Abstract
NUT midline carcinoma (NMC) is an aggressive subset of squamous cell carcinoma, genetically defined by rearrangement of the NUT gene. The rearrangements most often take the form of BRD4-NUT fusions, and in a minority of cases, BRD3-NUT or NUT-variant fusions. The simple karyotypes of NMCs, in contrast to the complex ones of typical squamous cell carcinoma, suggest an alternate, genetic shortcut to squamous cancer. Although originally thought to be a disease of the mediastinum, NMC frequently (35 %) arises in the head and neck. Diagnosis is made simply by demonstration of nuclear immunoreactivity to NUT protein, and ancillary studies to characterize the fusion oncogene, though not required for diagnosis, are recommended. The prognosis is dismal, with a 6.7 month median survival, and treatment with conventional chemotherapeutic regimens is ineffective. The oncogenic mechanism of the dual bromodomains and the p300-binding portion of BRD4-NUT is to sequester p300 to localized regions of chromatin, leading to global transcriptional repression and blockade of differentiation. Two therapies which target this mechanism have emerged, including bromodomain inhibitors (BETi) and histone deacetylase inhibitors (HDACi), both of which induce differentiation and growth arrest of NMC cells, both in vitro and in vivo. BETi is available to adults with NMC through a phase I clinical trial, and clinical response to HDACi has been demonstrated in pediatric patients. The emergence of these promising targeted therapies gives hope that NMC may one day be effectively treated and provides a strong rationale for diagnostic testing for NMC.
Keywords: NUT, BRD4, BRD3, BETi, HDAC, NUT midline carcinoma, Squamous cell carcinoma, p300
NUT midline carcinoma (NMC) is a recently described form of poorly-differentiated squamous cell carcinoma defined by chromosomal rearrangement of the NUT gene on chromosome 15 [1]. Although this cancer affects the head and neck in one-third of cases, it is not restricted to any specific organ system, and can affect a variety of midline regions, most often the mediastinum [2]. Thus, like a growing number of carcinomas, it is a genetically-defined cancer and can only be diagnosed by means of demonstrating rearrangement of the NUT gene. Fortunately, demonstration of NUT rearrangement and the diagnosis of NMC has been made much easier by the commercial availability of a NUT specific antibody [3], discussed below.
Genetics
A unique feature of NMCs which distinguishes it from garden-variety squamous cell carcinomas of the head and neck or thorax is that it is characterized by very simple karyotypes. Typical squamous cell carcinomas have complex and multiple cytogenetic rearrangements, whereas NMCs usually possess a single translocation involving the NUT gene (Fig. 1a). In the majority of cases (2/3rd), NUT is fused to BRD4 in a t(15;19)(q14, p13.1) translocation (BRD4-NUT [4]. In the remaining cases, it is either fused to BRD3 [t(9;15)(q34.2;q14), BRD3-NUT], or it is fused to an as yet uncharacterized gene(s) (NUT-variant) (Fig. 1b) [5]. Thus, from a cytogenetic perspective, NMC more closely resembles leukemia/lymphoma, which is also often characterized by simple karyotypes and a single diagnostic translocation, than it does a squamous cell carcinoma. The findings raise the possibility that the pathogenetic mechanism of NMC differs significantly from that of typical squamous cell carcinoma. BRD-NUT may represent a short-cut to squamous cancer, bypassing the complex and poorly-understood process of years of environmentally-acquired accumulated mutations and genetic instability that are required to develop an invasive, metastatic phenotype. Nevertheless, understanding the pathogenesis of NMC may reveal a mechanism fundamental to the formation of not only NMC, but more common squamous cancers.
Fig. 1.
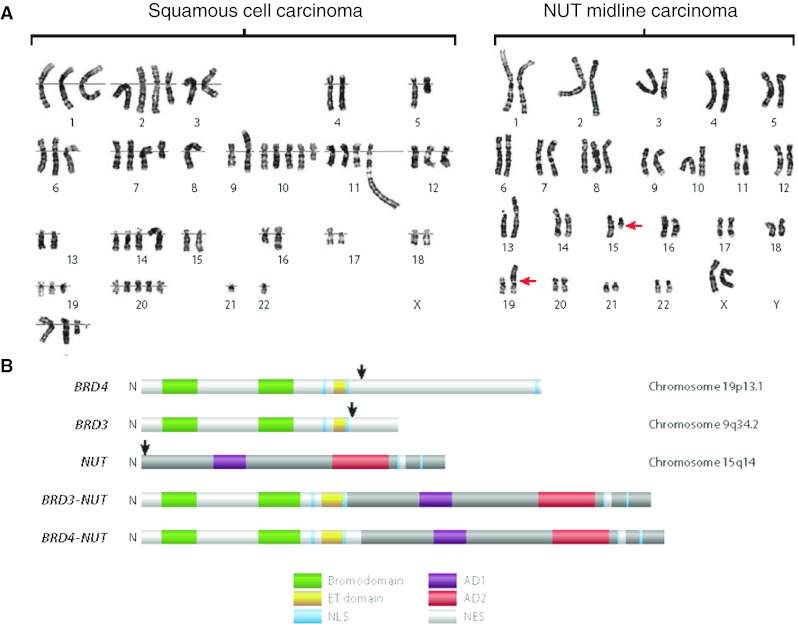
Genetic aberrations in NUT midline carcinoma (NMC). a Karyotypes of typical non–NUT midline carcinoma (NMC) squamous cell carcinoma (left), compared with (right) NMC. The red arrows denote chromosomal translocation between chromosomes 15 and 19. b Domain structures encoded by BRD-NUT fusions and native component genes. The black arrows indicate the locations of translocation-associated break points. AD1 acidic domain 1, AD2 acidic domain 2, ET extraterminal, NES nuclear export signal, NLS nuclear localization signal. a and b were taken from Annual Reviews of Pathology [7]
Diagnosis
The biggest challenge to the diagnosis of NMC is not the diagnosis itself, which is trivial, but knowing when to test for it. The presumed rarity of NMC, coupled with its recent description, lack of effective therapy, the frequent misconception that NMC only effects young people [2], and lack of pathognomonic morphologic characteristics, have resulted in a widespread lack of awareness of this disease, as well as a “why does it matter?” attitude. The consequence is that NMC is vastly underdiagnosed, as evidenced by its geographic distribution (Table 1) [2], which reveals a gradient of cases, most densely weighted in the U.S., whose epicenter is where this author practices! Recent developments in the targeted therapy of this disease make a strong case for why it does matter to make the diagnosis of NMC.
Table 1.
Geographical distribution of NUT midline carcinoma cases
| Location | n |
|---|---|
| United States | 41 |
| Massachusetts | 6 |
| Virginia | 5 |
| Minnesota | 4 |
| California | 3 |
| New York | 3 |
| Colorado | 2 |
| Connecticut | 2 |
| Georgia | 2 |
| Maryland | 2 |
| Pennsylvania | 2 |
| Washington | 2 |
| Florida | 1 |
| Idaho | 1 |
| Illinois | 1 |
| Kentucky | 1 |
| Maine | 1 |
| Michigan | 1 |
| New Mexico | 1 |
| Ohio | 1 |
| Italy | 5 |
| Australia | 4 |
| Sweden | 3 |
| Ireland | 2 |
| Japan | 2 |
| China | 1 |
| Croatia | 1 |
| Greece | 1 |
| Netherlands | 1 |
| New Zealand | 1 |
| Switzerland | 1 |
Taken from Clinical Cancer Research [2]
Morphologically, NMC is a poorly-differentiated squamous cell carcinoma and cannot reliably be distinguished from non-NMC squamous cancers. Nevertheless, there are a few unique features which should prompt consideration of NMC. The cells, which vary in size from small to medium, but not large, are conspicuously monotonous in appearance, and often have areas of focal “abrupt” squamous differentiation (Fig. 2a). This means that the tumors appear mostly undifferentiated, and usually lack an intermediate squamous differentiation component. The monotonous nuclei are typically round to slightly ovoid, and the cytoplasm is often clear, likely due to the presence of glycogen [6]. The lack of a pathognomonic appearance has led to the frequent misdiagnosis of this entity as garden variety squamous cell carcinoma [7], Ewing sarcoma [7], leukemia [7], sinonasal undifferentiated carcinoma [8, 9], and pancreatoblastoma [10], amongst other varied diagnoses.
Fig. 2.
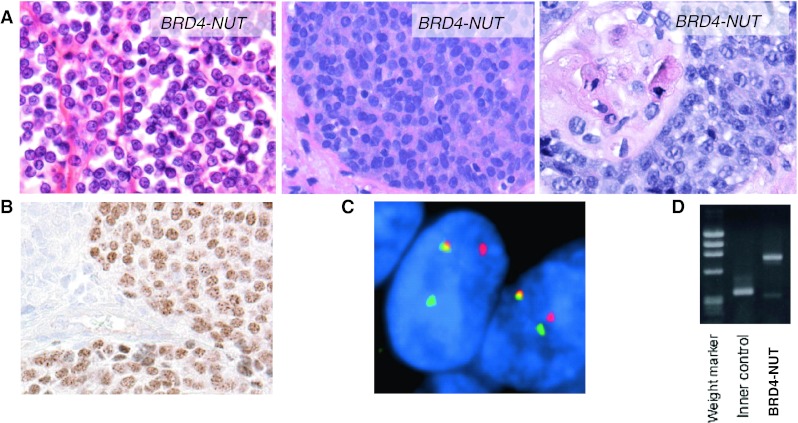
Diagnosis of NUT midline carcinoma (NMC). a H&E photomicrographs (400×) of three different NMCs reveal monotony and occasional abrupt squamous differentiation. Adapted from Annual Reviews of Pathology [7]. b Immunohistochemistry using a NUT-specific antibody (Cell Signaling Technologies) reveals speckled nuclear staining. c Fluorescent in situ hybridization (FISH) using a dual-color breakapart probe flanking the NUT gene. The split-apart red and green signals indicate rearrangement of the NUT locus. Taken from Cancer Research [2]. d Reverse-transcriptase polymerase chain reaction (RT-PCR) using primers that flank the coding sequence of the BRD4-NUT breakpoint and appropriate controls. Courtesy of Yukichi Tanaka, Mio Tanaka, Toru Horisawa, and Yutaka Saikawa, Kanazawa Medical University, Ishikawa, Japan
The diagnosis of NMC is made by immunohistochemical demonstration of nuclear reactivity for NUT using a monoclonal antibody available from Cell Signaling Technologies (Danvers, MA) [3]. Characteristically, the majority of nuclei stain and in a speckled pattern (Fig. 2b). Confirmatory fluorescence in situ hybridization (FISH), PCR, or cytogenetic analysis is no longer required for this diagnosis as the specificity of the NUT antibody is 100 % [3]. The sensitivity is excellent, at 87 % [3]. The only tumors which can also display nuclear NUT reactivity are germ cell tumors; however, the staining is very focal (<5 %), faint, and lacks the speckled pattern. Although not required for the diagnosis, further evaluation to characterize the fusion gene as either BRD4-NUT, BRD3-NUT, or NUT-variant, is recommended as this may impact treatment in the near future (see below). This can be accomplished by conventional cytogenetic analysis, FISH, or reverse-transcriptase-PCR (RT-PCR) (Figs. 1a, 2c, d). Cytogenetic analysis and RT-PCR require fresh or frozen tissue, whereas FISH can be performed on virtually any tissue preparation, including paraffin-embedded, formalin-fixed sections.
Clinico-pathologic Characteristics
NMC is well-known to oncologists who’ve treated it for its devastating clinical course. With a median survival of 6.7 months, NMC represents the most lethal subset of squamous cell carcinoma [2]. It affects people of all ages (range 0–78 years.), though a skew towards younger individuals may represent selection bias. NMC is named for its tendency to involve midline structures, but it doesn’t always do so. It affects primarily the thorax (57 %, most often the mediastinum), and head and neck (35 %), but has involved the adrenal gland (unpublished observations), major salivary glands [11, 12], pancreas [10], bladder [1], and lung [13].
Recent findings, based on a retrospective study of 54 patients, indicate that complete resection and radiation are independent predictors of improved progression-free (PFS) and overall survival (OS) [2]. Thus, local control of the primary mass, either by surgical resection and/or radiation, is recommended whenever possible. Unfortunately, in this same study, it was found that translocation type (BRD3-NUT, BRD4-NUT, NUT-variant) was not associated with a significant difference in PFS or OS, though it was intriguing that five of the seven longest living patients were either BRD3-NUT (n = 1) or NUT-variant (n = 4) patients. One of the more interesting findings in this largest ever NMC series was the significant association of BRD4-NUT translocation type with primary location in the head and neck (p = 0.04). The biological significance of this is unknown, but may be of relevance to the embryological development and cell of origin of this tumor. Probably the most important finding in this study was that no particular chemotherapeutic regimen (including platinum, alkylating agents, or anthracycline-based regimens) was superior. All but a few tumors were eventually unresponsive to chemotherapies of all types. Thus, it is recommended that novel treatments be explored in the treatment of this disease, as discussed below. In response to the rarity of NMC, and to learn how to better treat it, we have formed the International NUT Midline Carcinoma Registry to collect and prospectively track the outcomes of NMC patients. The Registry is a central repository for clinical outcomes and discarded patient tissue (www.NMCRegistry.org), and has become an important resource for patients, families, and caregivers.
Science
BRD4-NUT causes malignancy by blocking the differentiation of NMC cells and maintaining their proliferation, as evidenced by the rapid squamous differentiation that occurs following siRNA knockdown of the BRD4-NUT oncoprotein (Fig. 3a). How it does this remains poorly-understood. BRD4, a member of the dual bromodomain family of proteins (BET), binds acetylated chromatin with these bromodomains which thus tether BRD4-NUT to chromatin [5]. We know that this is essential to the oncogenic function of BRD4-NUT because chemical interference with this interaction, using acetyl-histone mimics (BETi), “unblocks” differentiation in NMC cells [14]. This has led to a novel targeted therapeutic approach to treating NMC, using pharmaceutical BETi. The adult phase I clinical trial for use of this class of drug in NMC patients by Glaxo-Smith Kline has been initiated and will be enrolling patients soon (http://www.clinicaltrials.gov/ct2/show/NCT01587703?term=NMC&rank=1). The availability of this new targeted therapy for NMC is another important reason why it is important to diagnose this disease.
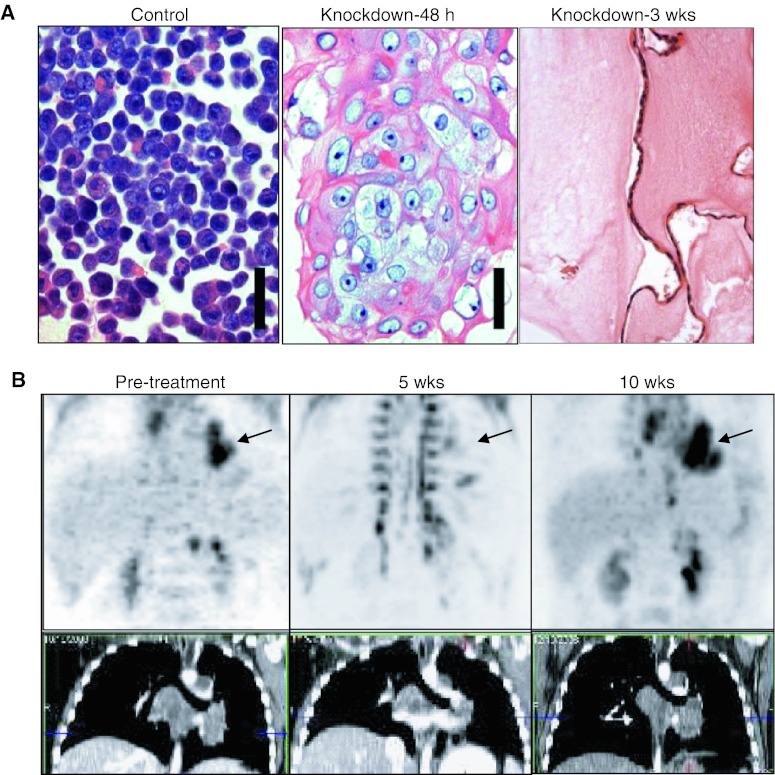
Fig. 3.
Targeting NUT midline carcinoma (NMC). a Small interfering RNA (siRNA) knockdown of BRD4-NUT in patient-derived NUT midline carcinoma cells (TC-797) using an siRNA directed against NUT [5] (scale bar 50 μm). Control consists of TC-797 cells 72 h after transfection with scrambled siRNA. Taken from Annual Reviews of Pathology [7]. b P18PF-fluorodeoxyglucose–positron emission tomography and computed tomography scan of the patient’s mediastinal tumor (arrow) reveals arrested growth five weeks following HDACi (vorinostat) treatment, then recurrence five weeks after the patient became intolerant to the drug and ceased taking it. Taken from Cancer Research [16]
The NUT portion of BRD4-NUT binds to and activates a histone acetyl-transferase, p300, which is hypothesized to acetylate regional chromatin, recruiting more local BRD4-NUT, and more local p300, leading to more local acetylation, in a self-perpetuating process that leads to the BRD4-NUT foci that can be seen by immunohistochemistry (Fig. 2b) [15]. The paradox is that these large aggregates of hyperacetylated chromatin, BRD4-NUT, and p300, rather than leading to increased transcription, actually act as p300 sinks that globally decrease acetylation and transcription [16]. Thus, genes required for differentiation are not expressed. This feed-forward mechanism can be reversed by treating cells with histone deacetylase inhibitors (HDACi), which artificially increase acetylation throughout the cell. HDACi treatment leads to globally increased transcription, differentiation, and arrested proliferation in NMC, both in vitro, and in animal models [16]. The findings have led to the recent treatment of pediatric patients with NMC using HDACi-containing regimens. Anecdotal results have been promising [16] (Fig. 3b). The potentially more effective treatment of NMC using HDACi provides yet another reason to diagnose this disease.
Conclusions
NMC is a genetically defined, extremely aggressive subset of squamous cell carcinoma that is under-recognized and under-diagnosed. Nevertheless, diagnosis is easy and several recent findings have led to the hope that this disease can be treated with targeted therapy that is now clinically available. More frequent recognition of this disease and prospective outcomes analysis are required to determine how to effectively treat NMC. Given the pathologic and clinical characteristics of NMC, we recommend immunohistochemical testing for NUT expression in all poorly-differentiated carcinomas without glandular differentiation arising in the chest, head, and neck [2]. Testing is not recommended at this time for those cancers with specific etiology, including EBV- or HPV-positive cancers.
Acknowledgments
This work was supported by a Dana Farber/Harvard Cancer Center 521 Nodal Award 5P30CA06516-44, US National Institutes of Health (NIH) grant 1R01CA124633, the Stanley L. Robbins Memorial Award, NIH Institutional National Research Service Award Grant T32-HLO7627, and the NIH National Cancer Institute Mentored Clinical Scientist Award 1K08CA128972.
References
- 1.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 2.Bauer D, Mitchell C, Strait K, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French CA, Miyoshi I, Kubonishi I, et al. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–307. [PubMed] [Google Scholar]
- 5.French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 6.Wartchow EP, Moore TS, French CA, et al. Ultrastructural features of NUT midline carcinoma. Ultrastruct Pathol. 2012;36:280–284. doi: 10.3109/01913123.2012.664613. [DOI] [PubMed] [Google Scholar]
- 7.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol Mech Dis. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 8.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–1221. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 10.Shehata B, Steelman CK, Abramowsky CR, et al. NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a two-year-old with a pancreatic/hepatic primary. Pediatr Dev Pathol. 2010;13:481–485. doi: 10.2350/09-10-0727-CR.1. [DOI] [PubMed] [Google Scholar]
- 11.den Bakker MA, Beverloo BH, van den Heuvel-Eibrink MM, et al. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. 2009;33:1253–1258. doi: 10.1097/PAS.0b013e3181abe120. [DOI] [PubMed] [Google Scholar]
- 12.Ziai J, French CA, Zambrano E. NUT gene rearrangement in a poorly-differentiated carcinoma of the submandibular gland. Head Neck Pathol. 2010;4:163–168. doi: 10.1007/s12105-010-0174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka M, Kato K, Gomi K, et al. NUT midline carcinoma: report of 2 cases suggestive of pulmonary origin. Am J Surg Pathol. 2012;36:381–388. doi: 10.1097/PAS.0b013e31824230a8. [DOI] [PubMed] [Google Scholar]
- 14.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reynoird N, Schwartz BE, Delvecchio M, et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 2010;29:2943–2952. doi: 10.1038/emboj.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]