Abstract
Sinonasal intestinal-type adenocarcinomas (ITACs) are rare neoplasms histologically resembling intestinal adenocarcinomas. Although a neuroendocrine differentiation in ITACs has been described, true mixed exocrine-neuroendocrine carcinomas, neoplasms in which each component represents at least 30 % of the lesion, are extremely rare and their molecular alterations are largely unknown. We describe herein the clinico-pathologic features, the methylation profile, chromosomal gains and losses, and mutation analysis of KRAS, BRAF and p53 in a nasal mixed exocrine-neuroendocrine carcinoma resected in a 79-year-old man. The tumor was composed of an ITAC and a poorly differentiated neuroendocrine carcinoma. Both exocrine and neuroendocrine components were CK8, CK20, CDX2 and p53 positive, and CK7 and TTF1 negative. The neuroendocrine component also showed immunoreactivity for chromogranin A, synaptophysin, serotonin and glicentin. Gains and losses were found at following chromosome regions: 17p13 (TP53), 14q24 (MLH3), 19q13 (KLK3), 5q21 (APC), 7q21 (CDK6), 9q34 (DAPK1), 12p13 (TNFRSF 1A, CDKN1B), 13q12 (BRCA2), 17p13.3 (HIC1), 18q21 (BCL2), and 22q12 (TIMP3). Aberrant methylation was detected only in the neuroendocrine component and involved APC and DAPK1 genes. No mutation of KRAS (exons 2–4), BRAF (exon 15), and p53 (exons 4–10) was found in both components. The results suggest a monoclonal origin of the tumor from a pluripotent cell undergoing a biphenotypic differentiation and that the neuroendocrine differentiation may be from an exocrine to an endocrine pathway. We have also reviewed the literature on sinonasal mixed exocrine-neuroendocrine carcinomas to give to the reader a comprehensive overview of these very rare tumor types.
Keywords: Intestinal-type adenocarcinomas, Neuroendocrine carcinoma, Nasal cavity, Mixed adenoneuroendocrine carcinoma, Molecular profile
Introduction
Sinonasal epithelial malignant tumors are rare neoplasms accounting for less than 1:100,000 population per year and represent about 0.2–0.8 % of all malignant neoplasms [1]. Among various different histological tumor types that can originate in the sinonasal region, intestinal-type adenocarcinoma (ITAC) is a peculiar tumor which histologically resembles intestinal adenocarcinoma and is associated with specific occupational exposure including wood and leather dusts. Although the frequency among primary sinonasal malignancies and the true incidence are difficult to ascertain due to the rarity of such cancer, a male predominance and a mean patient age of 58 years have been established [2].
As in intestinal adenocarcinomas, the recognition of a neuroendocrine differentiation has been also described in nasal ITACs [3–6]. In gut adenocarcinomas a wide spectrum of combinations of both neuroendocrine and exocrine components has been accurately described [7], but mixed exocrine-neuroendocrine carcinomas, now classified as mixed adenoneuroendocrine carcinomas (MANECs), are by definition neoplasms in which each component represents at least 30 % of the lesion [8]. Only seven nasal MANECs have been described in the literature to date [9–13], but this entity was not included in the WHO classification of tumors of the head and neck, probably due to its rarity [1]. However, considering the close analogies between intestinal and sinonasal adenocarcinomas and considering that in the gut MANEC is regarded as a specific entity [8] this may also represent a distinct clinico-pathologic type in the sinonasal cavity.
So far, only a few studies addressed the molecular alterations in ITACs and no data are available about the pathogenesis of this tumor type. Despite its histological similarity to colorectal carcinomas, the genetic and epigenetic changes frequently involved in colorectal tumorigenesis have been demonstrated at lower frequency in ITACs [14–17]. Other investigations reported a close relationship between dust exposure and specific gene alterations, including promoter methylation of p14 and p16 as well as TP53 mutation and/or loss of 17p13 region [18].
In this report, we describe the clinico-pathologic, genetic and epigenetic features of a sinonasal ITAC with an abundant neuroendocrine component and we review the literature of this subject with the aim of better characterizing this rare tumor type.
Case Report
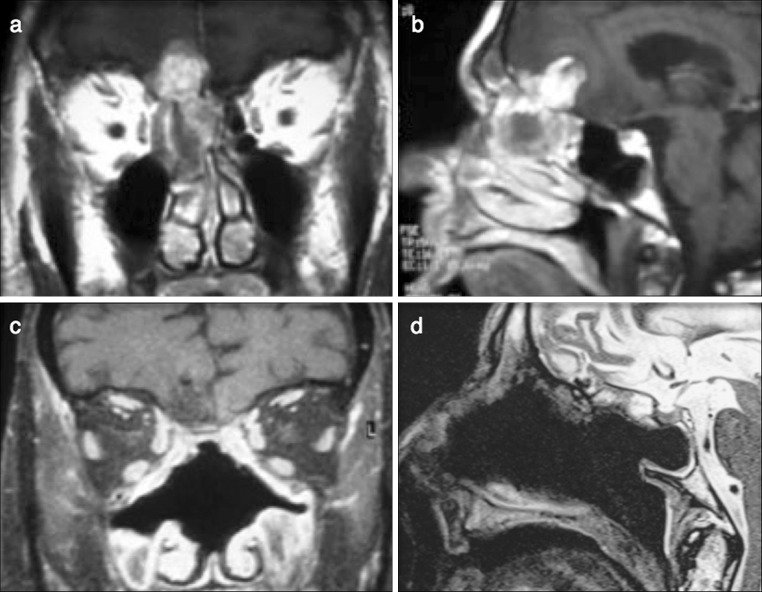
A 79-year-old patient underwent head and neck computer tomography (CT) scan because of a transitory ischemic attack and left arm weakness. The scan showed a right ethmoidal mass with intracranial involvement. The patient was a paperhanger and did not report respiratory airway obstruction, rhinorrhea, epistaxis or other nasal symptoms. To define the local extension of the disease more precisely, the patient also underwent head magnetic resonance imaging which showed an irregular hourglass shape lesion involving the ethmoidal complex and the right supraorbital recess, with a large intracranial extension apparently compressing the frontal lobe without infiltration of the brain parenchyma (Fig. 1). Endoscopic examination revealed a grayish ulcerated mass filling the right ostio-meatal complex, a biopsy was performed and the histological examination revealed an ITAC with a diffuse neuroendocrine component (see Results section for details). Staging of the disease included neck ultrasound, bone scintigraphy and total-body CT which did not indicate metastatic disease. The patient was considered eligible for a mini-invasive surgical treatment and underwent endoscopic endonasal tumor resection with trans-nasal craniectomy (Fig. 1). The disease was staged as pT4bN0M0 and postoperative radiotherapy was planned in a 54 Gy dose on the primary site of the tumor. No chemotherapy regimen was proposed because of the comorbity of the patient. The patient was free of disease until the 26th month after surgery when he developed bone metastases and died soon after. Autopsy was not performed.
Fig. 1.
The pre-operative magnetic resonance scan shows an irregular hourglass shape lesion involving the ethmoidal complex with a large intradural and intracranial extension (a, b). The post-operative magnetic resonance scan demonstrates the radical resection of the sinonasal and intracranial lesion (c, d)
Materials and Methods
Morphology and Immunohistochemistry
Tumor tissue was fixed in buffered formalin (formaldehyde 4 % w/v and acetate buffer 0.05 M) and routinely processed to paraffin wax. Serial sections were stained with hematoxylin and eosin (H&E) and Alcian-blue/periodic acid Schiff (AB-PAS) stains for the histopathologic evaluation. For immunohistochemistry, 5 μm-thick sections were mounted on poly-l-lysine coated slides, deparaffinized, quenched with 3 % hydrogen peroxide for 10 min and then incubated with primary antibodies (Table 1) at 4 °C for 18–20 h, followed by the avidin–biotin complex (ABC) procedure. Immunoreactions were developed using 0.03 % 3,3′diaminobenzidine tetrahydrochloride and then sections were counterstained with Harris’ hematoxylin.
Table 1.
Antibodies and antisera used
| Antibodies/antisera | P/M(Clone) | Dilution | Source |
|---|---|---|---|
| Synaptophysin | M (snp88) | 1:100 | BioGenex Laboratories, San Ramon, CA, USA |
| Chromogranin A | M (LK2H10) | 1:100 | Ventana, Tucson, AZ, USA |
| Cytokeratin | M (AE1/AE3) | 1:1 | Ventana, Tucson, AZ, USA |
| Cytokeratin 7 | M (OV-TL 12/30) | 1:200 | Dako, Glostrup, Denmark |
| Cytokeratin 8/18 | M (35BH11) | 1:100 | Dako |
| Cytokeratin 20 | M (Ks 20.8) | 1:100 | Dako |
| Glicentin | P | 1:7500 | Milab, Malmoe, Sweden |
| Somatostatin | P | 1:1500 | Dako |
| Pancreatic polypeptide | P | 1:4000 | Cambridge Research Biochemicals, Cambridge, UK |
| Serotonin | M (YC5) | 1:50 | Biogenesis, Bournemouth, UK |
| TTF1 | M (SPT24) | 1:100 | Novocastra, New Castle, UK |
| CDX2 | M (CDX2-88) | 1:100 | BioGenex Laboratories |
| CEA | M (TF3H8-1) | 1:1 | Ventana, Tucson, AZ, USA |
| APC | P | 1:800 | Santa Cruz Biotechnology Inc., Santa Cruza, CA, USA |
| P53 | M (D07) | 1:500 | Dako |
| Ki67 | M(MIB1) | 1:50 | Dako |
P/M polyclonal/monoclonal
Methylation –Specific Multiplex Ligation Probe Amplification (MS-MLPA)
Microspecimens of the exocrine and the endocrine component were manually microdissected from three 8 μm-cut histologic sections in order to obtain at least 70 % of tumor cells in both samples. DNA was extracted using a QIAamp® DNA FFPE Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Tumor DNA was obtained from formalin fixed and paraffin embedded tissue. The MS-MLPA assay was used to assess in both the tumor components the methylation status of 34 genes and copy number changes of 51 different DNA sequences (Table 2). The MS-MLPA analysis was performed using the two commercial kits SALSA MS-MLPA ME001 Tumor suppressor-1 Kit and SALSA MS-MLPA ME002 Tumor suppressor-2 Kit (MRC-Holland, Amsterdam, The Netherlands) according to the protocol previously reported [19]. Copy number and methylation variation were detected using Coffalyser V7 software (MRC-Holland). Identification of copy number changes was determined according to recommendations from MRC-Holland. Methylation dosage ratio (MR) was obtained by the following calculation: MR = (Px/Pctrl)Dig/(Px/Pctrl)Undig where Px is the peak area of a given target probe, Pctrl is the sum of the peak areas of all control probes, Dig stands for HhaI digested sample, and Undig stands for undigested sample. Based on our previous validation experiments aberrant methylation was scored as a categorical variable using a specific MR threshold for each gene corresponding to the highest level of accuracy of the test [19].
Table 2.
Copy number variations and promoter methylation analysis in the ITAC and NEC components
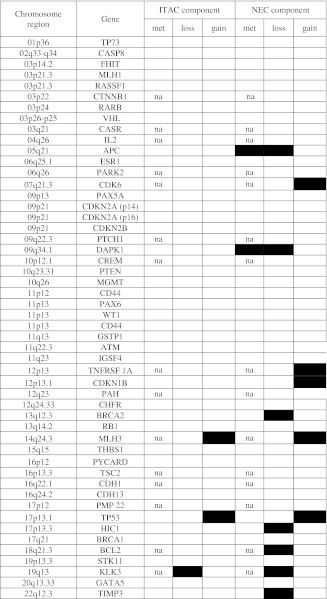
Met: methylation analysis; na: finding not available for the methylation status because the 15 reference probes do not contain HhaI digestion sites; the black boxes indicate the presence of methylation or of copy number variation depending on the specific column; the white boxes indicate the absence of methylation or of copy number variation
Mutational Analysis of KRAS, BRAF and TP53 Genes
KRAS mutations at codons 12, 13, 61 and 146 and BRAF at codon 600 were analyzed by pyrosequencing using an Anti EGFR MoAb response® (KRAS status) kit and an Anti EGFR MoAb response® (BRAF status) kit (Diatech Pharmacogenomics, Jesi, Italy). Exons 2–4 of KRAS and exon 15 of BRAF were PCR amplified according to the manufacturer’s instructions. Real-time reactions and post-PCR melting curve analysis were performed using a Rotor Gene 6000 (Corbett Research, Sydney, Australia). Pyrosequencing was carried out with Pyrogolds reagents on a PyroMark Q96 ID system (Qiagen) according to the manufacturer’s protocol. Pyrograms outputs were analyzed by the Pyromark Q24 software (Qiagen, Hilden, Germany) using the Allele Quantification software to determine the percentage of mutant versus wild-type alleles according to percentage relative peak height.
The specimens were also screened for the presence of TP53 mutations in exons 4-10 by direct sequencing as previously described [20]. Sequences were confirmed at least twice starting from independent PCR reactions, by comparing sense and antisense strands.
Results
The tumor was formed of two components which were very close to each other or even fused in some areas (Fig. 2). One component was a moderately differentiated ITAC with an abundant mucinous component; it was characterized by a proliferation of invasive glandular structures lined by stratified columnar cells with variably pleomorphic and hyperchromatic nuclei closely resembling colorectal adenocarcinoma (Fig. 3). Areas of mucinous lakes with glands inside were observed. The neuroendocrine component (Fig. 3), showing the typical features of a poorly differentiated neuroendocrine carcinoma [8, 21], was characterized by a proliferation of poorly differentiated small to intermediate cells forming solid nests. Cells showed hyperchromic and atypical nuclei with a high nuclear/cytoplasmic ratio, inconspicuous to absent nucleoli, and a high mitotic count (23 mitoses X 10HPF). The neuroendocrine component showed a diffuse immunoreactivity for synaptophysin and chromogranin A, which was lacking in the adenocarcinoma with the exception of a few scattered cells in some glandular structures identified with both antibodies. In addition, neuroendocrine tumor cells presented immunoreactivity for serotonin and glicentin, whereas they were negative for pancreatic polypeptide. Cytokeratin (CK) 8, CK20, CDX2 were expressed in both neoplastic components, which were conversely negative for CK7 and TTF1 (Fig. 3). Since each component represented more than 30 % of the tumor tissue the neoplasm was classified as a mixed exocrine-neuroendocrine carcinoma [22] or, following the recent proposal of the 2010 WHO classification [8], as a mixed adenoneuroendocrine carcinoma (MANEC). Peritumoral sino-nasal mucosa did not show any dysplastic lesion.
Fig. 2.
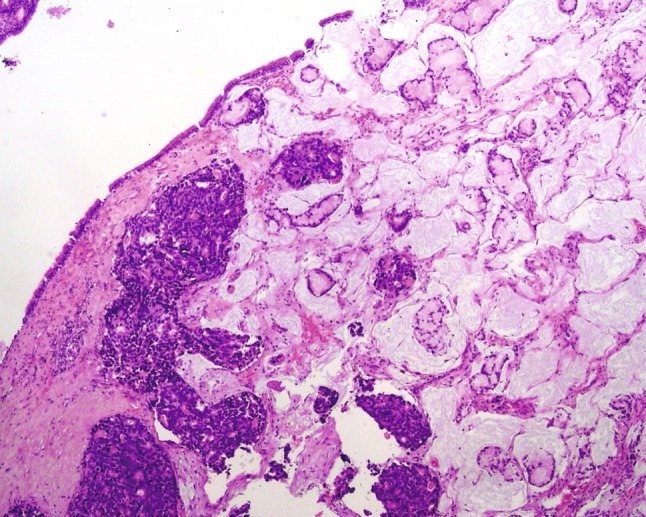
Panoramic view of the tumor that was formed of two components which were very close to each other. The exocrine component (right) was a moderately differentiated ITAC with an abundant mucinous component with areas of mucinous lakes with glands inside. The neuroendocrine component (left) was characterized by solid sheets of poorly differentiated neuroendocrine cells
Fig. 3.
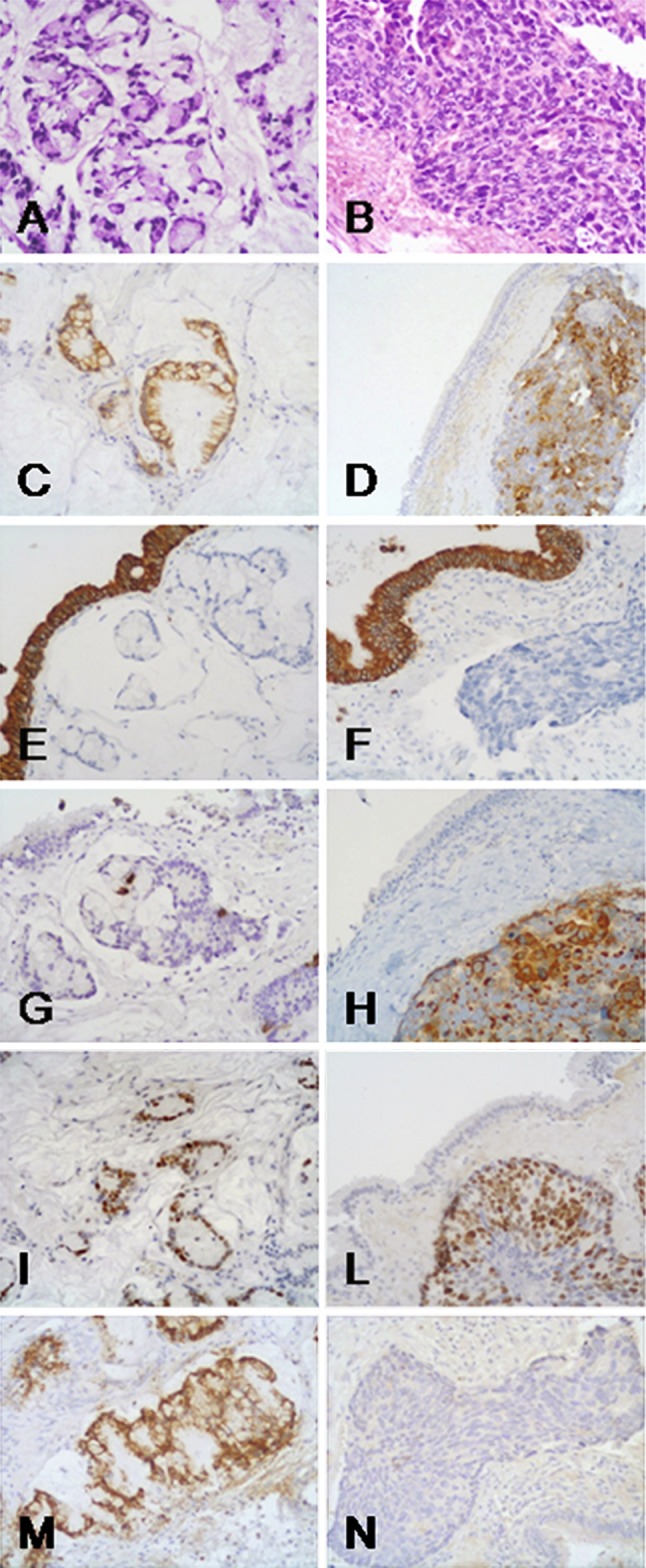
The exocrine component is a moderately differentiated ITAC characterized by a proliferation of invasive glandular structures lined by stratified columnar cells with variably pleomorphic and hyperchromatic nuclei, closely resembling colorectal adenocarcinoma (a). The neuroendocrine component, showing the typical features of a NEC, is characterized by a proliferation of poorly differentiated small to intermediate cells forming solid nests (b). Both components were immunoreactive for cytokeratin 20 (c, d), but not for cytokeratin 7 (e, f; the normal nasal epithelium serves as positive internal control). In the ITAC component only scattered chromogranin A positive cells were observed (g), while the NEC component showed an intense chromogranin A immunoreactivity (h). Both components were p53 positive (i, l). APC immunoreactivity was observed in the ITAC component (m), while it was lacking in the NEC component (n)
The MS-MLPA analysis was used to investigate the genetic and epigenetic profiles of the two tumor components. MS-MLPA results are summarized in Table 2. Concurrent copy number changes in the endocrine and exocrine components were observed at 17p13 (TP53), 14q24 (MLH3) and 19q13 (KLK3) regions. In agreement with the molecular result, an immunohistochemical p53 accumulation was observed in both neoplastic components (Fig. 3). Additional gains and losses were found to be restricted to the neuroendocrine component and included copy number variations at 5q21 (APC), 7q21 (CDK6), 9q34 (DAPK1), 12p13 (TNFRSF 1A, CDKN1B), 13q12 (BRCA2), 17p13.3 (HIC1), 18q21 (BCL2), 19q13 (KLK3) and 22q12 (TIMP3) regions. Aberrant methylation was detected only in the neuroendocrine component and involved APC and DAPK1 genes. Interestingly, the biallelic inactivation of the APC gene (by deletion and aberrant methylation, respectively) was observed only in the neuroendocrine component (Table 2). This result was confirmed by immunohistochemistry showing a diffuse immunoreactivity for APC protein in the ITAC component, which was lacking in the NEC component (Fig. 3). The mutational analysis of KRAS (codon 12, 13, 61 and 146), BRAF (codon 600) and TP53 (exons 4–10) did not find any mutation in each component of the tumor.
Discussion
In the last 40 years, the systematic application of immunohistochemical techniques to the study of tumors has led to the recognition that neuroendocrine cells occur rather frequently in exocrine neoplasms [23–25] and, especially, in adenocarcinomas of the digestive system [7, 26, 27]. It is now well known that there is a wide spectrum of combination of exocrine and neuroendocrine components, ranging from adenomas or carcinomas with interspersed neuroendocrine cells at one extreme to classical neuroendocrine tumors with focal exocrine component at the other [7, 26]. In addition, both exocrine and neuroendocrine components can show different morphological features ranging, for the former, from adenoma to adenocarcinoma with different degrees of differentiation and, for the latter, from well differentiated to poorly differentiated neuroendocrine tumors [7]. Although this spectrum of combinations of neuroendocrine and exocrine components is frequently observed in routine practice, mixed exocrine-neuroendocrine tumors are rare and are, by definition, neoplasms in which each component represents at least 30 % of the lesion [8, 22]. The clinical significance and the influence on survival of focal neuroendocrine differentiation in gut adenocarcinomas still remain controversial. Conversely, gastrointestinal mixed exocrine-neuroendocrine tumors can be stratified in different prognostic categories according to the grade of malignancy of each component [7]. Antibodies directed against chromogranin A and synaptophysin are the most frequently used to identify the neuroendocrine component.
Although the recognition of a neuroendocrine differentiation in nasal ITACs has been described [3–6], mixed adenoneuroendocrine carcinomas (MANECs) have been poorly reported accounting for only seven cases described in the English literature [9–13]. Moreover, this entity was not included in the recent WHO classification of tumors of the head and neck, probably because of its rarity [1]. However, considering the close analogies between intestinal and sinonasal intestinal-type adenocarcinomas and considering that in the gut MANEC is considered as a specific entity [8], it is reasonable to suggest that this is also a distinct clinico-pathologic type in the sinonasal cavity. From the review of the literature (Table 3) it appears that sinonasal mixed exocrine-neuroendocrine tumors can show different types of components. With the exception of one case [10] the neuroendocrine component was a poorly differentiated neuroendocrine carcinoma (NEC). The exocrine component was most often an ITAC, even though three not otherwise specified adenocarcinomas [9, 12, 13] and one inverted papilloma [9] were also reported. MANECs were found in males at a mean age of 60.6 years (range 51–79 years). Based on the limited available information, it would seem that sinonasal MANECs are not associated with occupational risks in the same way as sinonasal ITACs. Two tumors showed lymph node or distant metastases at the time of diagnosis, while other two cancers presented infiltration of the anterior cranial fossa. Two neoplasms developed bone metastases during follow-up. Survival information was available for five patients, all but one died of disease after a mean time of 16.5 months (range 4–30 months). From this analysis it appears that sinonasal MANECs can be considered aggressive neoplasms associated with poor prognosis. In this respect, a clear definition of a molecular profile may have a clinical influence in terms of tailored complementary therapy to associate with surgical procedure.
Table 3.
Literature review: sinonasal mixed adenoneuroendocrine carcinomas
| Histologic components | Sex | Age | Symptoms | Occupational exposure | Metastases at diagnosis | Therapy | Follow-up (months) | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Endocrine | Exocrine | |||||||||
| 1 | NEC | ADC | nk | nk | nk | No | Lung, kidney, adrenal, liver, node | nk | nk | [12] |
| 2 | NET | ITAC | M | 52 | Headache | No | No* | S + RT | DOD (30) | [10] |
| 3 | NEC | ADC | nk | nk | nk | nk | nk | nk | nk | [13] |
| 4 | NEC | ADC | M | 51 | nk | nk | No | C + RT | AWD (12) | [9] |
| 5 | NEC | Inv Pap | M | 57 | nk | nk | No | S | DOD (6) | [9] |
| 6 | NEC | ITAC | M | 50 | Stuffiness, rhinorrhea | No | Node | S + C + RT | nk | [11] |
| 7 | NEC | ITAC | M | 75 | Epistaxis, rhinorrhea, | No | No | S | DOD (4) | [11] |
| 8 | NEC | ITAC | M | 79 | TIA | No | No* | S + RT | DOD (26) | Present case |
ADC adenocarcinoma, ITAC intestinal-type adenocarcinoma, Inv Pap inverted papilloma, C chemotherapy, RT radiotherapy, S surgery, nk not known, M male, * infiltration of anterior cranial fossa, NEC poorly differentiated neuroendocrine carcinoma, NET neuroendocrine tumor, TIA transitory ischemic attack, DOD died of disease, AWD alive with disease
The immunophenotype of the exocrine component of the present tumor, including CK20, CK8, p53 and CDX2 immunoreactivity and the lack of TTF1 and CK7 expression, are in line with previous findings [3, 28], although CK7 expression has also been reported in some ITACs [11, 29]. The immunohistochemical profile of the neuroendocrine component largely overlapped with that of the exocrine one, but also showed a typical neuroendocrine phenotype including diffuse and intense immunoreactivity for chromogranin A and synaptophysin. These two neuroendocrine markers are generally widely applied to define the neuroendocrine nature of a tumor. Chromogranin A is present in the majority on neuroendocrine neoplasms even if tumors containing only a small number of secretory granules (i.e. NECs) may only exhibit a weak immunoreactivity for this marker. For this reason, synaptophysin expression should be included in the diagnostic panel of poorly differentiated carcinomas, because its immunoreactivity is independent of the presence of secretory granules and shows a higher sensitivity than chromogranin A expression [30]. Interestingly, the expression of glicentin, a hormonal peptide mainly expressed by neuroendocrine tumors of hindgut derivation [31], suggests a similarity between the neuroendocrine component and gut neuroendocrine neoplasms arising in the distal bowel. The CDX2 expression observed in the NEC component underlines the intestinal phenotype of the neuroendocrine component [19].
In agreement with these results, the molecular characterization of the tumor by the MS-MLPA assay strongly supported the involvement of the APC pathway in the progression of the cancer, confirming a genetic resemblance with colorectal carcinomas. Our data are consistent with previous studies demonstrating that ITACs share genetic similarities with microsatellite stable colorectal cancers [32, 33]. Until now, the involvement of APC in ITACs has been poorly examined. To our knowledge, a single mutational study of the gene has been performed, reporting only missense mutations, which might not affect the APC protein activity [32]. In this study, we demonstrated a concurrent hypermethylation and allelic loss of APC that determined lack of protein expression in the neuroendocrine component. By contrast, no gene or protein alterations were observed in the ITAC component (Fig. 2). This result suggests a causal role of APC in the progression of these tumors and outlines the potential utility of further analyses of APC promoter hypermethylation or loss of heterozygosity in ITACs and in MANECs. Promoter hypermethylation of DAPK1 was also found to be restricted to the neuroendocrine component. Interestingly, the aberrant methylation of DAPK1 is considered an important epigenetic event in nasopharyngeal carcinomas [34] and has been evaluated as an early molecular marker of subclinical presence of this neoplasm [35, 36].
Regarding the KRAS/BRAF pathway, our analysis did not find any mutation in the hotspot codons of the two genes. This result was not unexpected because, in several studies, a lower frequency of KRAS mutations has been reported in ITACs compared with colorectal carcinomas [17]. There is less information about BRAF mutation, which was not found in any of the 18 cases investigated in a recent published series [32].
In this study, a strong genetic relationship between the ITAC and NEC component was observed. Indeed, both components exhibited three concurrent copy number changes at 17p13 (TP53), 14q24 (MLH3) and 19q13 (KLK3) regions, while all the additional alterations of gene dosage were restricted to the NEC. These results strongly support the hypothesis of a monoclonal origin of the tumor from a pluripotent cell that undergoes a biphenotypic differentiation after carcinogenesis is initiated, as already proposed for MANECs of the lung [37] and other sites [38, 39]. In agreement with these previous molecular studies, the NEC component showed a more complex genetic profile than the adenocarcinoma, confirming that the neuroendocrine differentiation may be from an exocrine to an endocrine cell type and not vice versa.
In conclusion, extensive genetic and epigenetic analyses of heterogeneous tumors exhibiting early and more aggressive components are very informative approaches to define the clonality and timing of the molecular events underlying tumor progression. In these applications, MS-MLPA appears to be a powerful tool to detect copy number variation and hypermethylation of a large set of genes simultaneously using small amounts of DNA, also deriving from archival tissues.
Acknowledgments
This study was supported in part by a grant from the University of Insubria, Varese.
References
- 1.Barnes L, Eveson JW, Reichart P, Sidransky D. WHO classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 2.Franchi A, Santucci M, Wenig BM. Adenocarcinoma. In: Eveson JW, Reichart P, Sidransky D, Barnes L, editors. WHO classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 20–23. [Google Scholar]
- 3.Abecasis J, Viana G, Pissarra C, et al. Adenocarcinomas of the nasal cavity and paranasal sinuses: a clinicopathological and immunohistochemical study of 14 cases. Histopathology. 2004;45:254–259. doi: 10.1111/j.1365-2559.2004.01949.x. [DOI] [PubMed] [Google Scholar]
- 4.Batsakis JG, Mackay B, Ordonez NG. Enteric-type adenocarcinomas of the nasal cavity. An electron microscopic and immunohistochemical study. Cancer. 1984;54:855–860. doi: 10.1002/1097-0142(19840901)54:5<855::AID-CNCR2820540516>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.McKinney CD, Mills SE, Fraquemont DW. Sinonasal intestinal-type adenocarcinoma: immunohistochemical profile and comparison with colonic adenocarcinoma. Mod Pathol. 1995;8:421–426. [PubMed] [Google Scholar]
- 6.Simard LC, Jean A. Adenocarcinoma with argentaffin cells of the nasal cavity, giving widespread metastases. Cancer. 1953;6:699–703. doi: 10.1002/1097-0142(195307)6:4<699::AID-CNCR2820060408>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.La Rosa S, Marando A, Sessa F, et al. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers. 2012;4:11–30. doi: 10.3390/cancers4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rindi G, Arnold R, Bosman FT, et al. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, et al., editors. WHO classification of tumours of the digestive system. Lyon: IARC Press; 2010. pp. 13–14. [Google Scholar]
- 9.Babin E, Rouleau V, Vedrine PO, et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. J Laryngol Otol. 2006;120:289–297. doi: 10.1017/S0022215106000594. [DOI] [PubMed] [Google Scholar]
- 10.Bonato M, Frigerio B, Capella C, et al. Composite enteric-type adenocarcinoma-carcinoid of the nasal cavity. Endocr Pathol. 1993;4:40–47. doi: 10.1007/BF02914488. [DOI] [PubMed] [Google Scholar]
- 11.Jain R, Gramigna V, Sanchez-Marull R, et al. Composite intestinal-type adenocarcinoma and small cell carcinoma of sinonasal tract. J Clin Pathol. 2009;62:634–637. doi: 10.1136/jcp.2009.065433. [DOI] [PubMed] [Google Scholar]
- 12.Silva EG, Butler JJ, Mackay B, et al. Neuroblastomas and neuroendocrine carcinomas of the nasal cavity. A proposed new classification. Cancer. 1982;50:2388–2405. doi: 10.1002/1097-0142(19821201)50:11<2388::AID-CNCR2820501126>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Smith SR, Som P, Fahmy A, et al. A clinicopathological study of sinonasal neuroendocrine carcinoma and sinonasal undifferentiated carcinoma. Laryngoscope. 2000;110:1617–1622. doi: 10.1097/00005537-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Ariza M, Llorente JL, Alvarez-Marcos C, et al. Comparative genomic hybridization in primary sinonasal adenocarcinomas. Cancer. 2004;100:335–341. doi: 10.1002/cncr.11931. [DOI] [PubMed] [Google Scholar]
- 15.Douglas EJ, Fiegler H, Rowan A, et al. Array comparative genomic hybridization analysis of colorectal cancer cell lines and primary carcinomas. Cancer Res. 2004;64:4817–4825. doi: 10.1158/0008-5472.CAN-04-0328. [DOI] [PubMed] [Google Scholar]
- 16.Korinth D, Pacyna-Gengelbach M, Deutschmann N, et al. Chromosomal imbalances in wood dust-related adenocarcinomas of the inner nose and their associations with pathological parameters. J Pathol. 2005;207:207–215. doi: 10.1002/path.1819. [DOI] [PubMed] [Google Scholar]
- 17.Llorente JL, Pérez-Escuredo J, Alvarez-Marcos C, et al. Genetic and clinical aspects of wood dust related intestinal-type sinonasal adenocarcinoma: a review. Eur Arch Otorhinolaryngol. 2009;266:1–7. doi: 10.1007/s00405-008-0749-y. [DOI] [PubMed] [Google Scholar]
- 18.Perrone F, Oggionni M, Birindelli S, et al. TP53, p14ARF, p16INK4A and H-ras gene molecular analysis in intestinal-type adenocarcinomas of the nasal cavity and paranasal sinuses. Int J Cancer. 2003;105:196–203. doi: 10.1002/ijc.11062. [DOI] [PubMed] [Google Scholar]
- 19.La Rosa S, Marando A, Furlan D, et al. Colorectal poorly differentiated neuroendocrine carcinomas (NECs) and mixed adenoneuroendocrine carcinomas (MANECs): insights into the diagnostic immunophenotype, assessment of methylation profile and search for prognostic markers. Am J Surg Pathol. 2012;36:601–611. doi: 10.1097/PAS.0b013e318242e21c. [DOI] [PubMed] [Google Scholar]
- 20.Miyaki M, Iijima T, Yasuno M, et al. High incidence of protein-truncating mutations of the p53 gene in liver metastases of colorectal carcinomas. Oncogene. 2002;21:6689–6693. doi: 10.1038/sj.onc.1205887. [DOI] [PubMed] [Google Scholar]
- 21.Kao HL, Chang WC, Li WY, et al. Head and neck large cell neuroendocrine carcinoma should be separated from atypical carcinoid on the basis of different clinical features, overall survival, and pathogenesis. Am J Surg Pathol. 2012;36:185–192. doi: 10.1097/PAS.0b013e318236d822. [DOI] [PubMed] [Google Scholar]
- 22.Solcia E, Klöppel G, Sobin LH, et al. Histological typing of endocrine tumours. WHO international histological classification of tumours. 2. Berlin: Springer; 2000. [Google Scholar]
- 23.Brambilla E, Lantuejoul S, Sturm N. Divergent differentiation in neuroendocrine lung tumors. Semin Diagn Pathol. 2000;17:138–148. [PubMed] [Google Scholar]
- 24.di Sant’Agnese A. Divergent neuroendocrine differentiation in prostatic carcinoma. Semin Diagn Pathol. 2000;17:149–161. [PubMed] [Google Scholar]
- 25.Sapino A, Righi L, Cassoni P, et al. Expression of the neuroendocrine phenotype in carcinomas of the breast. Semin Diagn Pathol. 2000;17:127–137. [PubMed] [Google Scholar]
- 26.Volante M, Rindi G, Papotti M. The grey zone between pure (neuro)endocrine and non-(neuro)endocrine tumours: a comment on concepts and classification of mixed exocrine-endocrine neoplasms. Virchows Arch. 2006;449:499–506. doi: 10.1007/s00428-006-0306-2. [DOI] [PubMed] [Google Scholar]
- 27.Klöppel G. Mixed exocrine-endocrine tumors of the pancreas. Semin Diagn Pathol. 2000;17:104–108. [PubMed] [Google Scholar]
- 28.Franchi A, Massi D, Baroni G, Santucci M. CDX-2 homeobox gene expression. Am J Surg Pathol. 2003;27:1390–1391. doi: 10.1097/00000478-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Resto VA, Krane JF, Faquin WC, et al. Immunohistochemical distinction of intestinal-type sinonasal adenocarcinoma from metastatic adenocarcinoma of intestinal origin. Ann Otol Rhinol Laryngol. 2006;115:59–64. doi: 10.1177/000348940611500109. [DOI] [PubMed] [Google Scholar]
- 30.Komminoth P, Walch A, Werner M, et al. Methods in cellular and molecular pathology. In: Lloyd RV, et al., editors. Endocrine pathology. Differential diagnosis and molecular advances. New York: Springer; 2010. pp. 1–44. [Google Scholar]
- 31.Fiocca R, Rindi G, Capella C, et al. Glucagon, glicentin, proglucagon, PYY, PP and proPP-icosapeptide immunoreactivities of rectal carcinoid tumors and related non-tumor cells. Regul Pept. 1987;17:9–29. doi: 10.1016/0167-0115(87)90029-2. [DOI] [PubMed] [Google Scholar]
- 32.Frattini M, Perrone F, Suardi S, et al. Phenotype-genotype correlation: challenge of intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Head Neck. 2006;28:909–915. doi: 10.1002/hed.20433. [DOI] [PubMed] [Google Scholar]
- 33.Yom SS, Rashid A, Rosenthal DI, et al. Genetic analysis of sinonasal adenocarcinoma phenotypes: distinct alterations of histogenetic significance. Mod Pathol. 2005;18:315–319. doi: 10.1038/modpathol.3800315. [DOI] [PubMed] [Google Scholar]
- 34.Wong TS, Chang HW, Tang KC, et al. High frequency of promoter hypermethylation of the death-associated protein-kinase gene in nasopharyngeal carcinoma and its detection in the peripheral blood of patients. Clin Cancer Res. 2002;8:433–437. [PubMed] [Google Scholar]
- 35.Hutajulu SH, Indrasari SR, Indrawati LP, et al. Epigenetic markers for early detection of nasopharyngeal carcinoma in a high risk population. Mol Cancer. 2011;10:48. doi: 10.1186/1476-4598-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong TS, Kwong DL, Sham JS, et al. Quantitative plasma hypermethylated DNA markers of undifferentiated nasopharyngeal carcinoma. Clin Cancer Res. 2004;10:2401–2406. doi: 10.1158/1078-0432.CCR-03-0139. [DOI] [PubMed] [Google Scholar]
- 37.Fukui T, Tsuta K, Furuta K, et al. Epidermal growth factor receptor mutation status and clinicopathological features of combined small cell carcinoma with adenocarcinoma of the lung. Cancer Sci. 2007;98:1714–1719. doi: 10.1111/j.1349-7006.2007.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furlan D, Cerutti R, Genasetti A, et al. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003;83:963–971. doi: 10.1097/01.LAB.0000079006.91414.BE. [DOI] [PubMed] [Google Scholar]
- 39.Huang J, Behrens C, Wistuba II, et al. Clonality of combined tumors. Arch Pathol Lab Med. 2002;126:437–441. doi: 10.5858/2002-126-0437-COCT. [DOI] [PubMed] [Google Scholar]