History
A 74-year-old Asian female presented to her primary care physician with a 3-month history of cough, rhinorrhea, and post-nasal drip. Physical examination revealed the presence of bilateral firm, fixed, non-tender anterior cervical masses. The patient was treated for rhinosinusitis while radiographic studies were obtained.
Radiographic Features
Computed tomography (CT) and magnetic resonance (MR) images of the head and neck demonstrated a wedge-shaped hyperechoic mass in the right fossa of Rosenmuller and bilateral level II lymphadenopathy (Fig. 1). There was no evidence of bony invasion or neural compression.
Fig. 1.
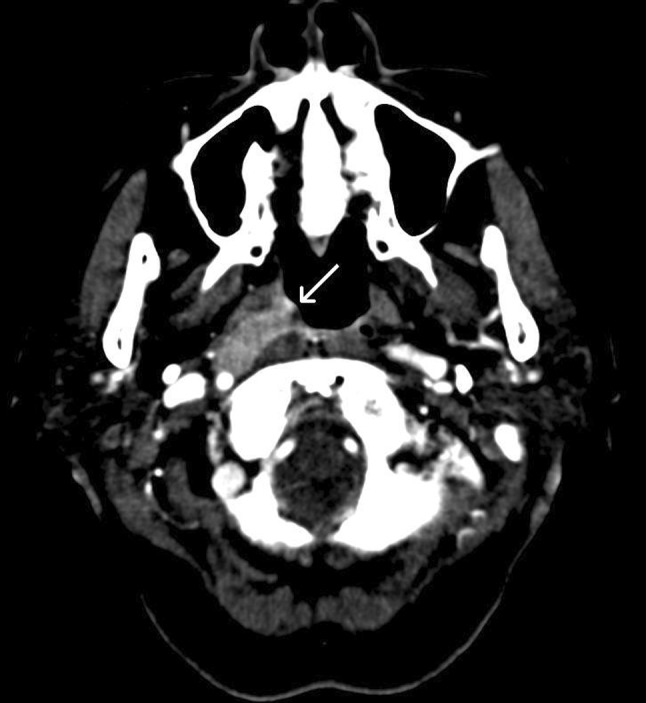
Computed tomography (CT) image showing a wedge-shaped mass in the right nasopharynx (arrow)
Diagnosis
Microscopic examination of a biopsy of the nasopharyngeal mass demonstrated multiple nests of round cells with a syncytial growth pattern, prominent nucleoli, scant cytoplasm with nuclear overlap, and an absence of keratinization (Figs. 2, 3). Immunohistochemical stains for AE1/AE3, CAM 5.2, and epithelial membrane antigen (EMA) were positive, while p16 was negative. Furthermore, the tissue was strongly reactive for Epstein–Barr virus encoded RNA (EBER) by in situ hybridization (Fig. 4).
Fig. 2.
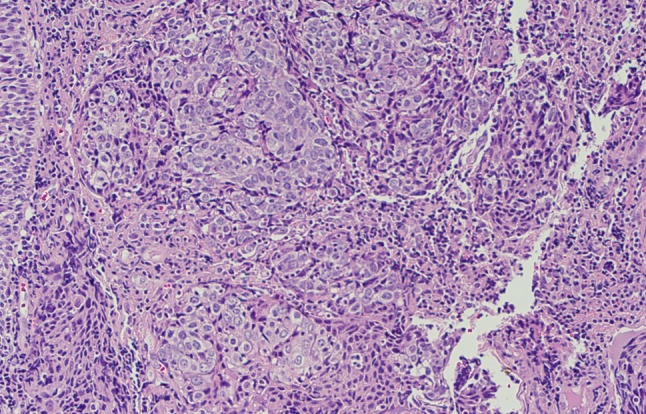
Medium-power view demonstrating large cells exhibiting a syncytial growth pattern with associated lymphoid stroma and an absence of keratinization
Fig. 3.
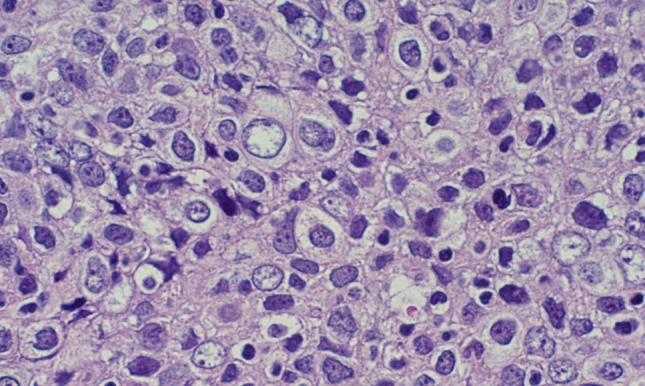
High-power view demonstrating cells with large round vesicular nuclei and prominent nucleoli
Fig. 4.
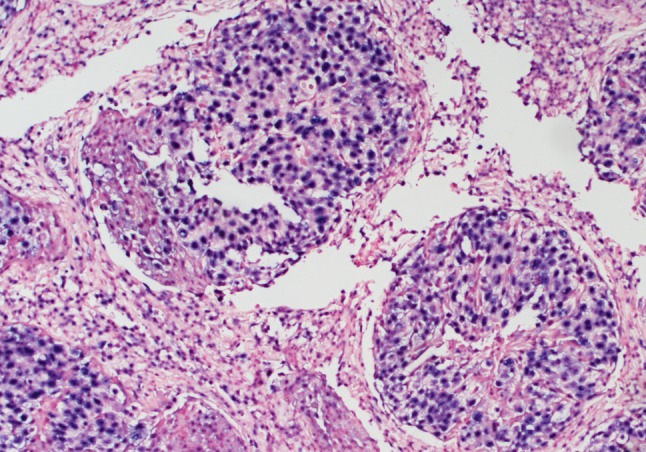
In-situ hybridization for Epstein–Barr virus encoded RNA (EBER) demonstrating diffuse nuclear labeling
Discussion
Nasopharyngeal carcinoma (NPC) is a rare entity in most parts of the world, representing <1 % of all cancers. However, in certain areas such as Southeast Asia and North Africa, the incidence is much higher, approaching 25 % of all cancers [1]. To explain this disproportionate global distribution, many etiologic factors have been proposed, including genetic susceptibility, dietary exposure to nitrosamine and high-salt fish [2], and infection with Epstein–Barr virus (EBV) [3]. NPC can occur at any age, most commonly between the ages of 40 and 60, and has a 3:1 male to female predominance [4]. Patients commonly present with neck swelling, symptoms of nasal obstruction, or serous otitis media (due to obstruction of the Eustachian tube). Less common presentations include cranial nerve palsy, headache, and tinnitus.
NPC is broadly divided by the World Health Organization into keratinizing and nonkeratinizing subtypes [5]. The subtypes may overlap, with approximately a quarter of cases demonstrating features of more than one subtype [6]. The histologic features of keratinizing NPC are similar to those of conventional squamous cell carcinomas arising elsewhere in the aerodigestive tract. These features include abundant keratinization, eosinophilic glassy cytoplasm, keratin pearls, and intercellular bridging. Nonkeratinizing NPC, characterized by solid sheets, irregular islands, or trabeculae of carcinoma with an absence of keratinization, is further subclassified into differentiated and undifferentiated subtypes. The differentiated subtype demonstrates some level of cellular stratification and pavementing, often described as resembling transitional cell carcinoma of the bladder. The undifferentiated subtype, such as the case presented here, has a characteristic syncytial growth pattern consisting of large cells with round to oval vesicular nuclei and prominent nucleoli. The tumor cells are often associated with a lymphoid stroma, which may vary from occasional scattered lymphocytes to an abundant infiltration of the tumor nests obscuring the epithelial nature of the cells (termed “lymphoepithelial carcinoma”). Other features occasionally seen include scattered spherical amyloid globules, epithelioid granulomas, and admixed eosinophils.
In addition to its histologic characteristics, nonkeratinizing NPC has several key clinical features which distinguish it from keratinizing NPC. Nonkeratinizing NPCs are relatively more frequent in high-incidence areas, where they represent >95 % of all NPCs (compared with approximately 75 % of NPCs in low-incidence areas) [5]. Nonkeratinizing NPCs also have a high association with positive EBV serologies, are more radiosensitive, and are more frequently associated with lymph node and distant metastasis [7].
The immunoprofile for keratinizing and nonkeratinizing NPCs is similar. Both show strong diffuse staining for pan-cytokeratin and high molecular weight cytokeratins, and weak or patchy staining for low molecular weight cytokeratins. In nonkeratinizing NPCs with a prominent lymphocytic infiltrate, the cytokeratin immunostain highlights the epithelial cells and reveals a distinctive meshwork pattern of staining. EMA staining is often focal. Staining for p16 is negative in NPC, in contrast to oropharyngeal carcinoma which is characteristically positive for p16.
An important diagnostic feature of NPC is its association with Epstein–Barr virus. While nearly 100 % of nonkeratinizing NPCs are associated with EBV, keratinizing NPC only demonstrates a strong association in high-incidence areas. The most reliable method of detecting EBV is with in situ hybridization for EBV-encoded early RNA (EBER), which is detected in cells infected by EBV. Nonkeratinizing NPCs show nuclear labeling in nearly all cells, while in keratinizing NPCs the signal is usually limited to the basal cells. Other methods for detecting EBV, including polymerase chain reaction and staining for EBV latent membrane protein-1 (LMP-1), are unreliable for diagnosis.
The differential diagnosis for NPC depends on its subtype. The diagnosis of keratinizing NPC is relatively straightforward, aside from difficulties in determining site of origin (i.e. nasopharyngeal vs. other head and neck sites). The differential for nonkeratinizing NPC is broader. In biopsies with a profound lymphocytic infiltrate, or in metastatic lymph node biopsies, confusion with Hodgkin lymphoma or large cell lymphoma is a common pitfall. Features common to these entities include large epithelioid tumor cells with prominent nucleoli in a background of mixed inflammatory cells. In this situation, immunohistochemical stains are invaluable. Staining with pan-cytokeratin will demonstrate the epithelial origin of NPC, while staining with leukocyte common antigen would favor a lymphoid origin. Furthermore, negative in situ hybridization for EBER would effectively rule out nonkeratinizing NPC.
Due to the rich lymphatic drainage of the nasopharynx, NPC has a notoriously high rate of regional lymphatic metastasis. Hematogenous spread may also occur, with bone, lung, and liver representing common sites for distant metastasis. Treatment for NPC typically involves radiation therapy, though the use of neoadjuvant or concurrent chemotherapy has gained support [5]. The overall 5-year survival for NPC has improved over the past few decades, and now exceeds 75 % [5]. Adverse prognostic factors include older age at diagnosis, male gender, keratinizing histology, and distant metastasis.
Footnotes
Disclaimer: The opinions and assertions expressed herein are those of the author and are not to be construed as official or representing the views of the Department of the Navy or the Department of Defense.
References
- 1.Pathmanathan R, Prasad U, Chandrika G, et al. Undifferentiated, nonkeratinizing, and squamous cell carcinoma of the nasopharynx. Variants of Epstein–Barr virus-induced neoplasia. Am J Pathol. 1995;146:1355–1367. [PMC free article] [PubMed] [Google Scholar]
- 2.Poirier S, Ohshima H, de The G, Hubert A, Bourgade MC, Bartsch H. Volatile nitrosamine levels in common foods from Tunisia, South China and Greenland, high-risk areas for nasopharyngeal carcinoma (NPC) Int J Cancer. 1987;39:293–296. doi: 10.1002/ijc.2910390305. [DOI] [PubMed] [Google Scholar]
- 3.Nicholls JM, Agathanggelou A, Fung K, et al. The association of squamous cell carcinomas of the nasopharynx with Epstein–Barr virus shows geographical variation reminiscient of Burkitt’s lymphoma. J Pathol. 1997;183:164–168. doi: 10.1002/(SICI)1096-9896(199710)183:2<164::AID-PATH919>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 4.Slootweg PJ, Richardson M. Squamous cell carcinoma of the upper aerodigestive system. In: Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. 2. Philadelphia: Elsevier; 2009. pp. 54–57. [Google Scholar]
- 5.Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumors. Pathology and genetics of head and neck tumors. Lyon: IARC Press; 2005. [Google Scholar]
- 6.Shanmugaratnam K, Chan SH, de The G, et al. Histopathology of nasopharyngeal carcinoma. Correlations with epidemiology, survival rates, and other biological characteristics. Cancer. 1979;44:1029–1044. doi: 10.1002/1097-0142(197909)44:3<1029::AID-CNCR2820440335>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Fandi A, Cvitkovic E. Biology and treatment of nasopharyngeal cancer. Curr Opin Oncol. 1995;7:255–263. doi: 10.1097/00001622-199505000-00011. [DOI] [PubMed] [Google Scholar]


