Abstract
Hyalinizing clear cell carcinoma (HCCC) is a unique low-grade tumor composed of cords and nests of clear cells in a hyalinized stroma that was first reported by Milchgrub et al. It was recognized as a separate entity from clear cell variants of epithelial-myoepithelial carcinoma, myoepithelial carcinoma and mucoepidermoid carcinoma. HCCC is included in a long list of clear cell-containing tumors of salivary gland, as well as odontogenic tumors and metastases (renal cell carcinoma). Up until now, it has been considered a diagnosis of exclusion, despite its very distinctive appearance, and labeled as “not otherwise specified” by the World Health Organization. The emergence of molecular data in salivary gland tumors, including HCCC now allow for a more rigorous appraisal of its spectrum. The EWSR1-ATF1 fusion has proven the concept of a “mucinous HCCC” and removes mucin as an exclusion criterion for this tumor. It has also proven a genetic link between clear cell odontogenic carcinoma and HCCC. Molecularly-proven cases have also highlighted variant morphologies and shown that cases with overt squamous differentiation are true HCCC. This gives further weight to the classification of this tumor as squamous or adenosquamous in differentiation and as a specific entity rather than an “NOS” tumor.
Keywords: Hyalinizing clear cell carcinoma, EWSR1, ATF1, Salivary gland
Introduction
Within the salivary gland and seromucous gland sites of the head and neck there is a long list of benign and malignant salivary type tumors that a general or head and neck pathologist may encounter. Most of these are rare and there is considerable overlap in morphology and immunohistochemistry between the different entities. Over the years, some of these tumors have been found to have special variants, such as the oncocytic variant of mucoepidermoid carcinoma (MEC) [1]. This has made the differential diagnosis that much more difficult to navigate. In fact, oncocytic change and clear cell change are ubiquitous findings that have been reported in nearly every salivary tumor, either focally or even commonly and diffusely. Similarly, squamous differentiation, mucous cells and ducts can be found in varying proportions in most salivary tumors, particularly those resembling or arising from the large duct system (striated and excretory ducts). The finding of occasional clear cells in most salivary tumors has led to a number of review articles on clear cell salivary tumors, with one of these aptly named “clearing up clear cell tumors” [2]. The differential diagnosis of clear cell tumors of salivary gland and head and neck in general is a common problem and there have been few reports offering new insights into their distinction and pathogenesis. This is perhaps where molecular pathology will come to the rescue.
The finding of specific and recurrent translocations, point mutations and amplifications in solid tumors has led to additional help in diagnosis and in some cases has offered new hope in prognostication and treatment [3–5]. There are now four recurrent translocations known in malignant salivary gland tumors: the MYB-NFIB fusion of adenoid cystic carcinoma (AdCC) [5], the CRTC1-MAML2 fusion in MEC [3], the ETV6-NTRK3 fusion in the recently discovered mammary analog secretory carcinoma (MASC) [6] and the EWSR1-ATF1 fusion in HCCC [7]. This is likely an evolving list as many low-grade salivary tumors that lack precursor lesions and are generally homogenous from case to case can be speculated to also have translocations, e.g., epithelial-myoepithelial carcinoma (EMC).
This review will focus on the discovery of “hyalinizing clear cell carcinoma (HCCC)” as a distinct entity, the recent discovery of the recurrent EWSR1-ATF1 fusion transcript found in the majority of HCCC, and how this translocation has proven and disproved previous conceptions about HCCC. In particular, it will focus on what new things it has taught us about the entity. The review is not intended to provide an exhaustive morphologic description of the entity or discuss the entire differential diagnosis.
Discussion
Hyalinizing clear cell carcinoma (HCCC) was first reported almost 20 years ago by Milchgrub et al. [8] and since then has been followed-up by numerous case reports and reviews on the subject. Until recently, however, there was little new information provided on the subject. It is typically accepted, in its classical form, as a low-grade carcinoma composed of clear cells embedded in a hyalinized stroma and showing cords and nests of tumor cells (Fig. 1). Milchgrub reported this tumor, in part, to separate it from the “monophasic” variant of EMC lacking ducts, from clear cell myoepithelial carcinoma (MyoEC), and from mucin-depleted MEC [8]. This was done by the consistent absence of myoepithelial immunohistochemical staining and mucin, respectively. In fact, both of these were considered exclusion criterion for this entity. The tumors were found to rarely recur or metastasize to lymph nodes and to never metastasize hematogenously or to lead to mortality.
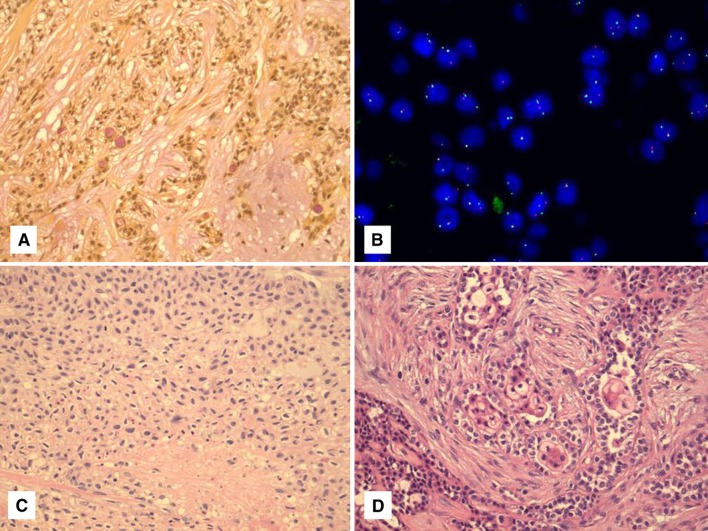
Fig. 1.
Typical appearance of HCCC composed of clear cells embedded in a hyalinized stroma arranged in cords and nests
Recently, we investigated the possible relationship of HCCC to “soft tissue myoepithelial tumors (SMET)”, which were found to harbor a EWSR1 rearrangement in 45 % of cases by Antonescu et al. [9]. The partner genes identified in this subset of tumors differed from other sarcomas and consisted of PBX1, ZNF444 and POU5F1 [9]. There are likely other partners as many of the EWSR1 rearranged tumors did not have one of these three gene partners by fluorescence in situ hybridization (FISH). It is perhaps a contradiction in terms to compare HCCC (where myoepithelial staining is not allowed) to SMET, which by definition is supposed to be myoepithelial in differentiation. The rationale for the comparison is twofold. First, SMET is considered to be myoepithelial based largely on S100 and GFAP staining, and an extensive study of true myoid markers has not been performed to date (in contrast, HCCC is always negative for S100, GFAP and all myoid markers). Second, there was frequent clear cell differentiation in SMET, particlarly those with a EWSR1-POU5F1 fusion [9]. Given the single report of a “poorly-differentiated MEC” of parotid and eccrine adnexal tumors of skin with this identical fusion [10], it seemed reasonable to investigate this in salivary gland tumors in general as well.
In our study of 23 cases of HCCC, 82 % showed a EWSR1 rearrangement; however, there were no cases with a POU51 rearrangement by FISH, thus disproving the link to SMET [7]. One case was successfully investigated by 3′RACE (rapid amplification of copy DNA ends), which found ATF1 as the partner gene. This gene was further confirmed as the consistent partner gene in HCCC by subsequent ATF1 FISH. There was a single case which lacked this partner gene and this may suggest an alternative, as yet undiscovered, partner gene for EWSR1. The finding of a number of different tumors in soft tissue, skin, salivary gland and other sites all harboring EWSR1 gene rearrangements, suggests an important role in oncogenesis for this gene. Within salivary gland however, the EWSR1 rearrangement by FISH serves as a specific marker and important new diagnostic tool to help finally resolve the “clear cell dilemma”. No other salivary tumor has convincingly shown this change [7, 11]. The finding of EWSR1 gene rearrangement, and EWSR1-ATF1 in particular, has also led to some new insights into this tumor that may help dispel some myths about the entity in general. Although a similar fusion is seen in clear cell sarcoma, clear cell sarcoma-like tumor of the gastrointestinal tract, and some angiomatoid fibrous histiocytomas, the completely different morphology, presence of squamous differentiation and complete lack of myoid (desmin) or melanocytic (HMB45, Melan-A) differentiation easily separates this tumor from other EWSR1-ATF1 fusion-positive entities.
Hyalinizing Clear Cell Carcinoma Can Have Minimal Clear Cell Differentiation and/or Lack Significant Hyalinization/Sclerosis
We have had the opportunity to examine several dozen cases of HCCC now with most having molecular confirmation. One recent example presented with a core biopsy of the neck in the “submandibular area”. It consisted of a solid and vaguely nested eosinophilic tumor with low-grade features, myxoid change and plasmacytoid cells (Fig. 2a) and showed no specific staining other than for pankeratin. The differential diagnosis was between a primary salivary tumor (benign or malignant) versus a metastatic carcinoma (with the additional red herring of p16 expression). The excision specimen clearly demonstrated its submandibular gland origin rather than a lymph node metastasis and showed a largely solid tumor composed of a reticular pattern of eosinophilic cells (Fig. 2b, c) with focal myxoid areas, focal palisading of cells and focal clear cells. There was rare sclerosis and inflammation in the background but there was no convincing pattern of the typical dense hyalinization with cords and small nests of cells expected in HCCC. At this point, the tumor did not have the typical appearance of any specific salivary tumor, with some thought given to an unusual acinic cell carcinoma. The focal clear cells prompted a FISH study for EWSR1 which was positive and suggested the possibility of HCCC. Subsequent FISH for ATF1 was performed and was also positive confirming the diagnosis. Incidentally, a repeat of the p16 staining was negative in the tumor resection and focal CK5 staining was found as well, perhaps suggesting some squamous differentiation.
Fig. 2.
a Core biopsy of a neck tumor showing sheets and nests of eosinophilic cells with minimal hyalinization. b, c The resection specimen of this case showed a largely solid tumor composed of eosinophilic cells and forming a reticular pattern. This tumor showed both EWSR1 and ATF1 rearrangement by FISH (not shown). d Another example of HCCC showing a basaloid-like morphology with focal clearing of the central cells within the nests
Our original study also described two examples of HCCC with basaloid-like features [7]. One was a pure tumor with this finding, which showed clear cells in the center of the nests (Fig. 2d). Another showed areas of classic HCCC and others with this same basaloid change. We have seen a third such case without sufficient material for molecular confirmation. This basaloid change from low-power imparted a “high-grade” appearance but, interestingly, the cases showed minimal mitotic activity and the atypical nuclei were uniformly enlarged rather than truly pleomorphic. This suggests the tumors were still low-grade, although a formal grading system does not exist for HCCC. We have also seen a case with what we called “high-grade transformation” [12] that initially presented as a solid, partially clear cell-containing tumor with mitotic activity and necrosis and lacking squamous differentiation by immunohistochemistry. This tumor re-presented as a typical HCCC in the same site within 1 year of radiation therapy. Both were confirmed by EWSR1 FISH. A few recent cases in our practice with radiological evidence of lung metastases or very large presentations of neck metastases with no known primary, have also suggested the need for prognostic markers or a grading system in the future. Whether solid growth and/or lack of clear cells actually indicate a worse prognosis, higher-grade, or tendency for atypical presentation, is not known at this time.
One obvious question that this latter discussion raises is: what makes an entity distinct? Its morphologic and immunohistochemical profile, its molecular findings, or both? Could the case example illustrated above be something else with the same molecular finding? Is the focal clear cell differentiation coupled with this molecular finding enough to include the case within the HCCC spectrum or call it a variant morphology? This is a difficult question to tackle, and will become more debated as new molecular markers are found in other salivary tumors and overlap between entities is discovered. At this time, it appears that the EWSR1-ATF1 is unique to HCCC with no other tumor of salivary gland sharing this marker [7, 11].
Intracellular Mucin is Not an Exclusion Criterion for HCCC and Does Not Warrant Classification as Mucoepidermoid Carcinoma in These Tumors
In addition to excluding myoepithelial differentiation in HCCC, Milchgrub et al. [8] argued that it did not represent a “mucin-depleted” form of MEC. The rationale was the lack of mucin or squamous differentiation in these tumors. The suggestion that mucin was not allowed in HCCC stuck and was considered an exclusion criterion until recently [7]. In fact, some consider focal mucin to represent the clear cell variant of MEC rather than HCCC. The literature even shows that the vast majority of “clear cell MECs” are found in the oral cavity (like HCCC) despite the fact that half of conventional MEC arise in major salivary gland [13] and most oncocytic MEC arise in the parotid [1]. Although MEC certainly can have focal clear cells, it was our theory that a pure clear cell MEC may not exist and that mucin is frequently seen in tumors that are otherwise identical to HCCC. This would also explain the oral predilection of “clear cell MEC”.
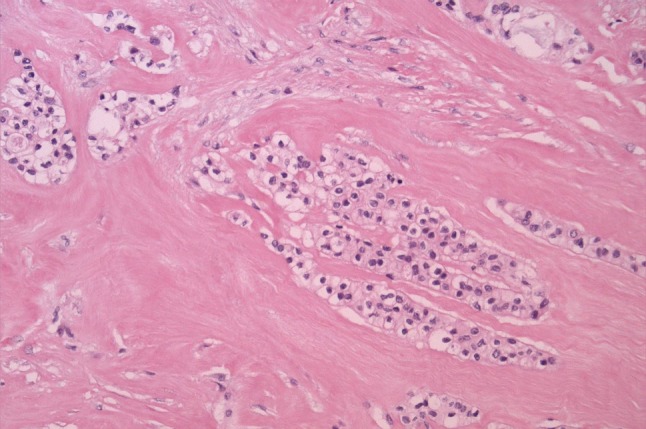
In investigating this concept, we found that amongst tumors called HCCC, nearly half showed mucinous differentiation [7]. Many of these were focal dot-like intracellular mucin but a number of them also showed diffuse mucinous differentiation (Fig. 3a). Almost all of them showed EWSR1 and ATF1 rearrangement by FISH confirming their relationship to HCCC rather than MEC (Fig. 3b). Moreover, none of these tumors showed MAML2 rearrangement, typical of a large proportion of conventional and oncocytic MEC [14, 15]. This is not surprising as conventional and oncocytic MEC rarely have the dense sclerosis and cord-like growth of tumor cells that HCCC has. In addition, none of these mucinous HCCC showed cysts lined by goblet cells, a feature typical of low-grade MEC. The idea that mucin should be an exclusion criterion for any tumor is also without basis in evidence, as mucin can be seen in classic examples of polymorphous low-grade adenocarcinoma (PLGA), salivary duct carcinoma (SDC), MASC [16], and other tumors, in our experience. Incidentally, there was focal mucin in a small proportion of cases of HCCC originally described by Milchgrub et al. [8], which is surprising given the argument they used to exclude this tumor from “mucin-depleted MEC”. Milchgrub also suggested this separation based on the lack of squamous differentiation, which is also not supported by the evidence they presented or which has subsequently come to light (see more below). Mucin cannot be considered an exclusion criterion for HCCC.
Fig. 3.
a Almost half of all HCCC cases (44 %) show mucinous differentiation either focally or diffusely. b The same case as above showed EWSR1 and ATF1 rearrangement by FISH (EWSR1 FISH shown here). Cells showing one normal fused yellow signal and one “break apart” signal with separated green and red signals indicating EWSR1 rearrangement. c, d Extensive squamous differentiation or focal squamous pearls can both be seen in molecularly proven HCCC
Hyalinizing Clear Cell Carcinoma has Both Squamous and Occasional Glandular Differentiation and Represents a Special Type of Adenosquamous Carcinoma
In their original description, Milchgrub et al. [8] refer to a lack of squamous differentiation in HCCC. However, they specifically mention the high molecular weight keratin positivity in the tumors. This has been found in most reviews of HCCC along with p63 and CK5/6 staining [7, 17, 18], highly suggestive of squamous differentiation. In addition, the electron microscopy in the original description highlights cytoplasmic pools of glycogen, tonofilaments of keratin and desmosomes [8]. These are in fact all features of squamous differentiation, which is shared with squamous carcinoma, HCCC and MEC. It could not, therefore, have been used as a distinguishing finding between HCCC and MEC. Dardick and Leong [19] reviewed the original ultrastructural features from the original electron micrographs and concluded that HCCC was indeed a squamous lesion, a theory supported by Bilodeau et al. [20]. Our study of 23 cases, like Bilodeau et al.’s, showed frequent squamous pearls and occasional keratinization in HCCC. One recent case also showed extensive squamous differentiation (Fig. 3c) and was proven by molecular findings. In fact, the majority of EWSR1 rearranged tumors in our cohort have shown both 34βE12 and p63 expression [7]. The tumor that originally was used for 3′RACE to discover EWSR1-ATF1 was one of them (Fig. 3d).
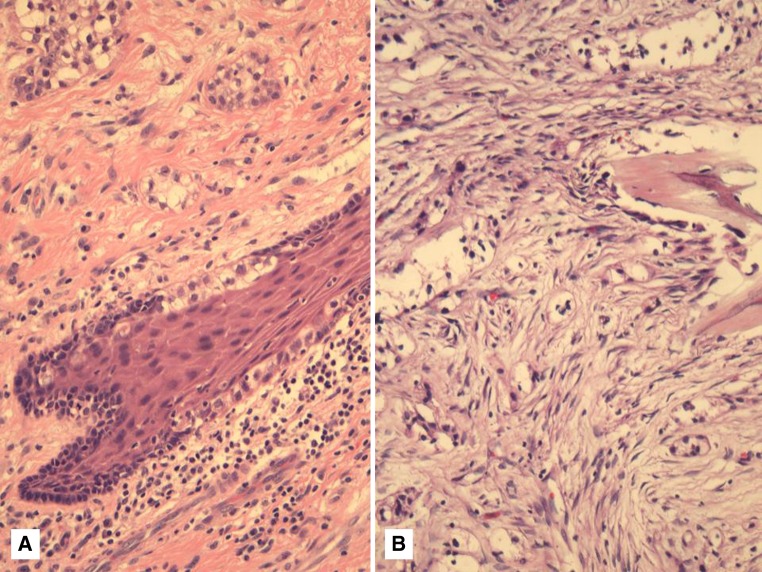
In addition to squamous differentiation and oral predominance, HCCC has frequent connection to the surface mucosal epithelium [7] (Fig. 4a). This is sometimes in the form of pagetoid extension of cells. Whether HCCC could actually be of mucosal origin rather than salivary gland is an interesting discussion. However, cases in parotid, submandibular gland, and central examples [21] have been reported and these cases do not support this concept entirely. Dardick’s suggestion of HCCC representing a squamous lesion is only partly accurate. Occasional glands can be seen and the fact that almost half of cases have intracellular mucin (proven by molecular) suggests that the tumor may represent a special type of adenosquamous carcinoma. HCCC is certainly not a pure adenocarcinoma, as the current fascicle of the Armed Forces Institute of Pathology [22] suggests by labeling it as “(hyalinizing) clear cell adenocarcinoma”. It is also not appropriate anymore to label this tumor a “clear cell carcinoma, not otherwise specified” which was adopted by the WHO “blue book” on head and neck tumors [13]. The consistent morphology, typical oral cavity location, and recurrent translocation mean that this tumor is very much a specific entity, whereas “NOS” suggests a waste basket category for any salivary tumor with clear cells that cannot be further classified. This latter category may remain useful for tumors that do not fit any category, including HCCC, and are translocation-negative.
Fig. 4.
a Extension of clear cells into the overlying squamous epithelium is a common finding in oral cavity examples of HCCC. b A CCOC within bone, which is morphologically, immunohistochemically and molecularly identical to HCCC and, therefore, represents a “central HCCC”
Clear Cell Odontogenic Carcinoma Represents HCCC Arising Within Bone (“Central HCCC”)
In a study comparing HCCC and clear cell odontogenic carcinoma (CCOC), Bilodeau et al. [20] showed that there was no reliable morphologic or immunohistochemical finding that could serve as a distinguishing factor (Fig. 4b). This included staining with squamous markers. They concluded that the distinction rested with location, with CCOC arising within bone from the dental lamina and HCCC arising within submucosal minor salivary glands. Those tumors that involved both would of course continue to be difficult to separate. Following our discovery of the EWSR1-ATF1 in HCCC, Bilodeau et al. [23] performed the molecular testing to further investigate this relationship. Of the 12 cases of CCOC tested, 8 showed successful hybridization with EWSR1 and, of those, 5 showed EWSR1 rearrangement. One of these cases was subsequently tested for ATF1, which was also positive, confirming the molecular overlap between these entities. Two of the EWSR1-negative cases were also reclassified as clear cell calcifying epithelial odontogenic tumors after amyloid was confirmed by Congo red staining [23]. Congo red is always negative in HCCC [8]. The frequency of EWSR1 rearrangement in CCOC is therefore 83 % (5/6) confirming the overlap with HCCC. CCOC therefore represents a central variant of HCCC as originally reported by Berho and Huvos [21]. CCOC can therefore be considered an odontogenic analog of HCCC.
Conclusions
Although classified as a clear cell carcinoma, NOS and references to it being a diagnosis of exclusion have long persisted [13], we now know from molecular evidence that HCCC is in fact a specific and reproducible entity [7]. It is distinct from MEC even when it contains mucin and is essentially the same entity as CCOC [7, 23]. It should be recognized as such in the next iteration of the WHO “blue book” for head and neck tumors [13]. The molecular findings have further allowed us to recognize new features that may eventually amount to variant morphologies (reticular, basaloid, etc.) and that not all HCCC have clear cells, hyalinization, or are low-grade. Although not all HCCC are clear cell dominant, we have yet to encounter one without at least focal clear cells and this finding should prompt FISH analysis for EWSR1 when the diagnosis is not readily apparent. This test is useful not only because it is sensitive and specific to HCCC but also because it is widely available in many labs owing to its use in other tumors in soft tissue. With the emergence of additional translocations and mutations associated with salivary gland neoplasms, it may in time be possible to generate a clinically-relevant and morphologically-consistent “molecular classification of salivary gland cancer”.
Acknowledgments
We would like to acknowledge all the efforts of Dr. Cristina R. Antonescu, Lei Zhang and Yun Shao Sung from the Memorial Sloan-Kettering Cancer Center for all their efforts in our identification of the EWSR1-ATF1 fusion, without which this manuscript would not be possible.
References
- 1.Weinreb I, Seethala RR, Perez-Ordoñez B, Chetty R, Hoschar AP, Hunt JL. Oncocytic mucoepidermoid carcinoma: clinicopathologic description in a series of 12 cases. Am J Surg Pathol. 2009;33(3):409–416. doi: 10.1097/PAS.0b013e318184b36d. [DOI] [PubMed] [Google Scholar]
- 2.Barber B, Côté D, Seikaly H. Clearing up clear cell tumours of the head and neck: differentiation of hyalinizing and odontogenic varieties. J Otolaryngol Head Neck Surg. 2010;39(5):E56–E60. [PubMed] [Google Scholar]
- 3.Tonon G, Modi S, Wu L, Kubo A, Coxon AB, Komiya T, O’Neil K, Stover K, El-Naggar A, Griffin JD, Kirsch IR, Kaye FJ. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33(2):208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 4.Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. 2010;16(8):2266–2274. doi: 10.1158/1078-0432.CCR-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persson M, Andrén Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proc Natl Acad Sci USA. 2009;106(44):18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, Starek I, Geierova M, Simpson RH, Passador-Santos F, Ryska A, Leivo I, Kinkor Z, Michal M. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 7.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, Perez-Ordoñez B, Have C, Asa SL, Leong IT, Bradley G, Klieb H, Weinreb I. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50(7):559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 8.Milchgrub S, Gnepp DR, Vuitch F, Delgado R, Albores-Saavedra J. Hyalinizing clear cell carcinoma of salivary gland. Am J Surg Pathol. 1994;18(1):74–82. doi: 10.1097/00000478-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, Edelman M, Rosenberg AE, Nielsen GP, Dal Cin P, Fletcher CD. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49(12):1114–1124. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Möller E, Stenman G, Mandahl N, Hamberg H, Mölne L, van den Oord JJ, Brosjö O, Mertens F, Panagopoulos I. POU5F1, encoding a key regulator of stem cell pluripotency, is fused to EWSR1 in hidradenoma of the skin and mucoepidermoid carcinoma of the salivary glands. J Pathol. 2008;215(1):78–86. doi: 10.1002/path.2327. [DOI] [PubMed] [Google Scholar]
- 11.Shah AA, LeGallo RD, van Zante A, Frierson Jr. HF, Mills SE, Berean KW, Mentrikoski MJ, Stelow EB. EWSR1 genetic rearrangements in salivary gland tumors: a specific and very common feature of hyalinizing clear cell carcinoma. Am J Surg Pathol (in press). [DOI] [PubMed]
- 12.Jin R, Craddock KJ, Irish JC, Perez-Ordonez B, Weinreb I. Recurrent hyalinizing clear cell carcinoma of the base of tongue with high-grade transformation and EWSR1 gene rearrangement by FISH. Head Neck Pathol. 2012;6(3):389–394. doi: 10.1007/s12105-012-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnes L, Eveson JW, Reichart P, et al., editors. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 14.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 15.García JJ, Hunt JL, Weinreb I, McHugh JB, Barnes EL, Cieply K, Dacic S, Seethala RR. Fluorescence in situ hybridization for detection of MAML2 rearrangements in oncocytic mucoepidermoid carcinomas: utility as a diagnostic test. Hum Pathol. 2011;42(12):2001–2009. doi: 10.1016/j.humpath.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Connor A, Perez-Ordoñez B, Shago M, Skálová A, Weinreb I. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum of a recently described entity. Am J Surg Pathol. 2012;36(1):27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 17.O’Sullivan-Mejia ED, Massey HD, Faquin WC, Powers CN. Hyalinizing clear cell carcinoma: report of eight cases and a review of literature. Head Neck Pathol. 2009;3(3):179–185. doi: 10.1007/s12105-009-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solar AA, Schmidt BL, Jordan RC. Hyalinizing clear cell carcinoma: case series and comprehensive review of the literature. Cancer. 2009;115(1):75–83. doi: 10.1002/cncr.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dardick I, Leong I. Clear cell carcinoma: review of its histomorphogenesis and classification as a squamous cell lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(3):399–405. doi: 10.1016/j.tripleo.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 20.Bilodeau EA, Hoschar AP, Barnes EL, Hunt JL, Seethala RR. Clear cell carcinoma and clear cell odontogenic carcinoma: a comparative clinicopathologic and immunohistochemical study. Head Neck Pathol. 2011;5(2):101–107. doi: 10.1007/s12105-011-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berho M, Huvos AG. Central hyalinizing clear cell carcinoma of the mandible and the maxilla a clinicopathologic study of two cases with an analysis of the literature. Hum Pathol. 1999;30(1):101–105. doi: 10.1016/S0046-8177(99)90308-8. [DOI] [PubMed] [Google Scholar]
- 22.Ellis GL, Auclair PL. Malignant epithelial neoplasms. In: Silverberg SG, Sobin LH, editors. Armed Forces Institute of Pathology (AFIP) Atlas of tumor Pathology. Tumors of the salivary glands (fourth series, fascicle 9). Maryland: ARP Press; 2008. p. 301–9.
- 23.Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, Barker B, Seethala RR. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding & biologic link to salivary clear cell carcinomas. Mod Pathol. 2012;25 (Supplement 2s):101:305A. [DOI] [PubMed]