Abstract
Fibro-osseous lesions of the maxillofacial bones should be classified based on their radiographic growth pattern. This method can simplify this category of lesions, which have considerable overlapping histologic features. These neoplasms can be grouped into three categories: (a) fibrous dysplasia; (b) ossifying fibroma; (c) and osseous dysplasia. Important lesions in the differential diagnosis are osteoblastoma and giant cell reparative granuloma.
Keywords: Fibrous dysplasia, Ossifying fibroma, Osseous dysplasia, Osteoblastoma, Giant cell reparative granuloma
Introduction
Fibro-osseous lesions of the maxillofacial bones are benign proliferations of spindle cells with varying amounts of woven bone. Many specific entities have been proposed based on histologic and radiographic features. However, because there is considerable overlap of histologic features in these lesions, the confusing nomenclature can be simplified by defining lesions based on their radiographic presentation. These neoplasms can all fit into one of three categories: (a) fibrous dysplasia; (b) ossifying fibroma; (c) and osseous dysplasia. These entities all have overlapping histologic features and are defined only by their growth pattern as apparent on plain radiographs or CT scans of the head and face.
Histopathologic Features
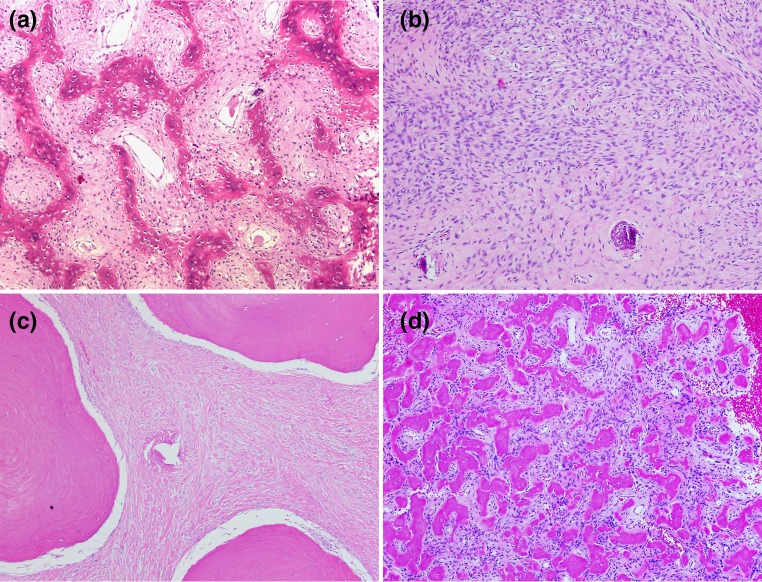
All fibro-osseous lesions of the jaw and face are variations of the same histologic pattern [1]. This pattern consists of a bland spindle cell population mixed with varying amounts of woven bone and occurs in fibrous dysplasia, ossifying fibroma, and osseous dysplasia. The most typical pattern is that seen with classic fibrous dysplasia. Spindle cells are intermixed throughout with woven bone. The woven bone is dispersed in the fibrous background in a pattern classically described as “Chinese Letters” (Fig. 1a). Almost always, there are no phenotypic osteoblasts seen synthesizing this bone. The amount of woven bone production in these lesions varies. In some cases, the amount of woven bone is minimal and the lesion consists predominantly of very cellular spindle cells (Fig. 1b). Other times, woven bone can be quite abundant and occurs as large islands of bone. Sometimes woven bone shows early transformation into lamellar bone (Fig. 1c). Another pattern of bone formation is the formation of small osteoid globules. These globules are often called “cementicles” (Fig. 1d). Lesions with abundant ossification in this matter have also been given the subtype as “cementoma”. This pattern of ossification should not warrant a separate diagnostic category, and the term cementoma should not be used. Cementum has the same chemical make-up as bone in its relationship of type 1 collagen to calcium hydroxyapatite crystals. When this tissue is not associated with the tooth root, as is normal cementum, it loses its identity as a specific tissue. Also, this globular pattern of bone formation is seen in lesions of fibrous dysplasia in the post cranial skeleton [1, 2]. It is also seen associated with other bone forming neoplasms such as osteoblastoma and osteosarcoma. Sometimes this pattern of mineralization occurs in the lining of unicameral bone cysts. For these reasons, we do not feel that this pattern of bone formation deserves a separate diagnostic category in the facial bones. Any of these three fibro-osseous lesions—fibrous dysplasia, ossifying fibroma, and osseous dysplasia—may have secondary aneurysmal bone cyst formation. This process can cause massive expansion of the lesions.
Fig. 1.
a A fibro-osseous pattern showing woven bone in the pattern of “Chinese letters” arranged in a spindle cell background (×40). b Fibro-osseous pattern with a predominance of spindle cells. Bone formation is minimal. c Fibro-osseous pattern showing broad plates of osteoid in a collagenized fibroblastic stroma. d Fibro-osseous pattern of globules of osteoid in a loose fibrous tissue background. This globular pattern has been called “cementum” (×40)
Fibrous Dysplasia
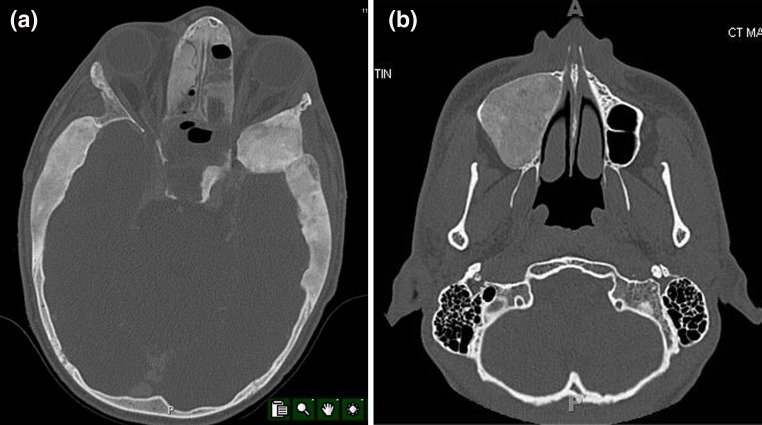
Fibrous dysplasia is a bone disorder wherein during skeletal growth normal bone is replaced by a dysplastic proliferation of fibrous tissue and woven bone. This disorder can occur focally or multifocally anywhere in the skeleton. It usually is first recognized in the second decade. Lesions of fibrous dysplasia may involve only one bone which accounts for 70 % of cases. Polyostotic fibrous dysplasia can involve random bones in the skeleton; however, lesions frequently occur in one extremity. Three percent of lesions are associated with skin pigmentation and hyperfunctioning endocrine disorders, which is known as the McCune–Albright syndrome. Fibrous dysplasia is caused by an activating point mutation in GNAS 1α [3]. This is a somatic mutation, and therefore, lesions are not passed to successive generations. Occasionally, identification of this mutation can be useful in distinguishing lesions of fibrous dysplasia from other fibro-osseous lesions. Radiographically, fibrous dysplasia in the postcranial skeleton is characterized by a long lesion in a long bone with a “ground glass” appearance. The ground glass texture is due to the radiodensity caused by the abundant woven bone production in the lesion. Lesions often have fuzzy transition zones. Rarely, lesions may expand the bone to cause structural weakening. The key radiographic feature of fibrous dysplasia in the jaw and facial bones is the diffuse nature of the process with poorly-defined borders (Fig. 2a). There is a texture of ground glass density in these lesions and often the calvarium is involved as well. The poorly-defined margin of fibrous dysplasia is a diagnostic clue. Also, fibrous dysplasia is the most likely diagnosis when there are lesions elsewhere in the skeleton. Therefore, patients with fibro-osseous lesions of the craniofacial skeleton should be evaluated with a bone scan for the presence of postcranial lesions. The identification of fibrous dysplasia is important in that treatment is generally conservative. Only if there is significant deformity might surgery be considered as an option in fibrous dysplasia.
Fig. 2.
a A fibrous dysplasia of the skull and sinuses. There is diffuse thickening of broad areas of the calvarium as well as an intrasinus lesion. The thickening is poorly-circumscribed and has a “ground glass” appearance. b Ossifying fibroma of the maxillary sinus. The lesion has a “ground glass” appearance but it is extremely well-demarcated. No other lesions in the face and skull are present
Ossifying Fibroma
Ossifying fibroma is a fibro-osseous lesion which can occur in any facial bone and can be distinguished from fibrous dysplasia in that it is usually well-demarcated (Fig. 2b). It is more common in the mandible than in the rest of the face. The term juvenile ossifying fibroma has been used for this lesion when patients are young [4]. However, most patients are affected in their third and fourth decade [5]. Radiographically, lesions are often expansile and destructive. In the sinonasal areas, large masses may develop. The most characteristic radiographic feature is sharp delineation from adjacent structures.
Two things help distinguish this lesion from classic fibrous dysplasia. First, lesions have been shown not to harbor the mutation in GNAS 1α [6, 7]. Also, patients with this fibro-osseous lesion generally do not have accompanying postcranial lesions.
Because of the expansile destructive nature of ossifying fibroma surgical excision is usually required. The recurrence rate is more common in younger patients.
Periapical Cemento-Osseous Dysplasia
The distinguishing feature of this disorder is that it is defined as a fibro-osseous lesion associated with the apex of the tooth. It may be unifocal or florid involving most of the mandible. The isolated periapical dysplasia is often an incidental radiographic finding (Fig. 3a). The more florid forms may be symptomatic [8]. One florid form occurs most commonly in middle-aged African-American females (Fig. 3b). Another form can be a large expansile lesion which has been called familiar gigantiform cementoma. This lesion has an autosomal dominant pattern of inheritance. Management of these lesions depends on their size. Usually, multifocal or unifocal periapical osseous dysplasia needs no treatment. Larger forms of the disorder may require surgery to prevent further destruction.
Fig. 3.
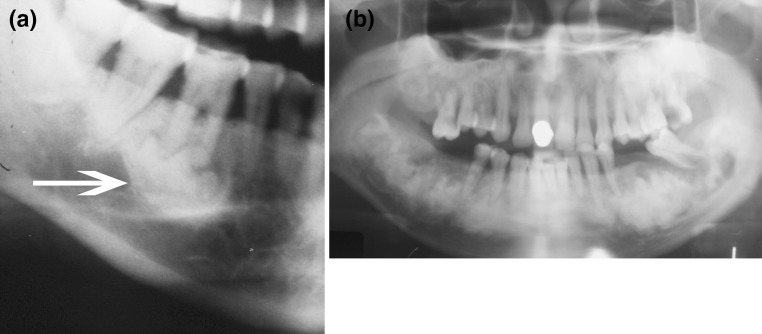
a Focal osseous dysplasia of the mandible. There is a periapical lucency filled with an irregular radiodensity (arrow). b Florid osseous dysplasia involving the apices of most of the teeth and the mandible. There is also involvement of the maxilla. The lesions are areas of radiolucency with central areas of irregular radiodensities
Differential Diagnosis
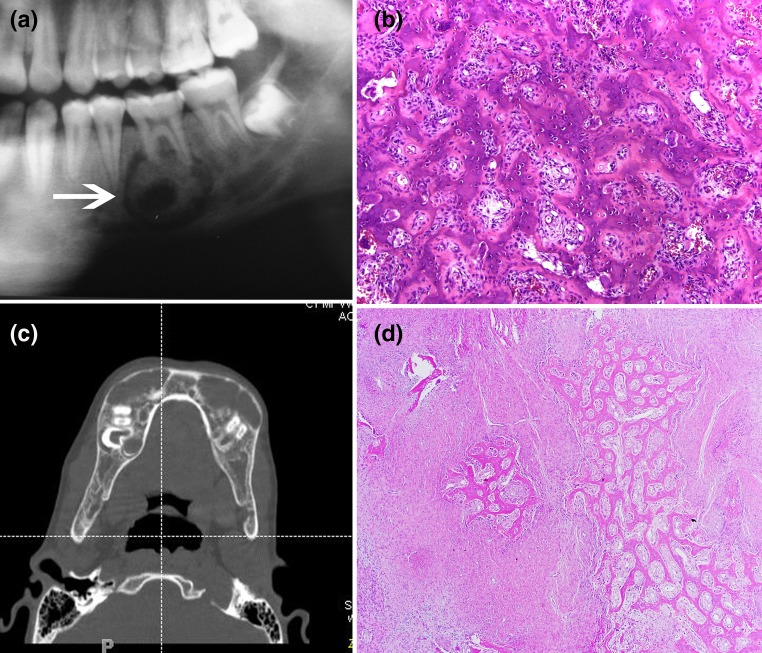
Fibro-osseous lesions of the jaw and face must be differentiated from other bone lesions which may mimic them histologically and radiographically. The most important lesions in the differential diagnosis are osteoblastoma and giant cell reparative granuloma. Osteoblastoma is a benign radiolytic bone-forming neoplasm which is most common in the postcranial skeleton, particular the posterior elements of the spine. It is a slow, but relentlessly growing, neoplasm which may destroy the structural architecture of the bone. Osteoblastomas also occur in the maxillofacial region [6, 9]. Radiographically, they are lytic lesions with focal radiodensity (Fig. 4a). In this area, they exhibit the same behavior of relentless growth that they do in the postcranial skeleton. Sometimes they grow very large and are regarded as “aggressive” osteoblastomas. Histologically, broad seams of interlacing osteoid are present with varying degrees of mineralization. The central feature to differentiate this pattern from fibro-osseous lesions is that the stroma does not consist of cellular spindle cells but rather a loose vascular stroma with numerous prominent epithelioid-type osteoblasts (Fig. 4b). This stromal component is the most important feature to differentiate osteoblastoma from a fibro-osseous process. Osteoblastomas must be curetted to stop their relentless growth.
Fig. 4.
a Osteoblastoma of the mandible. There is a poorly-defined radiolucency with interlesional radiodensities in the mandible. This lesion may be mistaken radiographically for osseous dysplasia. b Osteoblastoma. There are interlacing, lace-like, seams of osteoid in a background of loose fibrovascular tissue. Osteoblasts are prominent which indicate that this is an osteoblastic neoplasm. c Multiple giant cell reparative granulomas in the mandible known as cherubism. There is extreme symmetrical expansion of the mandible. d A giant cell reparative granuloma in the healing phase. There is a zonal deposition of reactive bone surrounding central areas of fibrous tissue. The giant cells are no longer present in this stage. This lesion may be mistaken for a fibro-osseous lesion
The second lesion which is often confused with a fibro-osseous process is giant cell reparative granuloma. In the craniofacial bones, this lesion is a well-defined lytic process. This lesion consists of multinucleated osteoclast-like giant cells, fibrous tissue and reactive bone in a zonal pattern. Often the giant cells are quite prominent and are associated with extravasated red blood cells. Giant cell reparative granuloma is a reactive process that is the result of the early repair of a large resorptive defect [10]. A florid form of multiple reparative granulomas, known as cherubism is an autosomal dominant inherited syndrome cause by mutation in the SH3BP2 gene on chromosome 4p16-3 [11]. Multiple expansile lytic lesions are present (Fig. 4c).
The natural history of both a single giant cell reparative granuloma and cherubism is to undergo spontaneous healing over time. As this healing process continues, the giant cells disappear leaving only the fibrous stroma and reactive bone in a zonal pattern. This zonal pattern is the most distinctive feature of giant cell reparative granuloma in both the early and in the healing phases and should distinguish giant cell reparative granuloma from a fibro-osseous lesion (Fig. 4d).
Another lesion which has been confused with fibro-osseous lesions is the so-called “sclerosing osteomyelitis”. This should not be regarded as a specific entity in the jaw. It is the same process of chronic osteomyelitis in any bone which is characterized radiologically by broad zones of sclerosis. Histologically, there is abundant reactive bone and the intervening space is filled with fibroinflammatory tissue. This fibroinflammatory tissue enables this lesion to be recognized as chronic infection. However, the diagnosis of osteomyelitis can only be rendered provided there has been an intraoperative culture that is positive for organisms. We do not make the diagnosis of osteomyelitis without a positive culture.
Finally, osteosarcomas may occur in the jaw. However, most osteosarcomas in the face and jaw are chondroblastic osteosarcomas and are rarely confused with a fibro-osseous process. On occasion, a conventional osteoblastic osteosarcoma may occur in the jaw. These are easily distinguished from a fibro-osseous lesion in that the stroma shows distinctly pleomorphic cells with abundant atypical mitotic figures.
Conclusion
Fibro-osseous lesions of the craniofacial skeleton should not be classified based on histologic features. There is considerable overlap of histologic changes in all these lesions, and sometimes all the different fibro-osseous patterns may be present in the same lesion. However, fibro-osseous lesions should be categorized based on radiographic appearance and growth pattern. Lesions that are poorly-delineated and involve large expanses of the craniofacial bones are most likely fibrous dysplasia. This pattern should be correlated with the presence of other lesions in the postcranial skeleton which would confirm the diagnosis of fibrous dysplasia. Unless there is considerable destruction of bone or the presence of aneurysmal bone cyst formation, lesions of fibrous dysplasia may be treated conservatively. The second pattern, ossifying fibroma, is a well-circumscribed process that may be radiolytic or have some degree of associated radiodensity. The expansile and well-defined growth pattern of this lesion, in the absence of other lesions in the skeleton, is the most important diagnostic feature. Finally, osseous dysplasia is best recognized by its association with the root of a tooth. Lesions may be focal or multifocal and, when possible, should be managed conservatively.
These patterns should help distinguish fibro-osseous lesions from other bone lesions in the face that have entirely different behaviors. Osteoblastoma must be recognized by its fibrovascular stroma with very prominent osteoblastic rimming of the osteoid seams. This is a lesion that must be treated aggressively. Giant cell reparative granuloma in its healing stage should be recognized by the zonal pattern of reactive bone. This lesion usually undergoes spontaneous regression and should only be surgically removed when there is extensive bony destruction.
References
- 1.Voytek TM, Ro JY, Edeiken J, Ayala AG. Fibrous dysplasia and cemento-ossifying fibroma. A histologic spectrum. Am J Surg Pathol. 1995;19:775–781. doi: 10.1097/00000478-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Sissons HA, Steiner GC, Dorfman HD. Calcified spherules in fibro-osseous lesions of bone. Arch Pathol Lab Med. 1993;117:284–290. [PubMed] [Google Scholar]
- 3.Tolman KG, Jubiz W, Sannella JJ, Madsen JA, Belsey RE, Goldsmith RS, Freston JW. Osteomalacia associated with anticonvulsant drug therapy in a pediatric outpatient population. Pediatrics. 1975;56:52. [PubMed] [Google Scholar]
- 4.Williams HK, Mangham C, Speight PM. Juvenile ossifying Fibroma. An analysis of eight cases and a comparison with other fibro-osseous lesions. J Oral Pathol Med. 2000;29:13–18. doi: 10.1034/j.1600-0714.2000.290103.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee RS, Weitzel S, Eastwood DM, Monsell F, Pringle J, Cannon SR, Briggs TWR. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J Bone Joint Surg Br. 2006;88:658–664. doi: 10.2106/JBJS.E.01156. [DOI] [PubMed] [Google Scholar]
- 6.Patel MM, Wilkey J, Abdelsayed R, D’Silva NJ, Malchoff C, Mallya SM. Analysis of GNAS mutations in cemento-ossifying fibromas and cemento-osseous dysplasias of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:739–743. doi: 10.1016/j.tripleo.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toyosawa S, Yuki M, Kishino M, Ogawa Y, Ueda T, Murakami S, Konoshi E, Iida S, Kogo M, Komori T, Tomita Y. Ossifying fibroma vs fibrous dysplasia of the jaw: molecular and immunological characterization. Mod Pathol. 2007;20:389–396. doi: 10.1038/modpathol.3800753. [DOI] [PubMed] [Google Scholar]
- 8.Brannon RB, Fowler CB. Benign fibro-osseous lesions: a review of current concepts. Adv Anat Pathol. 2001;8:126–143. doi: 10.1097/00125480-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Capodiferro S, Maiorano E, Giardina C, Lacaita MG, Lo Muzio L, Favia G. Osteoblastoma of the mandible: clinicopathologic study of four cases and literature review. Head Neck. 2005;27:616–621. doi: 10.1002/hed.20192. [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Cai Y, Zwahlen RA, Zheng Y, Wang S, Zhao Y. Central giant cell granuloma of the jaws: clinical and radiological evaluation of 22 cases. Skeletal Radiol. 2009;38:903–909. doi: 10.1007/s00256-009-0740-8. [DOI] [PubMed] [Google Scholar]
- 11.Ueki Y, Tiziani V, Santanna C, Fukai N, Maulik C, Garfinkle J, Ninomiya C, doAmaral C, Peters H, Habal M, Rhee-Morris L, Doss JB, Kreiborg S, Olsen BR, Reichenberger E. Mutations in the gene encoding c-Abl-binding protein SH3BP2 cause cherubism. Nat Genet. 2001;28:125–126. doi: 10.1038/88832. [DOI] [PubMed] [Google Scholar]