Abstract
This is a case report of an unusual odontogenic myxoma with calcifications, one of three reported in the literature. It had a typical radiographic appearance although it presented in an older patient. The presence of osteo-cementum-like calcification raises other differential diagnoses but does not in and of itself mitigate the diagnosis. The patient has not shown recurrence 14 months after resection and 11 months after reconstruction and continues to be closely monitored.
Keywords: Odontogenic myxoma, Diffuse calcification, Honeycomb appearance
Introduction
The odontogenic myxoma is a rare benign mesenchymal odontogenic neoplasm. It is diagnosed most frequently between the second and the fourth decade without gender predilection and approximately 60 % are located in the mandible [1, 2]. Smaller lesions are usually asymptomatic and discovered during a routine radiographic examination while larger lesions are more common in the mandible, are often associated with painless jaw expansion and perforation of the cortical plate.
Radiographically, the odontogenic myxoma appears as a unilocular or multilocular radiolucency with irregular or scalloped margins, and may displace or cause resorption of teeth [1, 3]. A “tennis racket” appearance [4, 5], and diffuse calcifications have been reported [6].
Microscopically, the tumor is composed of haphazardly arranged stellate and spindle-shaped cells within a loose mucinous or myxoid stroma that contains only a few collagen fibrils. Islands of odontogenic epithelium may or may not be present. However, two cases exist in the literature where calcifications were produced by the tumor [7, 8]. This is a case report of a third case of odontogenic myxoma with unusual calcifications.
Case Report
A 69-year old female with a medical history notable for hypothyroidism and hyperlipidemia was referred by her general dentist to a local oral and maxillofacial surgeon for evaluation of new onset right posterior mandibular discomfort. Based on findings of a plain film, an incisional biopsy of the right posterior mandible was completed and the patient was then referred to the Department of Oral and Maxillofacial Surgery at Massachusetts General Hospital (MGH) for management.
On presentation to MGH, the patient reported to be in her usual state of health 2 weeks earlier when she developed a sensation of “air” in the posterior right mandible. She denied unexplained weight loss, antecedent trauma, change in occlusion, paresthesia of the lip, mobility of her teeth or otalgia. Her prescription medications included atorvastatin and levothyroxine and she denied drug allergies. She had a 25 pack-year smoking history, having quit 19 years prior to presentation and consumed one alcoholic beverage daily. She was a recently retired office manager with a family history notable for pancreatic cancer in her mother as well as psoriasis in both her mother and a niece.
Extraoral examination revealed no facial asymmetry with a 2 cm area of induration noted on palpation of the right mandibular angle and posterior body. There was no cervical lymphadenopathy and the cranial nerve examination was unremarkable. Intraoral examination revealed heavily restored dentition in good repair with stable occlusion. There was a right mandibular buccal vestibular swelling adjacent to the retromolar trigone surrounding an incompletely healed biopsy site.
A recent panoramic radiograph provided by the referring oral and maxillofacial surgeon demonstrated a mixed radiolucent/radiopaque lesion with poorly defined borders encompassing the entire right mandibular body posterior to the second premolar as well as the angle and inferior ascending ramus, measuring approximately 3 × 3 cm (Fig. 1). A periapical radiograph from 1992 demonstrated a similar-appearing mixed radiolucent/radiopaque lesion with a delicate honeycomb appearance that appeared to be confined to the periradicular bone between teeth #30 and #31 (Fig. 2). A maxillofacial computerized tomography (CT) scan demonstrated a heterogeneously lytic and mildly expansile lesion, measuring 2.5 cm × 1.4 cm × 3 cm, of the right posterior mandible and ramus with a honeycomb appearance without evidence of cortical dehiscence or soft tissue involvement with scattered calcifications (Fig. 3).
Fig. 1.
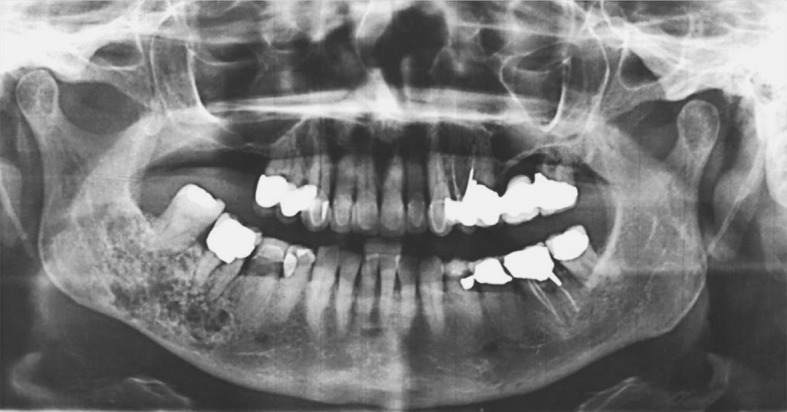
Panoramic radiograph showing “tennis racket” appearance of bone around the roots of teeth #30 and #31 with intact inferior border cortex (Courtesy of Dr. Toby Feldman, private practice, Cambridge, MA)
Fig. 2.
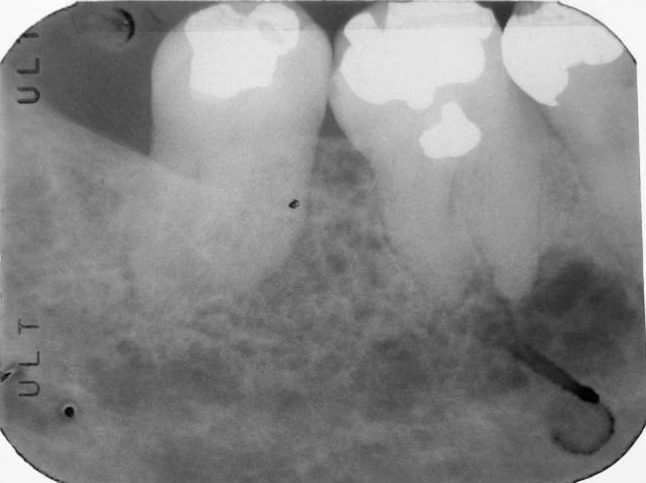
Periapical radiograph from 19 years prior to presentation demonstrating delicate honeycomb appearance (Courtesy of Dr. John Boyle, general practice, Lexington, MA)
Fig. 3.
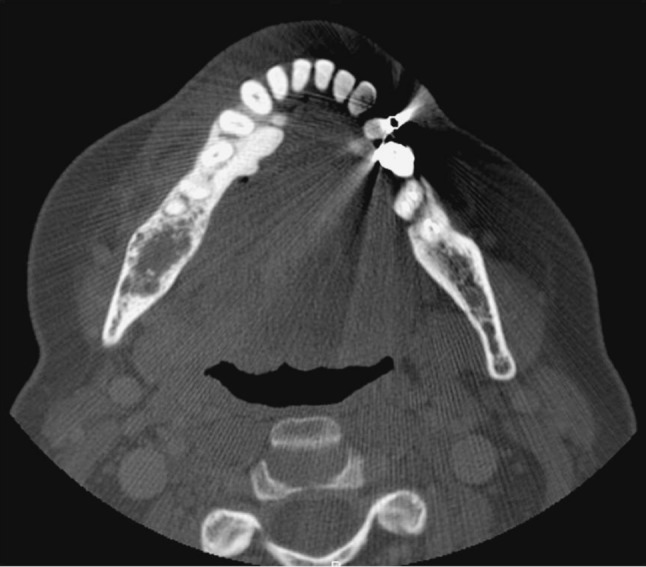
Axial view of mandibular CT demonstrating expansile, lytic lesion containing delicate radiopacities
The original biopsy had demonstrated a proliferation of spindled and stellate cells in a markedly mucinous or myxoid matrix containing delicate collagen fibers, islands of odontogenic epithelium and extensive areas of unusual calcifications that did not resemble osteoid or woven bone but rather dysplastic cementum (Fig. 4). The lesion was diagnosed as “fragments of odontogenic myxoma with unusual calcifications”. Based on the clinical, radiographic and histopathological findings, the patient underwent a segmental resection of the tumor via a combined transcervical and transoral approach with planned 1 cm margins. The resection spared the condyle but included teeth #29, #30, and #31 with the resection margin through the socket of extracted tooth #28. A cuff of mucosa at the previous biopsy site was also included in the resection specimen and the mandible was immediately reconstructed with a reconstruction plate. Postoperative radiographs demonstrated maintenance of the premorbid occlusion and right condylar position. Three months later the patient underwent autologous reconstruction with an iliac crest bone graft. There is no evidence of recurrent tumor 14 months after resection and 11 months after reconstruction.
Fig. 4.
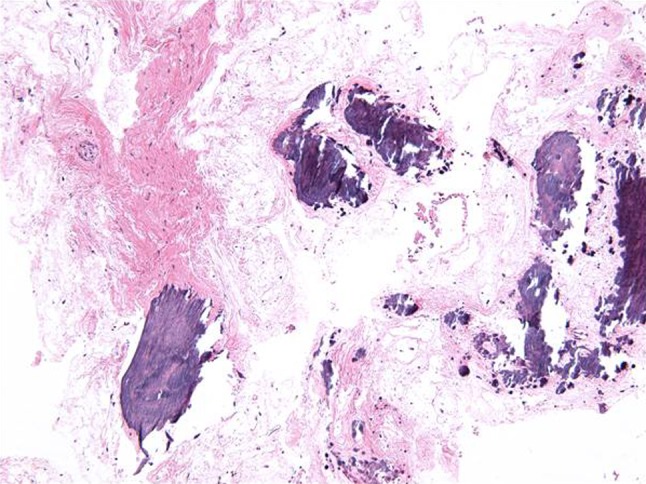
Photomicrograph of initial biopsy showing delicately collagenized myxoid tissue with spindle cells and large and small calcifications (Hematoxylin-eosin, magnification ×100)
Histopathologic Findings
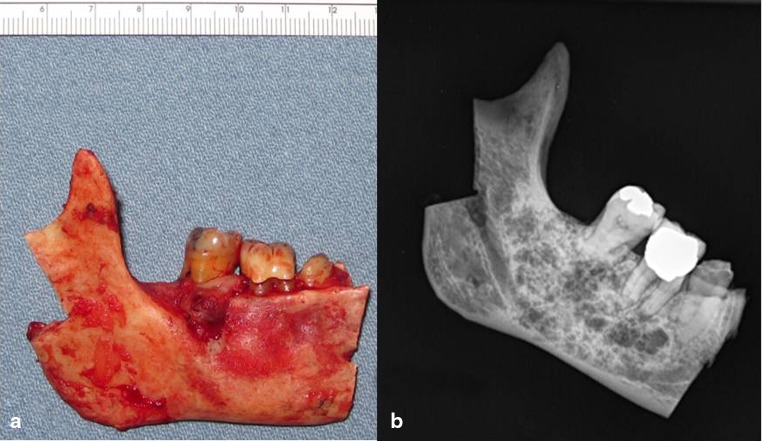
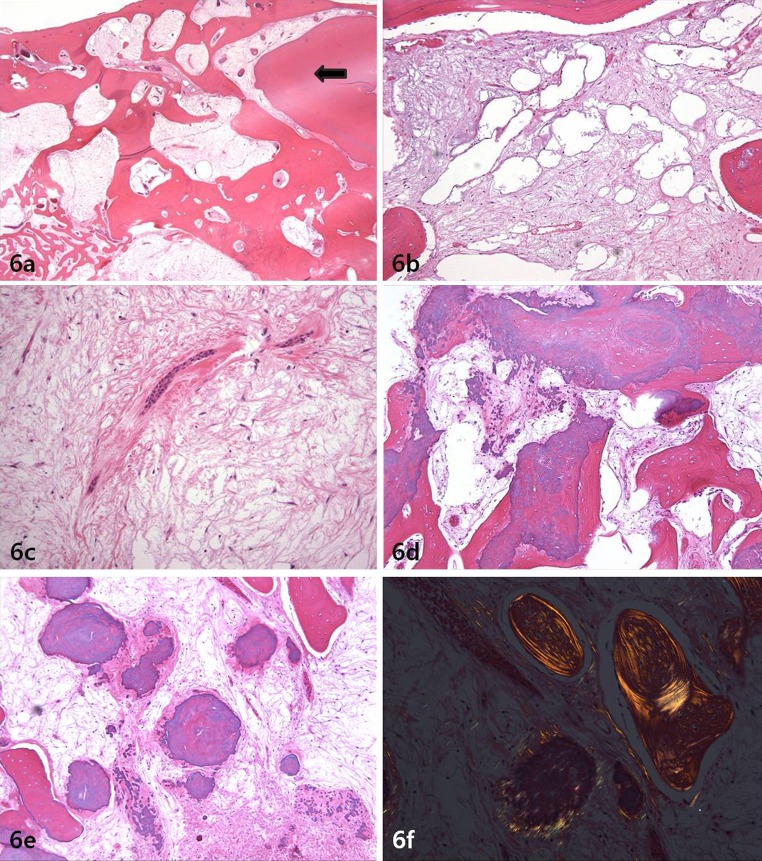
The gross specimen photograph and radiograph are presented in Fig. 5a, b. The radiograph showed fine bony trabeculations with a “tennis racket” or honeycomb appearance without a corticated margin. Gross examination of the cut surface revealed a gelatinous mass filling the medullary spaces. The histopathologic features of the main tumor were similar to the biopsy and had a permeating character, insinuating between bone trabeculae (Fig. 6a). There was a proliferation of stellate and spindled fibroblast-like cells or myxoblasts in a mucinous and myxoid stroma with areas that were delicately and densely collagenized; areas of pseudocystic change were present (Fig. 6b). The tumor cells had benign nuclei with dispersed chromatin and indistinct nucleoli; there were occasional binucleated cells and no mitoses were noted (Fig. 6c). Islands and strands of odontogenic epithelium were readily identified. The calcifications were hematoxyphilic and hypocellular to acellular and took the form of small spherules and larger masses, some of which were intimately associated with residual bony trabeculae (Fig. 6d). Some of the larger spherules resembled cementum (“cementicles”) with peripheral radiating collagen fibers, recapitulating Sharpey fibers (Fig. 6e). These calcifications did not polarize in the same manner as woven bone (Fig. 6f). All margins were free of tumor.
Fig. 5.
a Resected right mandible. b Gross specimen radiograph showing anterior margin to be free of tumor
Fig. 6.
a Photomicrograph showing tumor permeating bone and abutting the root of a tooth (arrow) (Hematoxylin-eosin; magnification ×20). b Photomicrograph showing pseudocystic change within tumor (Hematoxylin-eosin; magnification ×100). c Photomicrograph showing benign nature of the spindled and stellate cells in mucinous stroma (Hematoxylin-eosin; magnification ×400). d Photomicrograph showing hematoxyphilic calcifications resembling cementum fused with residual bone trabeculae (Hematoxylin-eosin; magnification ×100). e Photomicrograph showing spherules of dysplastic cementum with peripheral radiating collagen fibers suggestive of Sharpey fibers (Hematoxylin-eosin; magnification ×100). f Photomicrograph with polarized light comparing sweeping lamellae in residual lamellar bone compared to the short fibers in the osteo-cementum (Hematoxylin-eosin; magnification ×200)
Discussion
Odontogenic myxoma is classified by the World Health Organization as a mesenchymal odontogenic tumor that does not produce calcifications [9]. It is diagnosed most commonly in the 3rd decade with a predilection for the mandible (Table 1) [2, 6, 10–13].
Table 1.
Demographic data of series larger than 35 cases reported after 1997 in the English literature
| Author | Study period (population studied) | No of cases (M/F) | Age distribution | Location (max/man) |
|---|---|---|---|---|
| Martinez-Mata et al. [2] | Unknown (Mexico, Guatemala, Brazil) |
62 (19/43) | 9–71 year; mean 27.97 | 25/37 |
| Zhang et al. [6] | 1964–2005 (China) |
41 (22/19) | 4–63 year; most common in 3rd decade | 17/24 |
| Mosqueda-Taylor et al. [10] | 1960–1996 (Mexico) |
62 (20/41) (NS:1) |
5–59; no mean noted | 33/27 (NS:2) |
| Lu et al. [11] | 1952–1994 (China) |
64 (32/32) | Mean 19.6 | 31/33 |
| Adebayo et al. [12] | 1979–1998 (Nigeria) |
38 (10/28) | 9–70; mean 27 | 18/20 |
| Jing et al. [13] | 1952–2004 (China) |
76 (37/39) | Mean 25.3 | 34/42 |
M male; F female, Max maxilla, Man mandible, NO number, NS not specified
Odontogenic myxoma consists of spindled and stellate shaped cells in a delicately collagenized mucinous or myxoid stroma. On ultrastructural and histochemical study, the proliferating component of the tumor cell is similar to a secreting fibroblastic cell and myofibroblast and the tumor matrix consists of large amounts of mucopolysaccharides, chiefly hyaluronic acid and chondroitin sulfate [2, 14, 15].
Small islands of inactive-appearing odontogenic epithelial rests may be scattered throughout the myxoid ground substance, but they are not required for the diagnosis. The fibroblast or myxoblast putatively derives from intra-osseous pluripotent mesenchymal cells that may differentiate towards tissues of the dental papilla, the developing pulp. In fact, it is not uncommon to misdiagnose a fragment of dental papilla from a developing tooth as an odontogenic myxoma if the diagnostic layer of odontoblasts is not seen. It is conceivable that such cells may also produce other mesenchymal hard tissues such as cementum which composition closely resembles bone.
Radiographically, odontogenic myxoma presents as a multilocular or unilocular radiolucency often with a honeycomb or “tennis racket” appearance as was well-illustrated in this case [1, 4, 7]. Calcifications may be present in 13–20 % studies [6], and this was also noted in this case.
The differential diagnosis includes a central odontogenic fibroma and fibro-osseous lesion such as a cemento-ossifying fibroma. Central odontogenic fibroma is much more collagenized, although there may be focal myxoid areas, and may produce small and scattered deposits of “dentinoid”, an amorphous, eosinophilic material [5, 16]. The case reported here is diffusely myxoid with extensive calcifications. Cemento-ossifying fibroma is also more densely collagenized, is encapsulated, does not contain islands of odontogenic epithelium, and forms osteoid and cementum in various proportions [17]. Isolated foci of residual bone and dystrophic mineralization can be found in 25.8 % cases of odontogenic myxoma [2]. In this case however, the calcified structures were diffusely distributed throughout the lesions and did not show properties of either lamellar or woven bone but rather osteo-cementum with peripheral radiating collagen fibers.
Two other cases of odontogenic myxoma with calcifications have been reported in the literature. Both cases produced cementum-like material similar to this case. The case by Lin and Basile [7] and by Oygur et al. [8] both contained the calcifications composed of regularly-sized spherules similar to what is seen in cemento-ossifying fibromas although the stroma was myxoid/mucinous and more cellular than noted in our case. Osteo-cementum-like material surrounded by a rim of acellular osteoid that was birefringent under polarized light was present in one case [7], while the other was associated with an unerupted tooth and contained oval to round calcified bodies with peripheral collagenous radiations similar to that seen in this case [8]. Such osteo-cementum material recapitulating Sharpey fibers is not unexpected in “bone”-forming gnathic mesenchymal tumors if one believes that the tumors arise in pluripotent cells that during odontogenesis would have played a role in deposition of bone, cementum and collagen to produce the periodontal ligament.
This case is unusual in that it was diagnosed in a 69-year old patient while most odontogenic myxomas occur within the second and fourth decades of life. However, the size of the tumor and the fact that the lesion had been present on a periapical radiograph 19 years prior indicates that this lesion had already been present when she was in her fifth decade of life.
Because of high recurrence rate and permeative quality of the tumor, resection with a 1–1.5 cm bony margin is the treatment of choice. Enucleation or curettage are associated with a high incidence of recurrence but may be considered for palliation in patients unwilling or unable to undergo resection, or for recurrences [18, 19]. Conservative management for recurrent lesions followed by closed monitoring over the time has been proposed as has curettage with cryotherapy [20]. In spite of local recurrences, the overall prognosis is good, and metastases do not occur.
Acknowledgments
I would like to thank Dr. Toby Feldman of Arlington, MA, Dr. John J. Boyle of Arlington, MA and Dr. William Faquin of Massachusetts General Hospital, Boston, MA for their help with providing radiographs and the slides for this manuscript.
References
- 1.Kaffe I, Naor H, Buchner A. Clinical and radiological features of odontogenic myxoma of the jaws. Dentomaxillofac Radiol. 1997;26:299–303. doi: 10.1038/sj.dmfr.4600261. [DOI] [PubMed] [Google Scholar]
- 2.Martinez-Mata G, Mosqueda-Taylor A, Carlos-Bregni R, et al. Odontogenic myxoma: clinico-pathological, immunohistochemical and ultrastructural findings of a multicentric series. Oral Oncol. 2008;44:601–607. doi: 10.1016/j.oraloncology.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Hisatomi M, Asaumi J, Konouchi H, et al. Comparison of radiographic and MRI features of a root-diverging odontogenic myxoma, with discussion of the differential diagnosis of lesions likely to move roots. Oral Dis. 2003;9:152–157. doi: 10.1034/j.1601-0825.2003.01802.x. [DOI] [PubMed] [Google Scholar]
- 4.White SC, Pharoah MJ. Oral radiology: principles and interpretation. St. Louis: Mosby; 2004. [Google Scholar]
- 5.Koseki T, Kobayashi K, Hashimoto K, et al. Computed tomography of odontogenic myxoma. Dentomaxillofac Radiol. 2003;32:160–165. doi: 10.1259/dmfr/16752462. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Wang H, He X, et al. Radiographic examination of 41 cases of odontogenic myxomas on the basis of conventional radiographs. Dentomaxillofac Radiol. 2007;36:160–167. doi: 10.1259/dmfr/38484807. [DOI] [PubMed] [Google Scholar]
- 7.Lin YL, Basile JR. A case of odontogenic myxoma with unusual histological features mimicking a fibro-osseous process. Head Neck Pathol. 2010;4:253–256. doi: 10.1007/s12105-010-0189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oygur T, Dolanmaz D, Tokman B, et al. Odontogenic myxoma containing osteocement-like spheroid bodies: report of a case with an unusual histopathological feature. J Oral Pathol Med. 2001;30:504–506. doi: 10.1034/j.1600-0714.2001.030008504.x. [DOI] [PubMed] [Google Scholar]
- 9.Barnes EL, Eveson J, Reichart P, et al. Pathology and genetics of head and neck tumours. In: Kleihues P, Sobin LH, series editors. World Health Organization classification of tumours. Lyon, France: IARC Press; 2005.
- 10.Mosqueda-Taylor A, Ledesma-Montes C, Caballero-Sandoval S, et al. Odontogenic tumors in Mexico: a collaborative retrospective study of 349 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:672–675. doi: 10.1016/S1079-2104(97)90371-1. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Xuan M, Takata T, et al. Odontogenic tumors. A demographic study of 759 cases in a Chinese population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:707–714. doi: 10.1016/S1079-2104(98)90208-6. [DOI] [PubMed] [Google Scholar]
- 12.Adebayo ET, Ajike SO, Adekeye EO. A review of 318 odontogenic tumors in Kaduna, Nigeria. J Oral Maxillofac Surg. 2005;63:811–819. doi: 10.1016/j.joms.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Jing W, Xuan M, Lin Y, et al. Odontogenic tumours: a retrospective study of 1642 cases in a Chinese population. Int J Oral Maxillofac Surg. 2007;36:20–25. doi: 10.1016/j.ijom.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Lo Muzio L, Nocini P, Favia G, et al. Odontogenic myxoma of the jaws: a clinical, radiologic, immunohistochemical, and ultrastructural study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:426–433. doi: 10.1016/S1079-2104(96)80309-X. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Lu Y, Takata T, et al. Immunohistochemical and histochemical characterization of the mucosubstances of odontogenic myxoma: histogenesis and differential diagnosis. Pathol Res Pract. 1999;195:391–397. doi: 10.1016/S0344-0338(99)80012-0. [DOI] [PubMed] [Google Scholar]
- 16.Handlers JP, Abrams AM, Melrose RJ, et al. Central odontogenic fibroma: clinicopathologic features of 19 cases and review of the literature. J Oral Maxillofac Surg. 1991;49:46–54. doi: 10.1016/0278-2391(91)90265-N. [DOI] [PubMed] [Google Scholar]
- 17.Su L, Weathers DR, Waldron CA. Distinguishing features of focal cemento-osseous dysplasias and cemento-ossifying fibromas: I. A pathologic spectrum of 316 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:301–309. doi: 10.1016/S1079-2104(97)90348-6. [DOI] [PubMed] [Google Scholar]
- 18.Marx R, Stern D, editors. Chapter 14: Odontogenic tumors: hamartomas and neoplasms. Oral and maxillofacial pathology: a rationale for diagnosis and treatment. Chicago: Quintessence Publishing Co; 2003. p. 637–706.
- 19.Boffano P, Gallesio C, Barreca A, et al. Surgical treatment of odontogenic myxoma. J Craniofac Surg. 2011;22:982–987. doi: 10.1097/SCS.0b013e3182101400. [DOI] [PubMed] [Google Scholar]
- 20.Rocha AC, Gaujac C, Ceccheti MM, et al. Treatment of recurrent mandibular myxoma by curettage and cryotherapy after thirty years. Clinics (Sao Paulo) 2009;64:149–152. doi: 10.1590/S1807-59322009000200013. [DOI] [PMC free article] [PubMed] [Google Scholar]