping treatment with nevirapine, which decreased to 0.12 mg/L by day 14 (lower limit of quantification [LLOQ] of the assay was 0.05 mg/L; nevirapine concentrations were not measured for this child after day 14). Of the 18 remaining children, 16 had samples assayed with a more sensitive assay, 1 with an LLOQ of 0.15 mg/L, 6 with an LLOQ of 0.10 mg/L, and 9 with an LLOQ of 0.05 mg/L. At 7 days, 5 children had nevirapine plasma concentrations above the LLOQ for the assay but below 0.25 mg/L (0.057, 0.060, 0.060, 0.064, and 0.149 mg/L); of these children, 3 had nevirapine concentrations >10.0 mg/L at day 0. At 14 days, no children had a nevirapine concentration >0.25 mg/L, and only 1 child had a detectable plasma concentration above the LLOQ for the assay (described above).
The median efavirenz dose and median plasma drug concentration at treatment interruption were 12.3 mg/kg (range, 8.1–17.2 mg/kg) and 2.0 mg/L (range, 0.3–3.5 mg/L), respectively, for 12 children, including 2 with plasma concentrations less than the target therapeutic concentration of 1.0 mg/L [14]. Of 8 children, 4 had detectable efavirenz plasma concentrations >0.20 mg/L (0.40, 0.54, 0.61, and 4.07 mg/L) 7 days after stopping treatment (time from stopping treatment with refavirenz to stopping treatment with all drugs was 13, 18, 7, and 6 days, respectively). Among the 4 remaining children, all had samples assayed with a more sensitive assay (3 with an LLOQ of 0.05 mg/L and 1 with an LLOQ of 0.078 mg/L); 2 of these children had efavirenz plasma concentrations above the LLOQ for the assay but <0.20 mg/L at day 7 after stopping treatment (0.12 and 0.19 mg/L). The 2 children with the greatest efavirenz plasma concentrations >0.20 mg/L at day 7 also had plasma concentrations of 0.26 and 0.32 mg/L 14 days after interruption. One additional child, for whom plasma concentrations were not measured at day 7, had an efavirenz plasma concentration of 0.29 mg/L 14 days after stopping efavirenz (time from stopping efavirenz to stopping all drugs was 6 days). Two of the 3 children with detectable concentrations at day 14 were also tested at day 28, and no efavirenz was detected.
Viral load rebound
At day 14, 6 (38%) of 16 and 2 (17%) of 12 children interrupting treatment with nevirapine and efavirenz, respectively, had viral loads ≥50 copies/mL (range for the nevirapine group, 52–7000 copies/mL; range for the efavirenz group, 120–1600 copies/mL), which increased to 19 (95%) of 20 and 12 (92%) of 13 by day 28 (range for the nevirapine group, 300–4,897,788 copies/mL; range for the efavirenz group, <100 to 147,000 copies/mL) (table 2).
Table 2. Proportion (%) of children with HIV RNA load ≥50 copies/mL at 0, 14, and 28 days after interrupting a nevirapine- or efavirenz-containing HAART regimen with use of either a staggered stop (SS) or replacement stop (RS) strategy.
| Nevirapine group |
Efavirenz group |
|||||
|---|---|---|---|---|---|---|
| Treatment interruption strategy | Day 0 | Day 14 | Day 28 | Day 0 | Day 14 | Day 28 |
| SS and RS | 3/20 (15) | 6/16 (38) | 19/20 (95) | 3/14 (21) | 2/12 (17) | 12/13 (92) |
| SS | 2/11 (18) | 3/9 (33) | 11/11 (100) | 2/10 (20) | 2/7 (29) | 8/8 (100) |
| RS | 1/9 (11) | 3/7 (43) | 8/9 (89) | 1/4 (25) | 0/5 (0) | 4/5 (80) |
NNRTI resistance
HIV genotyping was performed at various times during the first 3 months after interruption of treatment with an NNRTI, depending on viral load values (for 1 child at week 2, 21 children at week 4, 10 children at week 8, and 2 children at week 12). For 1 child, cDNA could not be amplified. No new NNRTI resistance mutations were detected in virus from the 35 children interrupting treatment with an NNRTI-containing HAART regimen. One child who was receiving nevirapine had the NNRTI resistance mutation K103N detected 14 days after stopping treatment with nevirapine, but this child had a viral load of 700 copies/mL at day 0 (<50 copies/mL at the screening visit), and the K103N resistance mutation could also be detected in virus isolated from a stored sample taken at day 0. No lamivudine-associated resistance mutations were detected in virus obtained from any of the children.
DISCUSSION
In this substudy of virologically suppressed HIV-infected children enrolled in PENTA-11 trial and undergoing a PTI, we found that nevirapine and efavirenz plasma drug concentrations were below the detection threshold in the majority of children at 1 week (for those stopping treatment with nevirapine) and 2 weeks (for those stopping treatment with efavirenz) after treatment interruption and were below the lower limit of detection for all children at 2 weeks (for those stopping treatment with nevirapine) and 4 weeks (for those stopping treatment with efavirenz). No new NNRTI or lamivudine resistance mutations were detected.
We observed that efavirenz persisted longer than nevirapine in plasma. This result was apparent early in the trial, and we changed the management strategy to recommend staggered stop or replacement stop of efavirenz for 14 days rather than 7 days. At this time, a 7–14-day staggered stop strategy was recommended for children stopping treatment with nevirapine. The final analysis confirmed that efavirenz had longer persistence, an observation that was consistent with reports involving adults. For example, in a report describing 8 adults who interrupted treatment with efavirenz, median plasma concentrations were 0.31, 0.15, and 0.06 mg/L at 7, 14, and 21 days, respectively [3]. In a substudy of the Development of Antiretroviral Therapy in Africa (DART) trial, in which adults interrupted treatment with nevirapine using a 1-week staggered stop strategy, 5 (28%) of 18 patients had nevirapine concentrations >0.2 mg/L at 7 days, decreasing to only 1 (5%) of 19 patients by day 14 [4].
In our study, a staggered stop or replacement stop of 6–18 days (median duration, 14 days) was used for children interrupting treatment with efavirenz, and no new NNRTI resistance selection was observed as viral load increased. Despite this, the high percentage of children with efavirenz concentrations >0.20 mg/L at 14 days, coupled with the persistence of efavirenz for 3 weeks [3] and sometimes longer in adults [12], would suggest that a staggered stop or replacement stop duration >14 days should be advocated. The authors of the DART substudy suggest that a 7–10-day staggered stop strategy is adequate for African patients discontinuing treatment with nevirapine, for whom protease inhibitor replacement is not feasible. In our study, a staggered stop or replacement stop of 6–15 days (median duration, 9 days) was used for children interrupting treatment with nevirapine, and no NNRTI resistance was found. Of interest, whereas median nevirapine concentrations at treatment interruption were similar in our study and the DART study, concentrations 7 days after treatment interruption were generally higher in the adults participating in the DART study than in children participating in the PENTA-11 trial, possibly because of higher nevirapine clearance in the children.
A single-nucleotide polymorphism in the gene of the cytochrome P450 2B6 isoenzyme (CYP2B6 G516T) can reduce the oral clearance of efavirenz and, possibly, nevirapine [13, 14]; therefore, patients carrying the homozygous variant genotype may have a higher risk of selecting for NNRTI-resistant infection after treatment interruption. Indeed, predicted efavirenz plasma exposure after treatment interruption was found to be longer with the variant genotypes, suggesting that it may be difficult to develop a standard stopping strategy [15]. Our study did not include investigations of CYP2B6, although it would be expected that part of interpatient variability could be attributable to such polymorphisms.
It was not possible to compare the impact of the stopping strategies on viral load rebound with adjustment for the time between NNRTI treatment interruption and interruption of treatment with the other drugs, because this varied considerably among clinical sites, drug combinations, and strategies. For example, to maximize safety for children, some sites conducted real-time drug concentration assessments and stopped treatment with all drugs only when NNRTIs were undetectable. However, there is nothing in our observations that would favor one strategy over another, although a longer replacement stop strategy may be preferred over a longer staggered stop strategy to avoid the development of NRTI-resistant infection as the result of dual-NRTI therapy.
Comparing the persistence of NNRTIs between studies can be difficult because of the different LLOQs used. This raises the issue of drug concentration thresholds at which one expects an increased risk of selection of drug-resistant viruses. On the basis of the inhibitory concentration at which 50% of isolates are susceptible to nevirapine (0.01–0.02 mg/L), targeting a threshold lower than the 0.25 mg/L used in our study may have been preferable. However, although nevirapine plasma concentrations were barely detectable at 7 and 14 days (range, 0.05–0.25 mg/L) with use of the more sensitive assay, it appears that such very low concentrations were insufficient to induce selection of HIV resistance mutations. Indeed, with the stopping strategies used, significant viral load rebound did not appear until 14 days after stopping treatment with nevirapine.
Although no NNRTI resistance was detected using population sequencing techniques, minor resistant viral populations may have been selected and could have been detected using more-sensitive assays (e.g., Oligonucleotide-ligation [16], LigAmp [17] or Allele Specific-PCR [18]). Also, resistance mutations outside the DNA polymerase domain of HIV reverse transcriptase (i.e., connection and RNAse H domains) were not assessed but have recently been suggested to confer resistance to nevirapine [19]; however, the long-term clinical impact of such mutations and the minor populations of NNRTI-resistant viruses are uncertain.
Concerns regarding the selection of NNRTI resistance mutations after treatment interruption were originally raised with the use of single-dose nevirapine during labor for the prevention of mother-to-child transmission of HIV. Depending on the background prophylaxis regimen, the sensitivity of the resistance test, and the virus subtype, NNRTI resistance mutations have been detected in 20%–70% of women who received single-dose nevirapine [20, 21], and these mutations have proved to impact the success of subsequent NNRTI-based therapies, in particular when initiated within months of exposure [6]. However, viral loads in women at the time of single-dose nevirapine exposure are expected to be much higher than viral loads in children or adults receiving long-term nevirapine-based HAART with undetectable viral loads who undergo a PTI. Also, autoinduction of nevirapine clearance in patients receiving HAART results in a shortened half-life. As a consequence, the risk of selecting NNRTI-resistant viruses as part of a PTI is expected to be lower than that observed in the prevention of mother-to-child transmission of HIV setting.
In conclusion, in virologically suppressed children who interrupt an NNRTI-based HAART regimen during their first PTI cycle, the adoption in this trial of a staggered stop or replacement stop strategy for a median duration of 9 days for nevirapine and 14 days for efavirenz was not associated with the selection of drug resistance mutations. Whether a replacement stop or staggered stop is preferable could not be determined, although there are theoretical reasons to prefer a replacement stop strategy, particularly for efavirenz, which persists longer than nevirapine. However, our data are consistent with other studies in which a staggered stop strategy for nevirapine appeared to be adequate. We are continuing to monitor HIV load, drug resistance, and drug concentrations in children who undergo subsequent PTIs.
Acknowledgments
We thank all of the children, families, and staff from the centers participating in the PENTA-11 Treatment Interruption in Children with Chronic HIV-Infection Trial. In the PENTA-11 trial, children were enrolled from 9 countries: France (10 children), Germany (4), Italy (19), Poland (3), Spain (17), Switzerland (2), Thailand (23), the United Kingdom (28), and the United States (4).
Appendix
MEMBERS OF PENTA
Committees
PENTA Steering Committee: J.-P. Aboulker, A. Babiker, S. Blanche, A.-B. Bohlin, K. Butler, G. Castelli-Gattinara, P. Clayden, A. Compagnucci, J. H. Darbyshire, M. Debré, R. de Groot, M. della Negra, D. Duicelescu, C. Giaquinto (chairperson), D. M. Gibb, I. Grosch-Wörner, C. Kind, M. Lallemant, J. Levy, H. Lyall, M. Marczynska, M. J. Mellado Peña, D. Nadal, T. Niehues, C. Peckham, J. T. Ramos Amador, L. Rosado, C. Rudin, H. J. Scherpbier, M. Sharland, M. Stevanovic, P. A. Tovo, G. Tudor-Williams, N. Valerius, A. S. Walker, and U. Wintergerst.
PENTA-11 Executive Committee: J. P. Aboulker, A. Babiker, D. M. Burger, A. Compagnucci, J. H. Darbyshire, M. Debré, C. Giaquinto, D. M. Gibb, H. Green, L. Harper, N. Klein, M. Lallemant, H. Lyall, L. Mofenson, J. Moye, D. Nadal, and Y. Saïdi.
PENTA-11 Pharmacology Group: D. M. Burger, T. R. Cressey, E. Jacqz-Aigrain, S. Khoo, and J. M. Tréluyer.
PENTA-11 Immunology/Virology Group: M. Clerici, A. De Rossi, N. Klein, J. Moye, N. Ngo-Giang-Huong, M. A. Muñoz Fernandez, and D. Pillay.
PENTA-11 Data Safety and Monitoring Committee: C. Hill (Chair), P. Lepage, A. Pozniak, and S. Vella.
Trial centers
INSERM SC10, France: J. P. Aboulker, A. Compagnucci, V. Eliette, G. Hadjou, S. Léonardo, C. Pitrou, Y. Riault, and Y. Saïdi.
MRC Clinical Trials Unit, United Kingdom: A. Babiker, L. Buck, J. H. Darbyshire, L. Farrelly, D. M. Gibb, H. Green, L. Harper, D. Johnson, C. Taylor, and A. S. Walker.
Program for HIV Prevention and Treatment, Thailand: S. Chalermpantmetagul, T. R. Cressey, R. Peongjakta, S. Chailert, F. Fregonese, G. Jourdain, M. Lallemant, and N. Ngo-Giang-Huong.
Westat/National Institute of Child Health and Human Development, United States: D. Butler, C. Carlton, D. Collins, G. Kao, L. Mofenson, J. Moye, S. Van Buskirk, and S. Watson.
Clinical sites
Hôpital Edouard Herriot, Lyon, France: S. Corradini, D. Floret, and J. Laplace. Hôpital de l’Archet, Nice, France: F. Monpoux, J. Cottalorda, J. C. Lefebvre, and S. Mellul. Hôpital Cochin Port-Royal, Paris, France: N. Boudjoudi and G. Firtion. Hôpital Robert Debré, Paris, France: A. Faye, D. Beniken, and F. Damond. Hôpital Purpan, Toulouse, France: J. Tricoire. Hôpital Saint-Vincent de Paul, Paris, France: A. Krivine. Hôpital Necker, Paris, France: M. L. Chaix.
Universitäts-kinderkliniken, Munich, Germany: U. Wintergerst, G. Notheis, G. Strotmann, and S. Schlieben.
Università di Padova, Padua, Italy: C. Giaquinto, O. Rampon, and M. Zanchetta. Università di Genova, Genoa, Italy: R. Rosso, E. Repeto, and F. Vitale. Ospedale Bambino Gesù, Rome, Italy: G. Castelli-Gattinara, A. Martino, and S. Bernardi. Ospedale S. Chiara, Trento, Italy: A. Mazza and G. Rossetti.
Medical University of Warsaw/Regional Hospital of Infectious Diseases, Warsaw, Poland: M. Marczynska, S. Dobosz, A. Oldakowska, J. Popielska, M. Kaflik, J. Stanczak, T. Stanczac, and T. Dyda.
Hospital Universitario 12 de Octubre, Madrid, Spain: M. I. González Tomé and R. Delgado García. Hospital Carlos III, Madrid, Spain: M. José Mellado Peña, P. Martín-Fontelos, R. Piñeiro Pérez, A. Alimenti, and M. Penin. Hospital Universitario de Getafe, Madrid, Spain: J. T. Ramos Amador. Hospital General Universitario Gregorio Marañón, Madrid, Spain: D. Gurbindo, M. L. Navarro Gomez, J. L. Jimenez, C. Prieto, and M. A. Muñoz-Fernandez. Hospital Infantíl La Paz, Madrid, Spain: M. I. de José Gómez and M. C. García Rodriguez. Hospital Materno-Infantil, Málaga, Spain: D. Moreno Pérez and E. Núñez Cuadros. Hospital Infantil La Fe, Valencia, Spain: F. Asensi-Botet, A. Pérez, and M. D. Pérez Tamarit.
University Chidren’s Hospital, Switzerland: C. Kalhert and D. Nadal. National Laboratory for Retrovirus, Zurich, Switzerland: J. Schupbach.
HIV–Netherlands Australia Thailand Research Collaboration, Bangkok, Thailand: T. Bunupuradah, J. Ananworanich, P. Phanuphak, J. Intasan, and S. Ubolyam. Nakornping Hospital, Nakornping, Thailand: S. Kanjanavanit and T. Namwong.
St. Mary’s Hospital, London, United Kingdom: H. Lyall, G. Tudor-Williams, C. Foster, D. Hamadache, S. Campbell, C. Hanley, C. Walsh, and S. Kaye. Chelsea and Westminster Hospital, London, United Kingdom: H. Lyall, P. Seery, and D. Hamadache. Great Ormond Street Hospital for Children, London, United Kingdom: V. Novelli, D. M. Gibb, N. Klein, D. Shingadia, J. Flynn, M. Clapson, L. Farrelly, and M. Jacobsen. University Hospital of North Stafford, Stoke on Trent, United Kingdom: P. McMaster and E. Hawkes. Newham University Hospital, Newham, United Kingdom: S. Liebeschuetz, O. Sodeinde, D. Shingadia, and S. Wong. Birmingham Heartlands Hospital, Birmingham, United Kingdom: S. Walsh and Y. Heath. Royal Free and University College Medical School, London, United Kingdom: D. Pillay.
State University New York Upstate Medical University, Syracuse: L. Weiner and M. Famiglietti. Howard University Hospital, Washington, DC: S. Rana, P. Yu, and J. Roa. Children’s Diagnostic & Treatment Center, Ft. Lauderdale, Florida: A. Puga and A. Haerry.
Pharmacology laboratories
Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands: D. M. Burger. Istituto di Ricovero e Cura A Carattere Scientifico Policlinico San Matteo, Pavia, Italy: M. Regazzi and S. Villani. University of Liverpool, Liverpool, United Kingdom: S. Khoo and S. Gibbons. Hôpital Saint-Vincent de Paul, Paris, France: V. Jullien, E. Rey, and J. M. Treluyer. Hospital Carlos III, Madrid, Spain: S. Rodríguez Nóvoa. Chiang Mai University, Chiang Mai, Thailand: T. R. Cressey and Y. Tawon.
Footnotes
Financial support. PENTA is a coordinated action of the European Commission, supported by the Sixth Framework contract LSHP-CT-2006-018865 and Fifth Framework Program contract QLK2-2000-00150. The PENTA-11 trial is sponsored by the PENTA Foundation in Europe and Thailand and by National Institute of Child Health and Human Development in the United States. The trial is coordinated by 4 trial centers: the Medical Research Council Clinical Trials Unit, London, United Kingdom (with support from the MRC), INSERM SC10, Paris, France (supported by Agence Nationale de Recherche sur le SIDA), Program for HIV Prevention and Treatment, Chiang Mai, Thailand (with support from the PENTA Foundation and Institut de Recherche pour le Developpement–URI 174), and Westat, Maryland (supported by National Institute of Child Health and Human Development). Collaborating centers in the United Kingdom are supported by a grant from the MRC; those in Italy are supported by a grant from the Istituto Superiore di Sanità-Progetto Terapia Antivirale 2004, 2005. PENTA activities are also supported by the PENTA Foundation.
Potential conflicts of interest. The University of Liverpool HIV therapeutic drug monitoring service was developed into Delphic Diagnostics in 2005, and S.K. serves as nonexecutive director of Delphic Diagnostics. S.K. has also received research awards from Boehringer as part of 2 international therapeutic drug monitoring trials of tipranavir (Spring and Ticino). All other authors: no conflicts.
References
- 1.Arnedo-Valero M, Garcia F, Gil C, et al. Risk of selecting de novo drug-resistance mutations during structured treatment interruptions in patients with chronic HIV infection. Clin Infect Dis. 2005;41:883–90. doi: 10.1086/432881. [DOI] [PubMed] [Google Scholar]
- 2.Moore KH, Barrett JE, Shaw S, et al. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS. 1999;13:2239–50. doi: 10.1097/00002030-199911120-00006. [DOI] [PubMed] [Google Scholar]
- 3.Taylor S, Allen S, Fidler S, et al. Stop study: after discontinuation of efavirenz, plasma concentrations may persist for 2 weeks or longer [abstract 131]; Program and abstracts of the 11th Conference on Retroviruses and Opportunistic Infections; San Francisco, California. 2004. [Google Scholar]
- 4.Kikaire B, Khoo S, Walker AS, et al. Nevirapine clearance from plasma in African adults stopping therapy: a pharmacokinetic substudy. AIDS. 2007;21:733–7. doi: 10.1097/QAD.0b013e3280121801. [DOI] [PubMed] [Google Scholar]
- 5.Cressey TR, Jourdain G, Lallemant MJ, et al. Persistence of nevirapine exposure during the postpartum period after intrapartum single-dose nevirapine in addition to zidovudine prophylaxis for the prevention of mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2005;38:283–8. [PubMed] [Google Scholar]
- 6.Jourdain G, Ngo-Giang-Huong N, Le Coeur S, et al. Intrapartum exposure to nevirapine and subsequent maternal responses to nevirapine-based antiretroviral therapy. N Engl J Med. 2004;351:229–40. doi: 10.1056/NEJMoa041305. [DOI] [PubMed] [Google Scholar]
- 7.McIntyre J, Martnson N, Investigators for the Trial 1413 et al. Addition of short course combivir (CBV) to single dose viramune (sdNVP) for prevention of mother-to-child transmission (MTCT) of HIV-1 can significantly decrease the subsequent development of maternal NNRTI-resistant virus; Program and abstracts of the 15th International AIDS Conference; Bangkok, Thailand. 2004. [Google Scholar]
- 8.Dragsted UB, Gerstoft J, Pedersen C, et al. Randomized trial to evaluate indinavir/ritonavir versus saquinavir/ritonavir in human immunodeficiency virus type 1–infected patients: the MaxCmin1 Trial. J Infect Dis. 2003;188:635–42. doi: 10.1086/377288. [DOI] [PubMed] [Google Scholar]
- 9.Holland DT, DiFrancesco R, Stone J, Hamzeh F, Connor JD, Morse GD. Quality assurance program for clinical measurement of antiretrovirals: AIDS clinical trials group proficiency testing program for pediatric and adult pharmacology laboratories. Antimicrob Agents Chemother. 2004;48:824–31. doi: 10.1128/AAC.48.3.824-831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [Accessed 27 March 2008];Stanford University HIV database. Available at: http://hivdb.stanford.edu. [Google Scholar]
- 11. [Accessed 27 March 2008];HIV French Resistance Web site. HIV-1 genotypic drug resistance interpretation’s algorithms. AC11 ANRS algorithm. Available at: http://www.hivfrenchresistance.org. [Google Scholar]
- 12.Tariq Sadiq S, Fredericks S, Khoo SH, Rice P, Holt DW. Efavirenz detectable in plasma 8 weeks after stopping therapy and subsequent development of non-nucleoside reverse transcriptase inhibitor-associated resistance. AIDS. 2005;19:1716–7. doi: 10.1097/01.aids.0000186828.99032.60. [DOI] [PubMed] [Google Scholar]
- 13.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391–400. [PubMed] [Google Scholar]
- 14.Rotger M, Colombo S, Furrer H, et al. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Ribaudo HJ, Haas DW, Tierney C, et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group Study. Clin Infect Dis. 2006;42:401–7. doi: 10.1086/499364. [DOI] [PubMed] [Google Scholar]
- 16.Ellis GM, Mahalanabis M, Beck IA, et al. Comparison of oligonucleotide ligation assay and consensus sequencing for detection of drug-resistant mutants of human immunodeficiency virus type 1 in peripheral blood mononuclear cells and plasma. J Clin Microbiol. 2004;42:3670–4. doi: 10.1128/JCM.42.8.3670-3674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C, Eshleman SH, Jones D, et al. LigAmp for sensitive detection of single-nucleotide differences. Nature Methods. 2004;1:141–7. doi: 10.1038/nmeth713. [DOI] [PubMed] [Google Scholar]
- 18.Palmer S, Boltz V, Martinson N, et al. Persistence of nevirapine-resistant HIV-1 in women after single-dose nevirapine therapy for prevention of maternal-to-fetal HIV-1 transmission. Proc Natl Acad Sci USA. 2006;103:7094–9. doi: 10.1073/pnas.0602033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yap SH, Sheen CW, Fahey J, et al. N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Medicine. 2007;4:e335. doi: 10.1371/journal.pmed.0040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson JB, Becker-Pergola G, Guay LA, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14:F111–5. doi: 10.1097/00002030-200007280-00001. [DOI] [PubMed] [Google Scholar]
- 21.Eshleman SH, Church JD, Chen S, et al. Comparison of HIV-1 mother-to-child transmission after single-dose nevirapine prophylaxis among African women with subtypes A, C, and D. J Acquir Immune Defic Syndr. 2006;42:518–21. doi: 10.1097/01.qai.0000221676.22069.b8. [DOI] [PubMed] [Google Scholar]