Abstract
Abrus precatorius is highly regarded as a universal panacea in the herbal medicine with diverse pharmacological activity spectra. This experimental study on the mechanism of the anticancer activity of A. precatorius leaf extracts, may offer new evidence for A. precatorius in the treatment of breast cancer in clinical practice. Cell death was determined by using MTT assay. Further analyses were carried out by doing DNA laddering, PARP cleavage, FACS, semi-quantitative RT-PCR and detection of cellular reactive oxygen species (ROS) by DCFDA assay. A. precatorius showed very striking inhibition on MDA-MB-231 cells. MTT assay showed more than 75 % inhibition of the cells and treated cells indicated visible laddering pattern with thick compact band. PARP cleavage produced 89 kDa cleavage product which was associated with apoptosis. Flow cytometer exhibited a sub-G0/G1 peak as an indicative of apoptosis. mRNA expression level of apoptosis-related genes p21 and p53 was markedly increased in cells treated with the extract as compared to control. The up-regulation of p21 and p53 may be the molecular mechanisms by which A. precatorius extract which induces apoptosis. An increase in the concentration of A. precatorius extract does not generate ROS, instead it reduces ROS formation in MDA-MB-231 cells, as evident from the shift in fluorescence below untreated control. This is the first report showing that A. precatorius leaf extract exhibits a growth inhibitory effect by induction of apoptosis in MDA-MB-231 cells. Our results contribute towards validation of the A. precatorius extract as a potentially effective chemopreventive or therapeutic agent against breast cancer.
Keywords: Breast cancer cells, Abrus precatorius, Apoptosis, FACS, Semi-quantitative RT-PCR, PARP, ROS analysis
Introduction
Cancer is one of the most dreaded diseases. It is estimated that by 2030, 26.4 million people a year may be diagnosed with cancer and 17 million people will die from it (World Health Organization 2010). Among cancers, breast cancer is the most commonly diagnosed cancer and is a leading cause of mortality among females worldwide, with 23 % (1.38 million) of the total new cancer cases and 14 % (458,400) of the total cancer deaths recorded in 2008 (Source; GLOBOCAN 2008). It is estimated that half of the breast cancer cases and 60 % deaths due to cancer have been predicted to occur in economically developing countries (Jemal et al. 2011). Breast cancer, like many other cancers, tends to metastasize to other parts of the body, preferably to the lungs and bones (Solomayer et al. 2000). This process of neoplastic transformation, progression and metastasis involve alterations of normal apoptotic pathway (Bold et al. 1997). Apoptosis is a physiological cell suicide program critical to development and tissue homeostasis. Abnormal apoptosis is associated with a wide variety of human diseases such as cancer, auto-immune disease and neurodegenerative diseases (Joaquin and Gollapudi 2001). Hence the prerequisite for successful treatment of breast cancer is the susceptibility of the cancer cells to apoptosis (Sarath et al. 2007). Despite significant advances in the treatment for breast cancer, many patients suffer a systemic relapse. Therefore, there is a need to identify novel anticancer agents that induce apoptosis and to develop complementary and/or alternative treatments for the treatment of breast cancer and to significantly decrease obnoxious side effects (Norton 1999). Natural phytochemicals isolated from medicinal plants have gained significant recognition in the potential management of several human clinical conditions, including cancer (Mehta et al. 2010). There are more than 270,000 higher plants existing on this planet. India has one of the richest plant medical traditions in the world and around 25,000 effective plant-based formulations used in ethanobotanical communities in India. But only a small portion has been explored phytochemically (Helton 1996). Therefore, the search for alternative drugs that are both effective and non-toxic in the treatment of cancers is an important research line (Tang et al. 2003). In fact, increased efforts are being made to isolate bioactive products from medicinal plants for their possible utility in cancer treatment (Kinghorn et al. 2003). The mechanisms of tumor prevention by natural phytochemicals range from the inhibition of genotoxic effects, increased antioxidants, inhibition of proteases, anti-inflammatory activity, cell proliferation, protection of intracellular communications to modulate apoptosis and signal transduction pathways (Soobrattee et al. 2006). Many dietary supplements and plant-derived products are shown to be promising anti-cancer therapeutic agents and are now being assessed in pre-clinical and clinical studies (Cragg and Newman 2005).
Abrus precatorius belongs to the family (Fabaceae), popularly known as Crab’s eye, Indian liquorice, Jequirity, Rosary pea. It is originally native to India, but now commonly distributed throughout tropical and subtropical regions of the world (Naik 1998). People consume leaves of A. precatorius by chewing (or in tea) while the seeds contain a toxin called abrin which is a strong inhibitor of protein synthesis and lethal even at relatively low doses (0.1–1 μg/kg). A. precatorius extracts have been receiving attention as anticancer agents as it has been shown that various phytochemicals from A. precatorius have the property to induce apoptosis on various types of cancers (Hickman 1992). Several preliminary reports have documented that A. precatorius extract shows diverse pharmacological activity spectra especially, antitumoral (Hegde et al. 1991; Reddy and Sirsi 1969; Siddiqi et al. 2001), mitogenic (Kaufman and McPherson 1975), antifertility (Kamboj and Dhawan 1982), immunopotentiating (Ramnath et al. 2002), antimicrobial (Adelowotan et al. 2008), immunostimulant activity (Bhutia et al. 2009), antianaphylactic activity (Taur and Patil 2011), and anti-inflammatory activity (Anam 2001). In this study, we have explored the cytotoxic effect of leaf extracts from A. precatorius on human metastatic breast cancer cell line MDA-MB-231, and provide a rationale for using A. precatorius as a therapeutic agent against breast cancer.
Materials and methods
Plant materials
Fresh, disease free leaves of the A. precatorius were collected from Danvantrivanna, Jnanabharathi campus Bangalore, Karnataka, in March and May, washed thoroughly 2–3 times with tap water and once with sterile distilled water, shade dried then powdered and used for extraction (Fabrican and Farnsworth 2001). An authenticated voucher specimen of the plant is deposited in the herbarium of Molecular Diagnostic Laboratory, Department of Microbiology and Biotechnology, Bangalore University, Bangalore.
Preparation of plant extract
Aqueous crude leaf extracts of plants were prepared according to Sateesh (1998). Dried plant sample was grinded to fine powder in mixer-grinder and sieved. The powder was suspended in double distilled water at the rate of 5 gms in 30 ml, sonicated for 15 min and mixed with magnetic stirrer for overnight. Each extract was passed through two layered cheese cloth (Garg et al. 2007). The filtrate was then centrifuged at 5,000 rpm for 30 min. Lyophylization of the mother solution was done with a Speedvac (Savant Speedvac SC100 Centrifugal Evaporator, USA). Lyophilized aqueous extract was dissolved in PBS (sterile) to a stock concentration of 50 mg/ml and then passed through a 0.2 μm filter (Sartorius Stedim) for sterilization and was then used for cytotoxicity assays.
Cell lines and culturing of cells
Human breast cancer cell line MDA-MB-231 was procured from Indian Institute of Science (IISc), Bangalore (India). Cells were maintained in Dulbecco’s Modified Eagle’s medium (DMEM) with 10 % FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin, in a humidified atmosphere of 95 % air and 5 % CO2 at 37 °C. Once the cells had covered about 80 % of the surface, they were trypsinized and the titre was adjusted using haematocytometer for antiproliferative and cytotoxicity measurements.
Analyses of cell viability
The effect of aqueous extract of A. precatorius on the viability of cells was determined using the standard colorimetric MTT assay using the 3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyl tetrazolium bromide dye (Sigma, St. Louis, MO, USA), according to Carmichael et al. (1987). This assay is based on the reduction of MTT by the mitochondrial dehydrogenase of intact cells to a purple formazan product (Mosmann 1983). MDA-MB-231 cells (5 × 103 cells/well) were seeded to 96-well microtiter plates (Falcon, Becton–Dickinson, Franklin Lakes, NJ, USA). After 24 h of plating, cells were serum starved for 24 h. Respective concentrations of A. precatorius extracts were added to serum free medium and the assay was terminated after 48 h. Medium was removed and 200 μl DMSO was added and the amount of formazan formed was measured at 595 nm on a Model 680 Microplate Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
DNA fragmentation by agarose gel electrophoresis
DNA fragmentation was analyzed by the gel electrophoresis method according to Herrmann et al. (1994). MDA-MB-231 Cells (5 × 106 cells) were incubated with 600 μg/ml of the A. precatorius extract for 12 h at 37 °C. After treatment, cells were washed in cold PBS and lysed in a buffer containing 50 mM Tris–HCl (pH 8.0), 1 mM EDTA, 0.2 % Triton X 100 for 20 min at 4 °C. After centrifugation at 14,000×g for 15 min, the supernatant was treated with proteinase K (0.5 mg/ml) and 1 % SDS for 1 h at 50 °C. DNA was extracted twice with phenol and precipitated with 140 mM NaCl and 2 vol. of ethanol at −20 °C overnight. DNA precipitates were washed twice in 70 % ethanol, dissolved in TE buffer, and treated for 1 h at 37 °C with RNaseA (Peng et al. 2009). Finally, DNA preparations were electrophoresed in 1 % agarose gels, stained with ethidium bromide and visualized under UV light.
Western blot analysis of cell cycle regulatory protein-poly-ADP ribose polymerase (PARP) cleavage assay to detect apoptosis
For immunoblot analysis, total cell lysates were collected in lysis buffer [50 mM HEPES–KOH, pH 7.5, 1 % Triton X-100, 150 mM NaCl, and protease inhibitor cocktail (Roche, New York, NY, USA)]. The extracts were centrifuged at 12,000 rpm for 20 min, and then the clear supernatant was collected. The protein content was determined by Bradford method (Bradford 1976). Proteins (100 μg) were separated on 10 % sodium dodecyl sulphate (SDS)-polyacrylamide gel and electro-transferred to polyvinyldene fluoride (PVDF) membrane (Immunobilon-P, 0.45 mm; Millipore, Billerica, MA, USA). The membranes were blocked with 5 % (w/v) non fat dry milk and then probed with a relevant antibody (PARP at 1:1,000 dilution) for 12 h at 4 °C followed by detection using peroxidase-conjugated secondary antibodies (Santa Cruz Biotech, Santa Cruz, CA, USA). The membranes were then washed 4 times with Tris-Buffered Saline and Tween 20 (TBST), followed by detection using chemiluminescence ECL kit (GE Healthcare Biosciences, Piscataway, NJ, USA) and exposed to an X-ray film (Kodak, Rochester, NY, USA). For equal loading of proteins, the membrane was probed with appropriate loading controls. β-Actin was used as loading control (Zhou et al. 2010).
Cell cycle analysis by fluorescence-activated cell sorter (FACS)
MDA-MB-231 cells were incubated at 5 × 106 cells/well in 6-well plates, treated with A. precatorius extract for 48 h at 37 °C. The detached and attached cells were harvested and fixed in 70 % ice-cold methanol at −20 °C overnight. After fixation, cells were washed with PBS, resuspended in 1 ml PBS containing 1 mg/ml RNase (Sigma, St. Louis, MO, USA), and 50 μg/ml propidium iodide (Sigma, St. Louis, MO, USA), and incubated at 37 °C for 30 min in the dark (Ping et al. 2006). Samples of 10,000 cells were then analyzed for DNA content and cell cycle phase distributions by using FACS-Calibur and Cell-Quest software (Becton–Dickinson Biosciences).
Determination of the expression level of apoptosis-related genes (p21 and p53) by semi-quantitative reverse transcription polymerase chain reaction (RT-PCR)
Abrus precatorius extract treated cells were washed with PBS. Total RNA was isolated from MDA-MB-231 cells by Trizol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. After extraction, total RNA was measured at 260 nm for quantification. Before performing the RT reaction, total RNA was adjusted to the final concentration of 1 μg/μL, and RNA was treated with 10–5 U deoxyribonuclease I (DNase I) (Invitrogen, Carlsbad, CA, USA) for 30 min at 37 °C. DNase-I treated RNA was reverse transcribed by ImProm-IITM Reverse Transcription System (Promega, Madison, WI, USA) with oligo(dT)17 primer following the manufacturer’s protocol. For amplification of the cDNA, each desired DNA fragment was amplified for 35–40 cycles using each gene-specific preimer pair (Table 1). All PCR reactions were done at non-saturating cycle numbers, which were determined for each gene. The optimized PCR amplification program comprised an initial denaturation step at 94 °C for 2 min followed by denaturation at 94 °C for 45 s, annealing at 56 °C for 1 min, extension at 72 °C for 2 min and final extension step at 72 °C for 10 min (Lee et al. 2009a, b). RPL35a (a ribosomal protein coding RNA) was used as an internal control for integrity and equal amount of cDNA used in each PCR reaction. Doxorubicin (Dox) 20 μM was used as a positive control (Rethy et al. 2008). PCR products were analyzed on 2 % agarose gels.
Table 1.
Primer sequences of p21, p53, and Rpl35a
| S.No | Gene | Amplicon size | Primer sequence |
|---|---|---|---|
| 1 | p21 | 140 bps | RT-FORWARD:- GCCATTAGCGCATCACAGT |
| RT-REVERSE:- ACCGAGGCACTCAGAGGAG | |||
| 2 | p53 | 146bps | RT-FORWARD:- GCTCGACGCTAGGATCTGAC |
| RT-REVERSE:- CAGGTAGCTGCTGGGCTC | |||
| 3 | Rpl35a | 141bps | RT-FORWARD:- CTGGTTTTGTTTGGTTTGCC |
| RT-REVERSE:- AAGGGAGCACACAGCTCTTC |
2′,7′-Dichlorofluorescein diacetate (DCF-DA) assay for measurement of ROS generation
The percentage of cell population generating ROS was determined by flow cytometry analysis. The detection of ROS generation by flow cytometric analysis was performed by incubating MDA-MB-231 cells with the extract (600 μg/ml) for 30 min in serum free conditions. The cells were harvested by trypsinization and a single cell suspension of 1 × 106 cells/ml was made. The cells were then treated with 1 μM DCF-DA solution (in DMSO) for 15–20 min in dark at room temperature. The distribution of DCF-DA stained MDA-MB-231 cells were determined by using FACS-Calibur and Cell-Quest software (Becton–Dickinson Biosciences) in the FL-1 channel as reported previously (Ju et al. 2007).
Results
Cytotoxic activity of aqueous extract from leaves of A. precatorius against human breast cancer cells
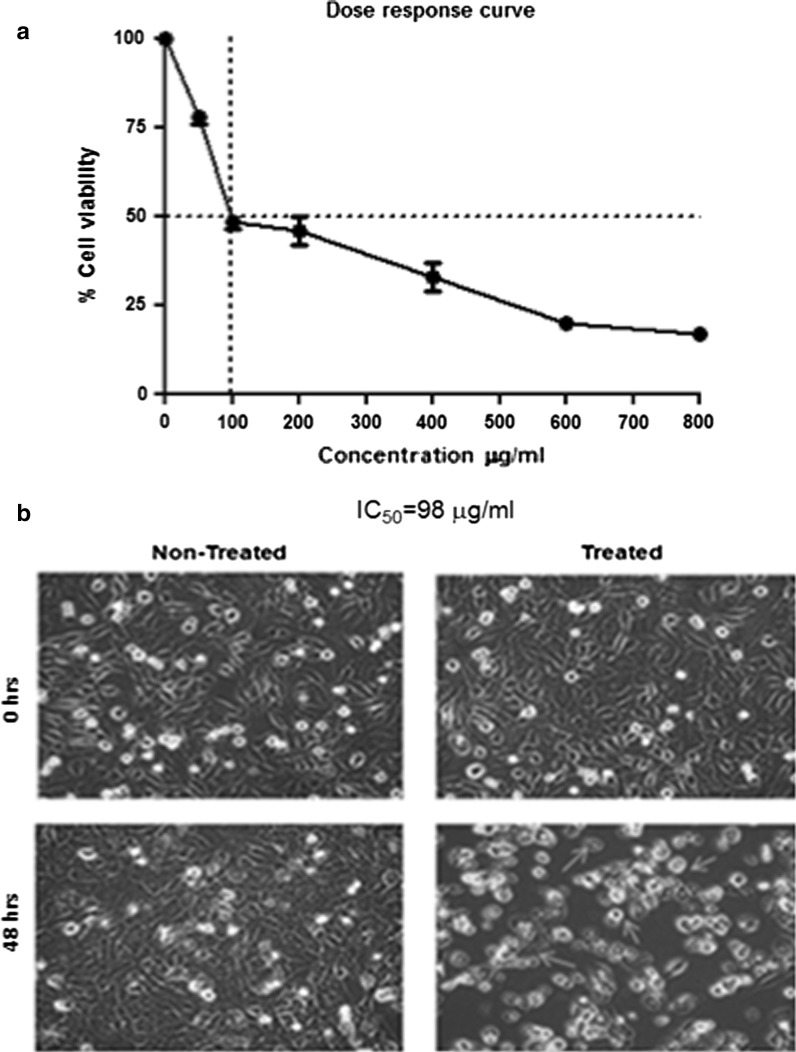
Aqueous extract from leaves of A. precatorius has strong dose and time-dependent anticancer activity against human breast cancer cells, with the maximal inhibition of cell growth (>75 %) obtained at 600 μg/ml after 48 h of incubation, with respect to the control as shown in (Fig. 1a). Microscopic studies show significant morphological changes such as shrinking of cytoplasm, condensation of nucleus and formation of membrane bound vesicles (apoptotic bodies) in treated MDA-MB-231 cells (1 × 106 cells/ml) with 600 μg/ml of the aqueous extract of A. precarious for 48 h as compared to control cells (Fig. 1b).
Fig. 1.
Effect of A. precatorius extract on the cell proliferation of MDA-MB-231 cells. a A dose dependent growth inhibition of MDA-MB-231 cells was observed at concentrations ranging from (200, 400, 600, 800 μg/ml) respectively. The maximal inhibition of cell growth (>75 %) was obtained at 600 μg/ml of the A. precatorius extract after 48 h of incubation. b Microscopic view of human breast cancer MDA-MB-231 cells (1 × 106 cells/ml), before treatment and after treatment with A. precatorius extract at 600 μg/ml at 0 and 48 h with magnification of ×10. The control cells had uniform nuclei and cytoplasm, while cells treated with A. precatorius extract exhibited the characteristic changes of apoptosis, with cell shrinkage, membrane blebbing, nuclear condensation and fragmentation, and formation of apoptotic bodies (arrows)
Abrus precatorius extract induces significant DNA fragmentation in MDA-MB-231 cells
Specific DNA cleavage and formation of DNA fragments of oligonucleosomal size (180–200 bp) is the hallmark of apoptosis in many cell types. This becomes evident in electrophoresis analysis by agarose gel electrophoresis (Enari et al. 1998). To elucidate whether the A. precatorius extract inhibits MDA-MB-231 cell proliferation through induction of apoptosis, cell death was examined by DNA fragmentation for 12 h treatment. Supernatants of control and apoptotic cell cultures were analyzed by agarose gel electrophoresis in the presence of marker 100 bp DNA ladder. Significant apoptosis was seen in the treated cultures with visible laddered pattern while thick compact band was seen in the control (Fig. 2).
Fig. 2.
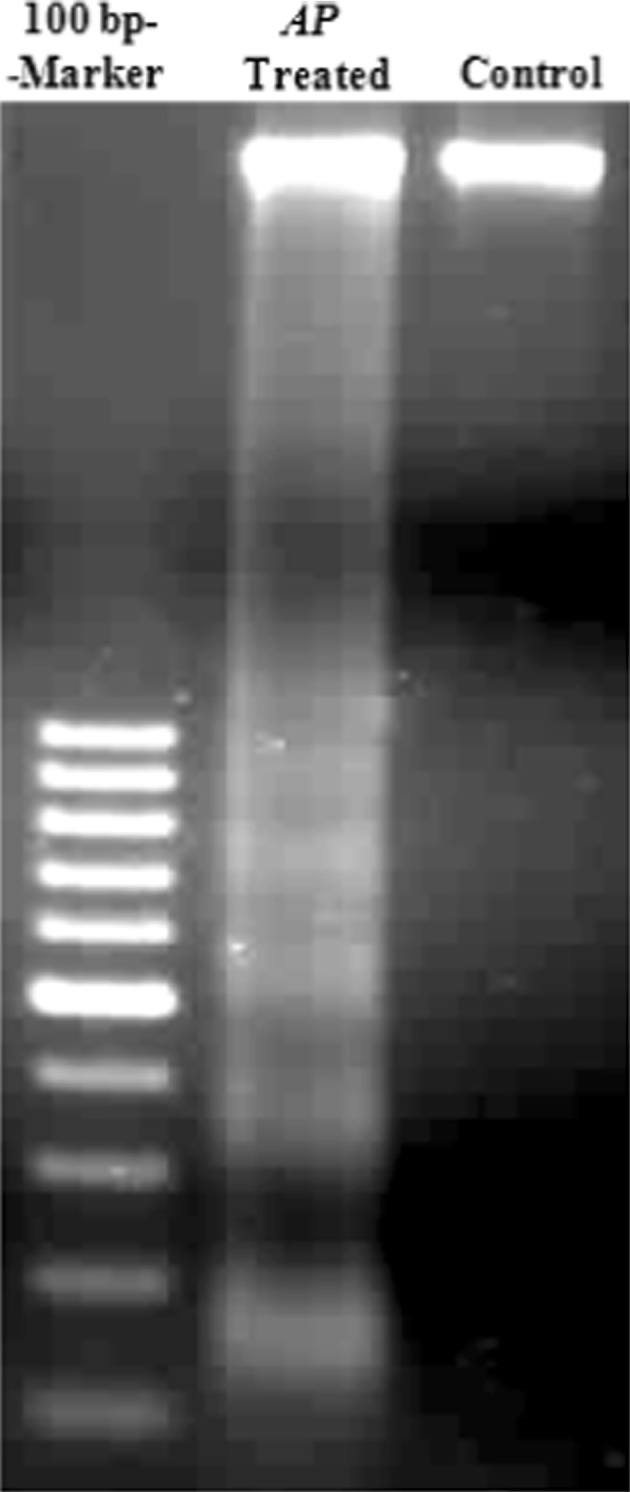
Induction of DNA fragmentation by A. precatorius extract in MDA-MB-231 cells. Cells were incubated with 600 μg/ml of the A. precatorius extract for 12 h at 37 °C. Control and apoptotic cultures were analyzed by agarose gel electrophoresis in the presence of a marker 100 bp DNA ladder. Lane-1 100 bp DNA molecular weight markers; Lane-2 DNA isolated from culture of A. precatorius extract treated cells; Lane-3 DNA isolated from culture of untreated control MDA-MB-231, human breast cancer cells
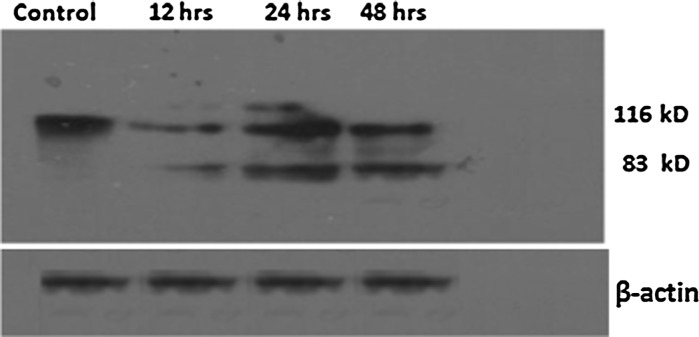
Western blotting clearly demonstrated a dose and time dependent inactivation of PARP in A. precatorius extract treated MDA-MB-231 cells
Apoptosis involves various initiator and executor caspases. Among the executor caspases, caspase-3 has been reported to cleave a number of substrates including PARP which acts in response to DNA strand breaks leading to apoptosis (Nicholson and Thornberry 1997). PARP cleavage occurs at Asp216 to generate 83 and 24 kDa apoptotic fragments during apoptosis (Soldani and Scovassi 2002). During apoptosis, caspase-3 inactivates PARP by cleaving it into 83 and 24 kd fragments. Results from immunoblot analysis using PARP antibody, that recognizes uncleaved PARP of 116 and the 83 kDa cleaved fragment clearly demonstrated a time dependent inactivation of PARP in A. precatorius extract treated MDA-MB-231 cells (Fig. 3).
Fig. 3.
Western blot analysis showing PARP cleavage in MDA-MB-231 cells treated with A. precatorius extract. PARP antibody recognizes both uncleaved PARP (116 kDa) and the larger cleavage fragment (83 kDa). Lane 1 Control cells; lane 2, 3 and 4: Cells treated with 600 μg/ml of the A. precatorius extract for 12, 24 and 48 h respectively. β-Actin was used as loading control
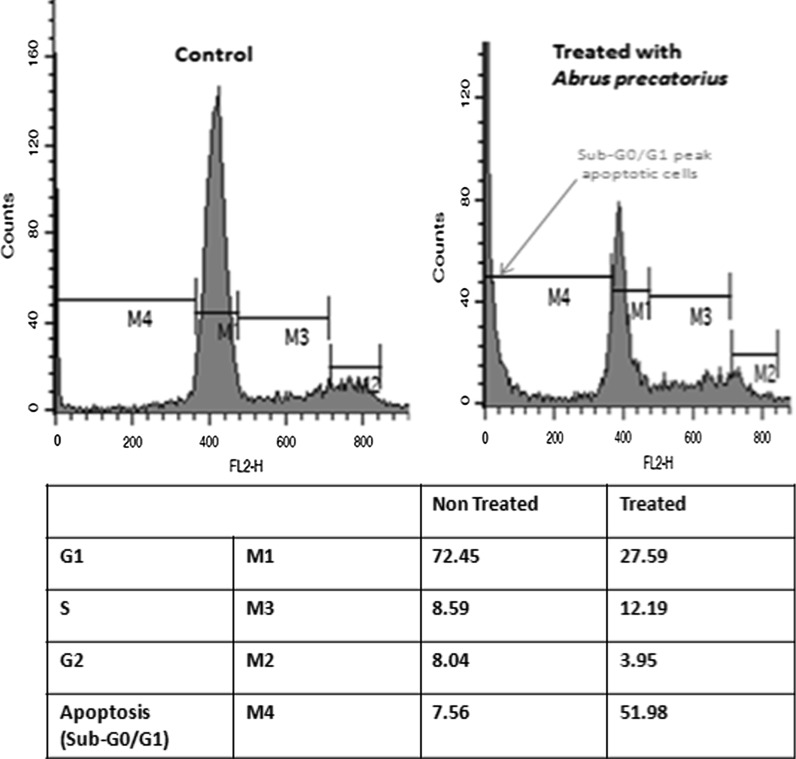
Effect of A. precatorius extract on cell cycle distribution by flow cytometry
Cell cycle control is a major regulatory mechanism of cell growth. Blockade of the cell cycle is considered as an effective strategy for the development of novel cancer therapies (McDonald and Deiry 2000). To gain insights into the mechanism by which cell reduction is achieved, we investigated the effect on cell cycle distribution by FACS analysis, and the results are summarized in (Fig. 4). An alteration in the percentage of cells in each stage of the cell cycle: G0/G1, S and G2/M, as compared to the control was observed. The results showed the increase of sub G0/G1 phase (apoptotic peak) of cell cycle in dose and time-dependent manner indicating that A. precatorius had a prominent ability to induce cell cycle arrest at G0/G1 phase and apoptosis in MDA-MB-231 cells (Fig. 4).
Fig. 4.
Effect of A. precatorius extract on MDA-MB-231 cell cycle distribution by flow cytometry. The values indicate the percentage of cells in the indicated phases of the cell cycle. Histograms show number of cells (vertical axis) versus DNA content (horizontal axis). Sub-G0/G1 peak as an indicative of apoptotic cells was induced in A. precatorius extract treated, but not in control cells
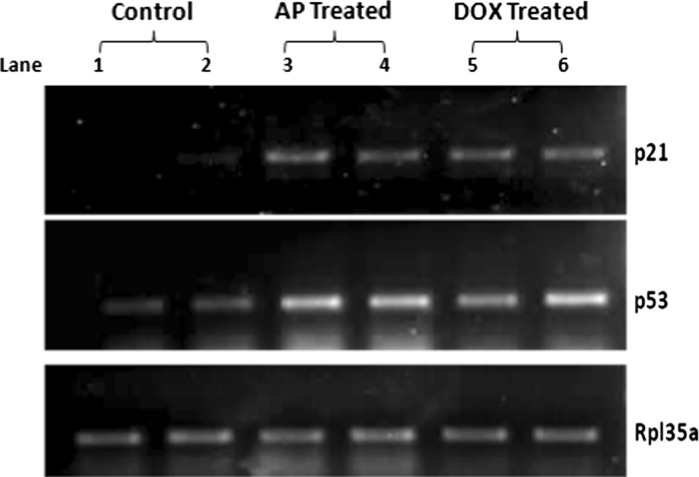
mRNA expression level of apoptosis-related genes p21 and p53 was markedly increased in A. precatorius extract treated MDA-MB-231 cells
The p53 gene codes for a protein that plays a key role in tumor suppression and cell cycle regulation. The loss of this protein due to mutation is a primary event in the formation of many types of leukemia’s, breast, colon, lung and liver cancer (Haupt et al. 2003). The p21 is activated by p53 protein, and an increased level of p21 is associated with cyclin containing complexes with decreasing cyclin-dependent activity in damaged cells destined to apoptosis (Harper et al. 1993). Previous reports also show marked increase in the expression of p53 at a 100 μg/ml concentration of A. precatorius seed extract. Also in the present study even control MDA-MB-231 cells did not exhibit expression of p53, but in the A. precatorius extract treated cells mRNA expression level was markedly increased and the mRNA expression level of p21 was also increased significantly. Expression of apoptosis-related genes, p21 and p53, was evaluated by RT-PCR. Control MDA-MB-231 cells did not exhibit their expression, but in the A. precatorius extract treated cells mRNA expression levels were markedly increased (Fig. 5).
Fig. 5.
Expression of apoptotic genes p53 and p21 in MDA-MB-231 cells treated with 600 μg/ml A. precatorius aqueous extract for 48 h. The expression levels of mRNAs were analyzed by RT-PCR, followed by an agarose gel electrophoresis. Lane 1-2 PCR product from control. Lane 3-4 PCR product from A. precatorius extract–treated. Lane 5-6 PCR product from positive control Dox (Doxorubicin)
ROS were not generated in A. precatorius extract treated cells, instead A. precatorius extract reduces ROS formation in MDA-MB-231 cells
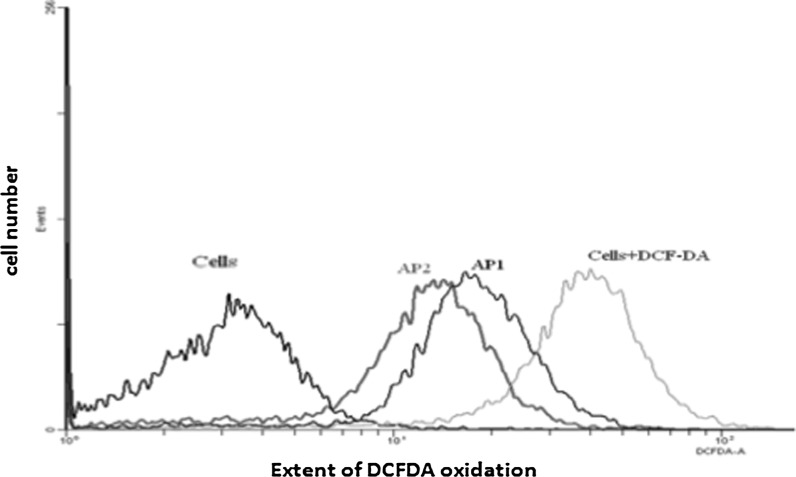
Anticancer phytochemicals have been shown to suppress or block cancer proliferation by acting as antiproliferative agents or antioxidants (Chang et al. 2008; Lee et al. 2009a, b). Apoptosis is carried out by a multistage chain of reactions in which ROS acts as trigger and essential mediator (Kerr et al. 1994). ROS such as super-oxide anion (O2−) and its reduced product hydrogen peroxide (H2O2) have been considered as cytotoxic by products of cellular metabolism, and the accumulation of ROS in cells may promote cell death. The dihydro-analogues fluorescent probe, 2′,7′-Dichlorofluorescein diacetate (DCF-DA) is commonly used to detect the generation of cellular reactive oxygen species (ROS) (Keston and Brandt 1965). Cell permeable DCF-DA can be oxidized by cellular ROS to generate fluorescent compound, Dichlorofluorescein having emission spectrum maxima at 525 nm (Esposti 2002). Cells treated with A. precatorius extract, were stained with ROS-specific dyes, 2′,7′-Dichlorofluorescein diacetate (DCF-DA). Altogether, these results indicate that the increasing concentration of A. precatorius extract does not generate ROS. Instead it reduces ROS levels in MDA-MB-231 cells, as evident from the shift in fluorescence in comparison to the control (Fig. 6).
Fig. 6.
A. precatorius treatment reduces ROS formation in dose dependent manner. MDA-MB-231 cells were treated with A. precatorius extract at concentration of (AP1) 300 and (AP2) 600 μg/ml concentration for 30 min and then incubated with DCF-DA (1 μM). A flow cytometric analysis was performed and differential distributions of cells showing fluorescence in FITC (fluorescein isothiocyanate) range are plotted. Increase in the concentration of A. precatorius does not generate ROS, instead it reduces ROS levels in MDA-MB-231 cells, as evidenced from the shift in fluorescence with respect to control
Discussion
The results of the present study showed that the extract of Abrus precatorius had a marked inhibitory effect on the proliferation of breast cancer cell line (MDA-MB-231). A. precatorius extracts markedly reduced cell viability in a concentration dependent manner. Previous preliminary studies on various cancer cell lines showed that various phytocompounds isolated from A. precatorius demonstrated marked inhibitory effects and have properties to induce apoptosis on various types of cancers (Hickman 1992). Glycyrrhizin, a triterpenoid saponins isolated from A. precatorius leaves and roots, induce apoptosis in many cell types including human hepatoma (HLE), promyelotic leukemia (HL-60) and stomach cancer (KATO III) and Kaposi sarcoma associated herpes virus-infected cells (Hibasami et al. 2005, 2006). The molecular study results showed suppression of cell growth induced by A. precatorius extract is due to induction of apoptosis rather than the inhibition of cell proliferation. In the present work various molecular events responsible for cytotoxicity were also studied. Significant apoptosis was seen in the treated cultures with visible ladder pattern while a thick compact band was seen in the control. Studies have shown that some proteins, including Abrin (ABR), purified from A. precatorius, induce apoptosis in various types of cancer cells (Shih et al. 2001; Narayanan et al. 2004). Also Abrus lectins are considered as potential anticancer agents due to their apoptosis-inducing activity (Lin et al. 1970). Abrins are more toxic to tumour cells than normal cells (Harborne et al. 1999). Not many studies have been done on the cytotoxic effect of A. precatorius on cancerous cells hence, molecular level investigation needs to be carried out. Results from immunoblot analysis using PARP antibody, recognizes the cleavage of PARP in A. precatorius treated MDA-MB-231 cells (Fig. 3). This confirmed A. precatorius induces apoptosis by cleaving cell cycle regulatory protein-poly-ADP ribose polymerase (PARP). Cell cycle analysis by flow cytometer exhibited a sub-G0/G1 peak in A. precatorius treated MDA-MB-231 cells. As compared to control, treated cells showed a significant increase of cells in sub-G1 phase, indicating the occurrence of an apoptotic event. The p53, known as guardian of human genome is a gene that codes for a protein that regulates the cell cycle and hence functions as a tumor suppressor. In cancerous cells, the expression of p53 is much suppressed (Bhutia et al. 2009). The p53 tumor suppressor gene is a cell cycle regulator able to induce cell cycle arrest to allow DNA repair or apoptosis (Vogelstein and Kinzler 1992). In the present study control MDA-MB-231 cells did not exhibit expression of p53, but in the A. precatorius extract treated cells mRNA expression levels were markedly increased and the mRNA expression level of p21 was also increased significantly. This up-regulation of p21 and p53 may be the molecular mechanisms by which A. precatorius extract induces apoptosis, however, further definitive studies are needed. Results from DCFDA assay showed increase in the concentration of A. precatorius extract does not generate ROS, instead it reduces ROS formation in MDA-MB-231 cells, as evident from the shift in fluorescence with respect to control. This showed reactive oxygen species play no role in apoptosis induction in MDA-MB-231 cells.
In conclusion, the present study clearly demonstrated the potential anticancer activity of A. precatorius extract against human breast cancer cell line MDA-MB-231. Also the results from our molecular studies clearly showed that the cell death was by apoptosis. A. precatorius extract have been reported to induce proliferation inhibition and cell death in various types of cancers (Reddy and Sirsi 1969; Hegde et al. 1991; Hickman 1992; Siddiqi et al. 2001). As apoptosis has become a promising therapeutic target in cancer research, these results confirm the potential of A. precatorius extract as an agent of chemotherapeutic and cytotoxic activity in human breast cancer cells. Phytochemical analysis and bioassay-directed isolation of bioactive components from the aqueous extract of the leaves of A. precatorius need to be done, and more detailed studies are required to determine the exact mechanism(s) of action behind the cytotoxic and apoptotic activities of A. precatorius extract.
Acknowledgments
The authors are grateful to the Department of Microbiology and Biotechnology, Bangalore University, Bangalore, Karnataka, India for support and facilities to carry out our research. We thank Dr. Sreekeerthy for critical review of the manuscript.
References
- Adelowotan O, Aibinu I, Adenipekun E, Odugbemi T. The in vitro antimicrobial activity of A. precatorius (L) fabaceae extract on some clinical pathogens. Niger Postgrad Med J. 2008;15:32–37. [PubMed] [Google Scholar]
- Anam EM. Anti-inflammatory activity of compounds isolated from the aerial parts of Abrus precatorius (Fabaceae) Phytomedicine. 2001;8:24–27. doi: 10.1078/0944-7113-00001. [DOI] [PubMed] [Google Scholar]
- Bhutia SK, Mallick SK, Maiti TK. In vitro immunostimulatory properties of Abrus lectins derived peptides in tumor bearing mice. Phytomedicine. 2009;16:776–782. doi: 10.1016/j.phymed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Bold RJ, Temuhlen PM, McConkey DJ. Apoptosis, cancer and cancer therapy. Surg Oncol. 1997;6:113–142. doi: 10.1016/S0960-7404(97)00015-7. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- Chang H, Mi M, Ling W, Zhu J, Zhang Q. Structurally related cytotoxic effects of flavonoids on human cancer cells in vitro. Arch Pharmacal Res. 2008;31:1137–1144. doi: 10.1007/s12272-001-1280-8. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Esposti MD. Measuring mitochondrial reactive oxygen species. Methods. 2002;26:335–340. doi: 10.1016/S1046-2023(02)00039-7. [DOI] [PubMed] [Google Scholar]
- Fabrican DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Darokar MP, Sundaresan V, Faridi U, Luqman SR, Khanuja SPS. Anticancer activity of some medicinal plants from high altitude evergreen elements of Indian Western Ghats. J Res Educ Indian Med XIII. 2007;3:1–6. [Google Scholar]
- Harborne JB, Herbert B, Gerard P. Phytochemical dictionary. London: Taylor and Francis; 1999. [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk- interacting protein is a potent inhibitor of Gl cyclin-dependent kinases. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-N. [DOI] [PubMed] [Google Scholar]
- Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis—the p53 network. J Cell Sci. 2003;116:4077–4085. doi: 10.1242/jcs.00739. [DOI] [PubMed] [Google Scholar]
- Hegde R, Maiti TK, Podder SK. Purification and characterization of three toxins and two agglutinins from A. precatorius seed by using lactamyl-sepharose affinity chromatography. Anal Biochem. 1991;194:101–109. doi: 10.1016/0003-2697(91)90156-N. [DOI] [PubMed] [Google Scholar]
- Helton LR. Folk medicine and health beliefs: an Appalachian perspective. J Cult Divers. 1996;3:123–128. [PubMed] [Google Scholar]
- Herrmann M, Lorenz HM, Voll R, Grunke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibasami H, Iwase H, Yoshioka K, Takahashi H. Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int J Mol Med. 2005;16:233–236. [PubMed] [Google Scholar]
- Hibasami H, Iwase H, Yoshioka K, Takahashi H. Glycyrrhizin acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int J Mol Med. 2006;17:215–219. [PubMed] [Google Scholar]
- Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–139. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Melissa M, Ferlay J, Ward E, Forman D. Global Cancer Statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Joaquin AM, Gollapudi S. Functional decline in aging and disease: a role for apoptosis. J Am Geriatr Soc. 2001;49:1234–1240. doi: 10.1046/j.1532-5415.2001.04990.x. [DOI] [PubMed] [Google Scholar]
- Ju W, Wang X, Shi H, Chen W, Belinsky SA, Lin Y. A critical role of luteolin induced reactive oxygen species in blockage of tumor necrosis factor activated nuclear factor-kappaB pathway and sensitization of apoptosis in lung cancer cells. Mol Pharmacol. 2007;71:1381–1388. doi: 10.1124/mol.106.032185. [DOI] [PubMed] [Google Scholar]
- Kamboj VP, Dhawan BN. Research on plants for fertility regulation in India. J Ethnopharmacol. 1982;6:191–226. doi: 10.1016/0378-8741(82)90004-6. [DOI] [PubMed] [Google Scholar]
- Kaufman SJ, McPherson A. Abrin and hurin: two new lymphocyte mitogens. Cell. 1975;4:263–268. doi: 10.1016/0092-8674(75)90174-9. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::AID-CNCR2820730802>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Keston AS, Brandt R. The Fluorometric Analysis of Ultramicro Quantities of hydrogen peroxide. Anal Biochem. 1965;11:1–5. doi: 10.1016/0003-2697(65)90034-5. [DOI] [PubMed] [Google Scholar]
- Kinghorn AD, Farnsworth NR, Soejarto DD, Cordell GA, Swanson SM, Pezzuto JM, Wani MC, Wall ME, Oberlies NH, David J, Keoll DJ, Kramer RA, Rose WC, Vite GD, Fairchild CR, Peterson RW, Wild R. Novel strategies for the discovery of plant-derived anticancer agents. Pharm Biol. 2003;41:53–67. doi: 10.1080/1388020039051744. [DOI] [Google Scholar]
- Lee HZ, Liu WZ, Hsieh WT, Tang FY, Chung JG, Leung HWC. Oxidative stress involvement in Physalis angulata induced apoptosis in human oral cancer cells. Food Chem Toxicol. 2009;47:561–570. doi: 10.1016/j.fct.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Jung YS, Lee SH, Chung HY, Park BJ. Isolation of a chemical inhibitor against K-Ras induced p53 suppression through natural compound screening. Int J Oncol. 2009;34:1637–1643. doi: 10.3892/ijo_00000294. [DOI] [PubMed] [Google Scholar]
- Lin JY, Tserng KY, Chen CC, Lin LT, Tung T. Abrin and ricin: new anti-tumour substances. Nature. 1970;227:292–293. doi: 10.1038/227292a0. [DOI] [PubMed] [Google Scholar]
- McDonald ER, Deiry WS. Cell cycle control as a basis for cancer drug development. Int J Oncol. 2000;16:871–886. [PubMed] [Google Scholar]
- Mehta RG, Murillo G, Naithani R, Peng X. Cancer chemoprevention by natural products: how far have we come? Pharm Res. 2010;27:950–961. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Naik VN. Flora of Marathwada (Ranunculaceae to Convolvulaceae) Aurangabad: Amrut Prakashan; 1998. [Google Scholar]
- Narayanan S, Surolia A, Karande AA. Ribosome inactivating protein and apoptosis: abrin causes cell death via mitochondrial pathway in Jurkat cells. Biochem J. 2004;377:233–240. doi: 10.1042/BJ20030797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/S0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Norton L. Adjuvant breast cancer therapy, current status, future strategies growth kinetics and the improved drug therapy of breast cancer. Semin Oncol. 1999;26:1–4. [PubMed] [Google Scholar]
- Peng BO, Hu Q, Liu X, Wang L, Chang Q, Li J, Tang J, Wang N, Wang Y. Duchesnea phenolic fraction inhibits in vitro and in vivo growth of cervical cancer through induction of apoptosis and cell cycle arrest. Exp Biol Med. 2009;234:74–83. doi: 10.3181/0806-RM-204. [DOI] [PubMed] [Google Scholar]
- Ping YH, Lee HC, Lee JY, Wu PH, Ho LK, Chi CW, Lu MF, Wang JJ. Anticancer effects of low-dose 10-hydroxycamptothecin in human colon cancer. Oncol Rep. 2006;15:1273–1279. [PubMed] [Google Scholar]
- Ramnath V, Kuttan G, Kuttan R. Immunopotentiating activity of abrin, a lectin from A. precatorius Linn. Indian J Exp Biol. 2002;40:910–913. [PubMed] [Google Scholar]
- Reddy VV, Sirsi M. Effect of Abrus precatorius L. on experimental tumors. Cancer Res. 1969;29:1447–1451. [PubMed] [Google Scholar]
- Rethy B, Hohmann J, Minorics R, Varga A, Ocsovszki I, Molnar J, Juhasz K, Falkay G, Zupko I. Antitumour Properties of Acridone Alkaloids on a Murine Lymphoma Cell Line. Anticancer Res. 2008;28:2737–2744. [PubMed] [Google Scholar]
- Sarath VJ, So CS, Won YD, Gollapudi S. Artemisia princeps var orientalis induces apoptosis in human breast cancer MCF-7 cells. Anticancer Res. 2007;27:3891–3898. [PubMed] [Google Scholar]
- Sateesh MK (1998). Microbiological investigations on die-back disease of neem (Azadirachta indica A. Juss.). Ph.D. thesis. Mysore University, Mysore
- Shih SF, Wu YH, Hung CH, Yang HY, Lin JY. Abrin triggers cell death by inactivating a thiol-specific antioxidant protein. J Biol Chem. 2001;276:21870–21877. doi: 10.1074/jbc.M100571200. [DOI] [PubMed] [Google Scholar]
- Siddiqi TO, Bhupinder D, Peerzada SHK, Iqbal M. Angiospermous seeds of medicinal importance. J Econ Taxon Bot. 2001;25:99–123. [Google Scholar]
- Soldani C, Scovassi AI. Poly (ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–328. doi: 10.1023/A:1016119328968. [DOI] [PubMed] [Google Scholar]
- Solomayer EF, Diel IJ, Meyberg GC, Gollan C, Bastert G. Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat. 2000;59:271–278. doi: 10.1023/A:1006308619659. [DOI] [PubMed] [Google Scholar]
- Soobrattee MA, Bahorun T, Aruoma OI. Chemopreventive actions of polyphenolic compounds in cancer. BioFactors. 2006;27:19–35. doi: 10.1002/biof.5520270103. [DOI] [PubMed] [Google Scholar]
- Tang W, Hemm I, Bertram B. Recent development of antitumor agents from Chinese herbal medicines; low molecular compounds. Planta Med. 2003;69:97–108. doi: 10.1055/s-2003-37718. [DOI] [PubMed] [Google Scholar]
- Taur DJ, Patil RY. Mast cell stabilizing and antiallergic activity of A. precatorius in the management of asthma. Asian Pacific Journal of Tropical Medicine. 2011;4:46–49. doi: 10.1016/S1995-7645(11)60030-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- World health report. Geneva: WHO; 2000. [Google Scholar]
- Zhou M, Zhao Y, Ding Y, Liu H, Liu Z, Fodstad O, Riker AI, Kamarajugadda S, Lu J, Owen LB, Ledoux SP, Tan M. Warburg effect in chemosensitivity: targeting lactate dehydrogenase—a re-sensitizes Taxol-resistant cancer cells to Taxol. Mol Cancer. 2010;9:33. doi: 10.1186/1476-4598-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]