Abstract
Previous report showed that leukemia cells’ differentiation could be induced by retinoic acid (RA), and prostate cancer cells’ proliferation could be inhibited by Vitamin D or its analog. This study aimed to examine whether RA and vitamin D analog EB1089 have synergistic effect on hepatocellular cancer cells’ apoptosis. The hepatocellular cancer cell lines’ viability was determined by MTT method after treating by RA and EB1089 alone or in combination, cell cycle of SSMC-7721 cell analyzed by FACS, mitochondrial membrane potential of SSMC-7721 under different treatments were detected using MitoTracker Red CMXRos. TUNEL analysis was also used for cell apoptosis detection. Real time-PCR and Western Blot assay were used to detect the expression of Bcl-2 and Bax. Moreover, hepatocellular cancer model was developed by subcutaneously (S.C.) challenging H22 cells to nude mice. In the combination group (10 μmol/L RA, 10 nmol/L EB1089), the viability of hepatocellular cancer cells decreased significantly compared with drugs used alone (P < 0.05). From the TUNEL analysis, SSMC-7721 cells have a higher apoptotic ratio in the combined drug group than in the groups for which the drugs were used separately. In a hepatocellular cancer model, the tumor weight of H22 tumor bearing mice was more reduced in the combined drug treated group when compared to the groups for which the drugs were used alone (P < 0.05), in addition, significantly prolonged survival was observed. Combination of RA and EB1089 exert synergistic growth inhibition and apoptosis induction on hepatocellular cancers cells.
Keywords: Retinoic acid, EB1089, Hepatocellular cancers, Apoptosis
Introduction
Retinoic acid (RA) is the biologically active form of vitamin A and its signaling plays an important role in development process via its vitamin A receptor (RAR). In recent years, the pharmaceutical research showed that vitamin A could be used for disease prevention or tumor treatment (Hou et al. 2008; Engedal et al. 2009; Chiu et al. 2008; Czeczuga-Semeniuk et al. 2009; Popadic et al. 2008; Park et al. 2008; Luo et al. 2009), such as vitamin A and its derivatives are used to treat various skin diseases, and for many other cancers (head and neck cancer, lung cancer, breast cancer, prostate cancer and bladder cancer) (Clarke et al. 2004). Wang Zhen-Yi’s group (Wang and Chen 2008) made a major breakthrough in application of all-trans retinoic acid treatment of human acute promyelocytic leukemia (APL). Meanwhile, vitamin A can inhibit chemically induced bronchial multi-squamous cell carcinoma and breast cancer proliferation in clinical treatment (Ng et al. 2010; Sadikoglou et al. 2009; Gao et al. 2010; Papi et al. 2009; Wang et al. 2009).
Vitamin D, a fat-soluble steroid hormone precursor, can bind to its receptor (vitamin D receptor, VDR) to achieve various functions, such as maintaining normal calcium metabolism, regulating nuclear gene expression, inhibiting tumor cell proliferation, and inducing tumor cell differentiation and apoptosis (González-Sancho et al. 2006; Spina et al. 2006; Pérez-López et al. 2009). Vitamin D and its analog have been assessed on prostate cancer ranging from basic to clinical research. Studies have shown that vitamin D and its analog at physiological concentrations can inhibit prostate cancer PC-3 and LNCaP cell proliferation (Guzey et al. 2002). At the same time, vitamin D and its analog can enhance the cytotoxicity of anticancer drugs such as paclitaxel, by synergistic inhibition of prostate cancer cell proliferation (McGrath 2001). However, high concentrations in vitamin D can cause hypercalcemia side effects of its clinical application in the field of anti-tumor treatment, moreover, combination of vitamin D or its analog and retinoids were used to induce cancer cells apoptosis such as of human leukaemia cells (James et al. 1997), pancreatic cancer cells (Pettersson et al. 2000), breast cancer cells (Stefanska et al. 2012), and prostate cancer cells (Elstner et al. 1999). Previous studies have found that the combination of all-trans retinoic acid and vitamin D can induce differentiation of tumor cells of the hematopoietic system (Muto et al. 1999), however, with lower efficiency than in studies on solid tumors.
Heptaocellular carcinoma is the most common type of malignant liver tumor, however, most of the patients do not receive a timely diagnosis, thus, resistance to chemotherapy and systemic side effects are often observed. Therefore, there is an urgent need to explore some new treatment options, to find efficient and low side effects of drugs for the treatment of hepatocellular cancer, and in addition non-chemotherapy drugs such as RA and vitamin D or its analog are low-cost-alternative drugs. Moreover, whether RA and vitamin D analog EB1089 could induce hepatocellular carcinoma cancer cell apoptosis is still unknown.
This article aims to explore the synergy of RA and vitamin D analog EB1089 induced hepatocellular cancer cell apoptosis and its mechanisms, to lay a theoretical foundation for the clinical application of RA and EB1089 associated with the treatment of hepatocellular cancer.
Materials and methods
Cells and culture conditions
Mouse hepatocellular cancer cell line H22, human hepatocellular cancer cell line SSMC-7721 and HepG2, human prostate cancer cell line PC-3, were purchased from the American Type Culture Collection (ATCC), RA and EB1089 were obtained from Sigma Chemical (St Louis, MO, USA). Cells were cultured in DMEM containing 10 % FBS, l-glutamine (4 mmol/L), penicillin (100 units/mL), and streptomycin (100 μg/mL), and incubated at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air cells were sub-cultured every other day.
MTT detection
MTT is taken up by cells and is reduced to a colored formazan product that can be detected by spectrophotometry (λmax = 562 nm). Reduction of MTT is dependent on the mitochondrial respiratory function, and thus measures the relative number of viable cells in the culture. The SSMC-7721, H22 or HepG2 cell concentration was adjusted to 2.0 × 106/mL. Then the cells were incubated in a 96-well plate, with 200 μL (4 × 105 cells per well) for each well. After adherence of cells for 24 h, RA (5, 7.5, 10, 15 μmol/L), EB1089 (5, 7.5, 10, 15 nmol/L), and their combinations (10 μmol/L RA, 10 nmol/L EB1089) were added for different times (24, 48, 72 and 96 h) respectively, with each concentration in three parallel wells, and a blank control group was established. After 24, 48, 72 or 96 h of culture, 20 μL newly prepared MTT (5 g/L) was added into each well, and incubation was continued for 4 h at 37 °C. Then, supernatant was removed, and 150 μL DMSO was added to completely dissolve the precipitate. Absorbance (A) at 490 nm was measured by automatic fluorescence microplate reader, and the growth inhibition ratio was calculated as follows: Cell Growth Inhibition Ratio = (1–A value of experimental well/A value of control well) × 100 %.
FCM (flow cytometry) analysis
SSMC-7721 cells were seeded on a 6-well plate the day before the drug treatment so that they were 60–80 % confluent at the time of treatment. The treatments were divided into 5 groups (group I, untreated; group II, 10 μmol/L RA; group III, 10 nmol/L EB1089; group IV, 10 μmol/L RA combined with 10 nmol/L EB1089; group V, 10 μg/mL cisplatin) for 48 h treatment, 1 × 106–5 × 106 SSMC-7721 cells were collected and centrifuged at 1,000 rpm for 5 min, and then the culture suspernant was removed; the cells were washed twice by 1 mL PBS, centrifuged, fixed with pre-cooled 70 % alcohol, and left overnight at 4 °C; after centrifugation, the suspernant was removed, 1 mL PI staining solution was added to stain cells for 30 min at 4 °C at a dark place, and then the solution were sieved by a 400-mesh nylon net. FCM was used to detect the cell cycle.
Detection of mitochondrial membrane potential
SSMC-7721 cells (4 × 105 cells per well in 96-well plate) were treated by RA (10 μmol/L), EB1089 (10 nmol/L) and its combinations for 48 h, then the membrane potential-dependent stain MitoTracker Red CMXRos (Invitrogen, Carlsbad, CA, USA) was used to assess the mitochondrial membrane potential. A total of 5 × 106 cells per well treated by RA, EB1089 or their combinations were incubated in complete DMEM containing a 0.1 μM final concentration of MitoTracker Red CMXRos dissolved in dimethyl sulfoxide for 20 min in a 37 °C, 5 % CO2 gas incubator. The cells were sedimented and washed in 150 mM NaCl-20 mM phosphate (pH 7.4; PBS) and then incubated in DMEM without dye for 30 min. Cells were sedimented, washed with PBS, and fixed with 4 % paraformaldehyde in PBS for 10 min at 4 °C. After another wash in PBS, the cells in the microplate were read by a spectrophotometer. Mean and standard deviation is plotted for 3 replicates from each condition.
RNA extraction, RT-PCR and Real-Time PCR
After the SSMC-7721 cells (6-well plate) treated by RA (10 μmol/L), EB1089 (10 nmol/L) and its combinations for 48 h, total RNA was isolated using RNeasy kit (QIAGEN, Mississauga, Ontario, Canada), followed by DNAse I treatment. Six hundred nanograms of total RNA were reverse transcribed in 30 mL reaction mixture containing 500 mM each of deoxynucleotide triphosphate, 12U ribonuclease inhibitor and MultiScribe reverse transcriptase using TaqMan reverse transcription reagents (Applied Biosystems, Branchburg, NJ, USA). The reaction mixture was incubated at 25 °C for 10 min, 48 °C for 30 min and 95 °C for 5 min. The PCR primers for Bcl-2, Bax or the house-keeping gene GAPDH are described in Table 1. Real-time PCR was performed on cDNA samples in triplicate using an ABI PRISM 7900HT sequence detection system (Applied Biosystems). SYBR Green PCR Master Mix (AB Applied Biosystems) was used according to the manufacturer’s protocol. PCR optimized reactions were performed in a 20 mL volume containing 0.5 mM primers and 1 μL cDNA under the following conditions: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The results were analyzed using a comparative method. The amount of target, normalized to the endogenous reference, is given by the formula 2−ΔΔCt (which indicates the copy number) where Ct represents the threshold cycle, indicating the fractional cycle number at which the amount of amplified target reaches a fixed threshold. All experiments were performed in triplicate.
Table 1.
Primer sequences of Bcl-2, Bax and GAPDH
| Gene | Accession | Primer sequence | Size (bp) |
|---|---|---|---|
| Bcl-2 F | NM_177410 | CTGCACCTGACGCCCTTCACC | 119 |
| Bcl-2 R | CACATGACCCCACCGAACTCAAAGA | ||
| Bax F | NM_007527 | TTGCTACAGGGTTTCATCCA | 298 |
| Bax R | CAGCCTTGAGCACCAGTTTG | ||
| GAPDH F | XM_001479371 | GGTCGGTGTGAACGGATTTG | 218 |
| GAPDH R | GGAAGATGGTGATGGGATTTC |
TUNEL assay
SSMC-7721 cells were grown to 75 % confluency on 12-well Falcon culture slides and incubated for 14 h with or without RA (10 μmol/L), EB1089 (10 nmol/L) or their combinations, and then cells were fixed with 4 % PFA. The TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling) assay was performed using the In Situ Cell Death Detection Kit (Roche Diagnostics, Barcelona, Spain), according to the manufacturer’s instructions. Negative controls were performed by substituting tris-buffered saline (TBS) for the TdT enzyme.
Protein isolation and western blotting
After the SSMC-7721 cells (6-well plate) treated by RA (10 μmol/L), EB1089 (10 nmol/L) and its combinations for 48 h, cells were lysed in lysis buffer (0.5 % sodium deoxycholate, 0.1 % sodium dodecyl sulfate, 1 % Nonidet P-40 and 1× PBS) containing proteinase inhibitors (100 mg/mL phenylmethylsulfonyl fluoride, 13.8 mg/mL aprotinin (Sigma, St Louis, MO, USA) and 1 mM sodium orthovanadate. Total cell lysates were incubated on ice for 30 min, followed by microcentrifugation at 10,000g for 10 min at 4 °C. Protein concentrations of the supernatants were determined by the Bio-Rad protein assay. Equal amounts of protein (50 mg) were mixed with 2× sodium dodecyl sulfate (SDS) sample buffer, boiled for 4 min and separated by 10 or 12 % SDS–polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes (Bio-Rad Laboratories). Nonspecific binding was blocked with 5 % non-fat milk in 1× TBST (Tris-buffered saline with 0.1 % Tween-20). Primary antibodies were directed against VDR (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), RAR (1:500, Abcam, Ma, USA), Bcl-2 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Bax (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or GAPDH (1:2,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After washing three times, each for 10 min in 1× TBST, blots were exposed to the secondary antibody (anti-mouse or anti-rabbit IgG-HRP, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:2,000 and visualized using the ECL chemiluminescence detection system (Amersham, Buckinghamshire, UK). Band intensities were quantified by scanning densitometry. All studies were performed in triplicate experiments.
Establishment of hepatocellular cancer model and its therapy studies
Female athymic BALB/c nude mice 6–8 weeks old were housed in autoclaved microisolator cages in an air-filtered laminar flow cabinet and were given food and water ad libitum. All procedures were performed under sterile conditions in a laminar flow hood. This animal experiment was approved by the Institutional Animal Care and Use Committee and was in compliance with all regulatory guidelines.
H22 cell suspension (5 × 106 cells in 100 μL of DMEM medium) was injected subcutaneously at several injection sites. The purpose of developing hepatocellular tumors was to generate histologically intact tumors for drug therapy. When the diameter of tumors reached about 1 cm, RA, EB1089 or their combinations were administrated by intraperitoneal injection per 3 days, twenty-five of these mice were randomly assigned into one of the following five groups (n = 5): (a) mice treated with DMEM medium, (b) mice treated with DMSO, (c) mice treated with RA (10 mg/kg), (d) mice treated with EB1089 (1 μg/kg) and (e) mice treated with RA + EB1089 (Collins et al. 1992). Body weight and tumor size at the site of inoculation were measured three times a week. Tumor size was assessed from two diameters, the greater diameter (a (mm), average size) and the lesser diameter (b (mm), average size) measured at right angles to each other. Tumor weight was estimated using the formula ab2/2.
Statistical analysis
The measurement data are indicated by mean ± standard deviation (x ± s), statistical software SPSS 11.5 was applied, and single factor analysis of variance and pairwise comparison analysis of multiple sample means were adopted. Significance level was assigned at P < 0.05.
Result
RAR (retinois acid receptor) and VDR (vitamin D receptor) expression on hepatocellular cancer cell lines
Retinoic acid (RA) and vitamin D play their role in signaling pathways via their respective receptor RAR and VDR. A previous report has shown that the analog of vitamin D (EB1089) at physiological concentrations inhibit the proliferation of the prostate cancer cells PC-3 expressing RAR and VDR and LNCaP via VDR transferring apoptosis signaling (Guzey et al. 2002). Therefore, we analyzed the expression of RAR and VDR on hepatocellular cancer cell lines H22, SSMC-7721 and HepG2 (PC-3 cells used as a RAR and VDR double expression cell line, Fig. 1). RAR and VDR expression on the hepatocellular cancer cell lines indicate that RA and vitamin D or its analog may have a role in the growth of these cancer cell lines.
Fig. 1.
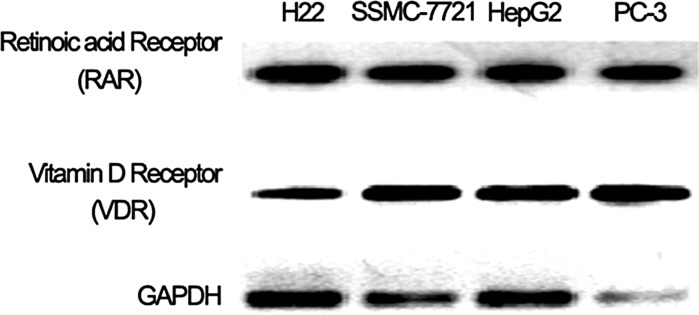
Retionic acid receptor (RAR) and vitamin D receptor (VDR) expression on hepatocellular cancer cell lines H22, SSMC-7721, HepG2 and prostate cancer cell line PC-3. GAPDH was used as an inner positive control
The inhibition effect of RA, EB1089 and its combination
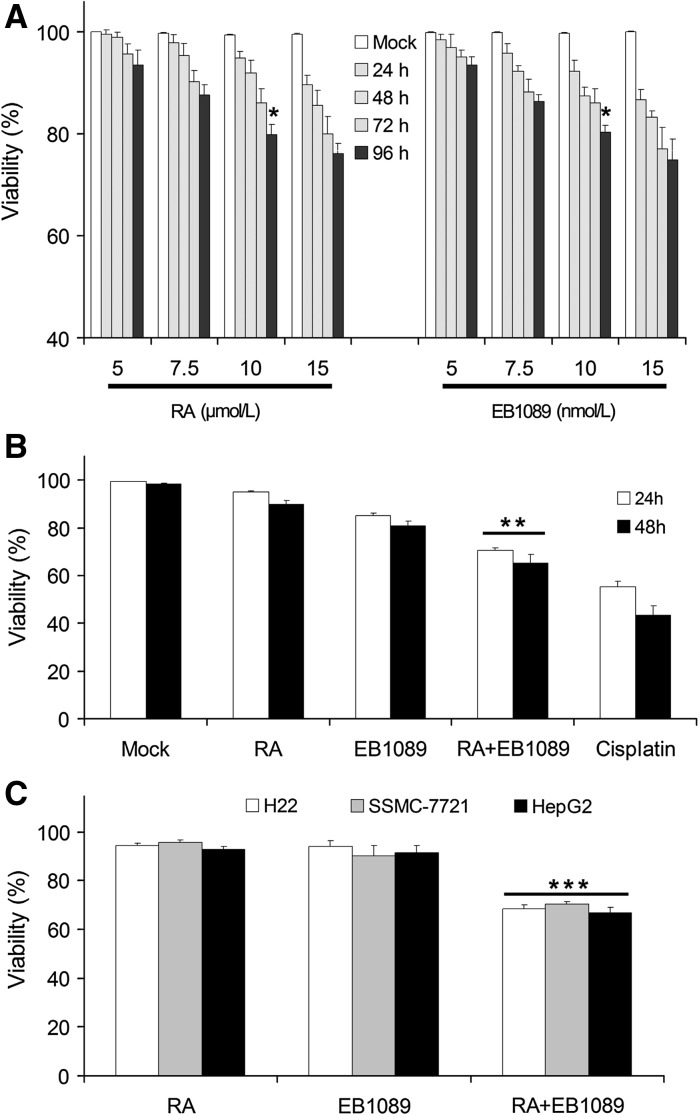
The results showed that RA and EB1089 inhibited SSMC-7721 cell growth in dependence of time and concentration (Fig. 2a). For each group the difference was statistically significant (P < 0.05) in comparison to the control group. In this study, 10 μmol/L RA and 10 nmol/L EB1089 were used for treating SSMC-7721 cells using different detection methods. From the data shown, the viability of SSMC-7721 cells was significantly decreased for the groups for which RA and EB1089 were used separately, the difference was statistically significance (P < 0.05) (Fig. 2b). Moreover, the combination of RA and EB1089 led to synergistic inhibition of SSMC-7721 growth. At the same time, different cells had different drug sensitivities towards treatment with RA combined with EB1089. SSMC-7721 cells were more sensitive (Fig. 2c), the difference was statistically significant (P < 0.05). From the data shown above, we found that the combination of RA and EB1089 can synergistically inhibit the growth of hepatocellular cancer cell lines.
Fig. 2.
Inhibition of RA and EB1089 on the growth of hepatocellular cancer cells. a Viability of SSMC-7721 cells in relation to different drug concentrations, viability of SSMC-7721 cells treated with RA (5, 7.5, 10 and 15 μmol/L), EB1089 (5, 7.5, 10 and 15 nmol/L) at 24, 48, 72 and 96 h. b RA (10 μmol/L), EB1089 (10 nmol/L) and its combination on SSMC-7721 cells with different time treatments, viability of combination group decreased significantly compared with the group treated separately with the drugs, P < 0.05. c Combination of RA and EB1089 on different hepatocellular cancer cell lines for 48 h, the viability of different cell lines decreased significantly in the drug combination group compared with the groups treated separately with the drug, P < 0.01
SSMC-7721 cells cell cycle changes and apoptosis by RA and EB1089 combination
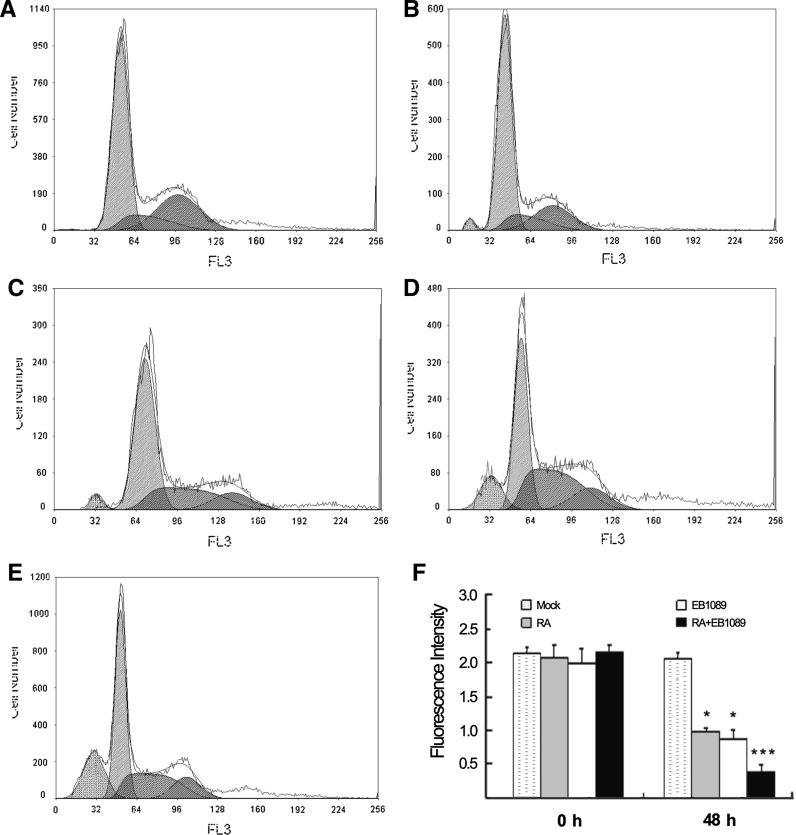
The proportions of apoptosis were quantitatively detected by flow cytometry. A hypodiploid peak was observed for the group treated with RA and EB1089 alone or in combination, the G1 peak appeared to the left of the hypodiploid cell population (apoptotic cells) peak (Fig. 3a–e). In the combination group, the apoptotic rate of SSMC-7721 cells was significantly increased compared for the groups treated separately with the drugs (Table 2).
Fig. 3.
Cell cycle and mitochondria potential of SSMC-7721 cells. a Mock treated cells. b 10 μmol/L RA treatment. c 10 nmol/L EB1089 treatment. d Treatment of RA (10 μmol/L) combined with EB1089 (10 nmol/L). e 10 μg/mL cisplatin treatment as positive control. f Mitochondrial membrane potential (MMP) assay with RA (10 μmol/L), EB1089 (10 nmol/L) and its combination, MMP of RA and EB1089 group decreased compared with mock treated group, P < 0.05. For the RA and EB1089 combination group it decreased significantly (P < 0.01)
Table 2.
Effect of RA and EB1089 on SSMC-7721 cells’ apoptosis (x ± s, n = 3)
| Group | Cell cycle | Apoptotic rate (%) | ||
|---|---|---|---|---|
| G0/G1 (%) | S (%) | G2/M (%) | ||
| Mock | 61.7 ± 1.3 | 14.2 ± 1.3 | 23.5 ± 1.7 | 0.7 ± 0.3 |
| RA | 58.7 ± 2.9 | 13.4 ± 3.9 | 21.7 ± 2.3 | 6.0 ± 2.4 |
| EB1089 | 46.1 ± 2.2 | 14.7 ± 5.2 | 26.2 ± 4.4 | 12.7 ± 3.0 |
| RA + EB1089 | 23.1 ± 2.4 | 31.5 ± 3.2 | 12.2 ± 2.6 | 33.2 ± 1.9 |
| Cisplatin | 12.4 ± 2.8 | 31.1 ± 1.4 | 10.8 ± 2.2 | 46.2 ± 4.7 |
Mitochondrial permeability transition is an important step in the induction of cellular apoptosis. During this process, the electrochemical gradient across the mitochondrial membrane collapses. The collapse is thought to occur through the formation of pores in the mitochondria by pro-apoptotic proteins. Therefore, the loss of mitochondrial membrane potential is a hallmark for apoptosis. The analysis of changes in mitochondrial membrane potential (MMP) that can occur during apoptosis provides precious information on the mechanisms. The membrane potential of mitochondria will be significantly reduced during apoptosis. In the combination group, mitochondrial transmembrane potential of the SSMC-7721 cells was significantly decreased (Fig. 3f), the difference was statistically significant (P < 0.05).
Data of cell cycle analysis by FACS and MMP assay indicated that SSMC-7721 cells apoptosis could be induced by RA and EB1089 combination.
Apoptosis and the expression of related genes
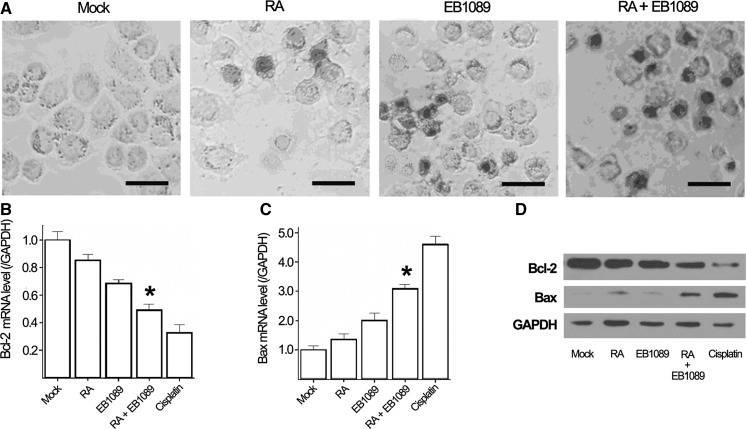
The intracellular endonucleases cut genomic DNA during the cell apoptosis and the 3′-OH ends of DNA are exposed, terminal deoxynucleotidyl transferases (TdT) is able to label blunt ends of double stranded DNA breaks independent of a template, and then colored with DAB. Experiments suggest that TUNEL assay is more specific for apoptosis detection. RA and EB1089 alone can induce SSMC-7721 cells apoptosis, cell shrinkage, varied shapes, nucleus shrinkage, dense chromatin cytoplasmic vacuolization, and apoptotic bodies, however the number is low. In the combination group, the number of apoptotic SSMC-7721 cells, detected by TUNEL, was significantly increased (Fig. 4a).
Fig. 4.
TUNEL analysis and expression of Bcl-2 and Bax in SSMC-7721 cells after treatment with RA and EB1089 for 48 h. a SSMC-7721 cells apoptosis analyzed by TUNEL. b Bcl-2 mRNA expressions by Real-time PCR, Bcl-2 mRNA level in the combination group was significantly decreased compared with RA group or EB1089 group, P < 0.05. c Bax mRNA expressions by Real-time PCR, Bax in the combination group increased compared with the drugs used separately, P < 0.05. d Bcl-2 and Bax protein expression by Western blotting, Bcl-2 protein decreased while Bax protein expression increased in the combination group compared with the group treated with the drugs separately
The expression of apoptosis-related genes was detected by real time-PCR and Western blot. The level of Bcl-2 and Bax mRNA (Fig. 4b, c) and protein expression (Fig. 4d) was largely reduced in the drug combination group (RA+EB1089), the difference was statistically significant (P < 0.05).
Effect of drug combination on tumor weight and survival of tumor bearing mice
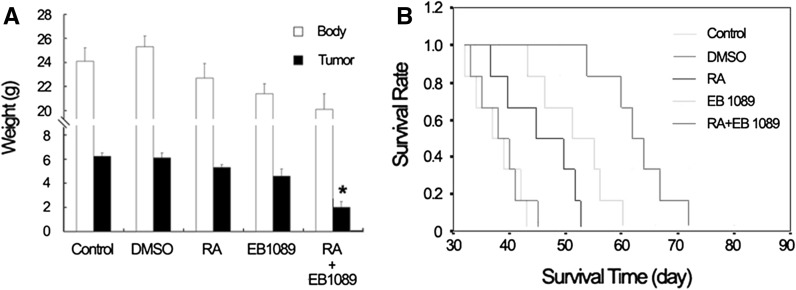
Mice inoculated with hepatocellular cancer tumor had lost about 10 % of their initial body weight by about 16 days after tumor inoculation. However, tumor weight decreased significantly in the RA and EB1089 combination group (P < 0.05) (Fig. 5a), moreover, tumor bearing mouse survived longer in drug combination (Fig. 5b).
Fig. 5.
Hepatocellular cancer model and its therapy effect. a Body weight and tumor weight under different treatment conditions, tumor weight of the combination group was significantly decreased compared with the RA group or EB1089 group, P < 0.05. b Survival of tumor bearing mice on different therapies, in the combination group, tumor bearing mice survived longer than when RA and EB1089 were separately used
Discussion
RA is the biologically active form of vitamin A acid, which has various biological activities such as regulating of cell proliferation and differentiation of a variety of normal tissues and epithelial cell. Recent studies, showed that RA has the ability to prevent and inhibit the development of tumors. Studies have shown that vitamin D can enhance the activity of some anticancer drugs and reduce their side effects (McGrath 2001). Research reports indicate that RAR and VDR can form heterodimers, VDR-RAR complex (Zinser et al. 2003; Zhang et al. 2001; González-Sancho et al. 2006; Spina et al. 2006; Bettoun et al. 2003), enhance or inhibit transcription of the target gene (Zhang et al. 2011). The Muto group confirmed that the all-trans retinoic acid and 1α, 25—dihydroxy vitamin D3 has a certain synergy to induce differentiation of acute promyelocytic leukemia cells UF-1. The mechanism of this synergistic effect may be signaled through the VDR-RAR heterodimer interaction enhancing gene expression targeted by VDR.
At present, the reports of RA in the treatment of hepatocellular cancer are still relatively few. Using different methods of detection, the objective was to find out if there is a certain degree of inhibition of proliferation of hepatocellular cancer cells by the combined treatment with RA and the vitamin D analog EB1089. Meanwhile, flow cytometry assay revealed that the combination of RA and EB1089 could significantly modify the cell cycle of hepatocellular cancer cells. Moreover, the TUNEL assay showed that the combination of RA and EB1089 could induce apoptosis of hepatocellular cancer cells, most importantly, tumor bearing mouse could survive longer after treatment with a combination of RA and EB1089. Effective synergy to reduce the dose of each drug, to improve efficacy, reduce adverse drug reactions, provides a new basis for induction of differentiation therapy for hepatocellular cancer.
In conclusion, RA and EB1089 have antiproliferation and induce apoptosis activity, and when the RA and EB1089 used in combination, there is a certain synergistic effect in tumor cell growth inhibition. This will be lain a theoretical foundation for treatment of hepatocellular cancer in clinical application.
Acknowledgments
The authors have no commercial, proprietary, or financial interest in the products or companies described in this article.
References
- Bettoun DJ, Burris TP, Houck KA, Buck DW, 2nd, Stayrook KR, Khalifa B, Lu J, Chin WW, Nagpal S. Retinoid X receptor is a nonsilent major contributor to vitamin D receptor-mediated transcriptional activation. Mol Endocrinol. 2003;17:2320–2328. doi: 10.1210/me.2003-0148. [DOI] [PubMed] [Google Scholar]
- Chiu HJ, Fischman DA, Hammerling U. Vitamin A depletion causes oxidative stress, mitochondrial dysfunction, and PARP-1-dependent energy deprivation. FASEB J. 2008;22:3878–3887. doi: 10.1096/fj.08-112375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke N, Germain P, Altucci L, Gronemeyer H. Retinoids: potential in cancer prevention and therapy. Expert Rev Mol Med. 2004;6:1–23. doi: 10.1017/S1462399404008488. [DOI] [PubMed] [Google Scholar]
- Collins MD, Eckhoff C, Chahoud I, Bochert G, Nau H. 4-Methylpyrazole partially ameliorated the teratogenicity of retinol and reduced the metabolic formation of all-trans-retinoic acid in the mouse. Arch Toxicol. 1992;66:652–659. doi: 10.1007/BF01981505. [DOI] [PubMed] [Google Scholar]
- Czeczuga-Semeniuk E, Bielawski T, Lemancewicz D, Rusak M, Wołczyński S. Vitamin A family compounds, estradiol, and docetaxel in proliferation, apoptosis and immunocytochemical profile of human ovary endometrioid cancer cell line CRL-11731. Folia Histochem Cytobiol. 2009;47:S127–S135. doi: 10.2478/v10042-009-0052-9. [DOI] [PubMed] [Google Scholar]
- Elstner E, Campbell MJ, Munker R, Shintaku P, Binderup L, Heber D, Said J, Koeffler HP. Novel 20-epi-vitamin D3 analog combined with 9-cis-retinoic acid markedly inhibits colony growth of prostate cancer cells. Prostate. 1999;40:141–149. doi: 10.1002/(SICI)1097-0045(19990801)40:3<141::AID-PROS1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Engedal N, Auberger P, Blomhoff HK. Retinoic acid regulates Fas-induced apoptosis in Jurkat T cells: reversal of mitogen-mediated repression of Fas DISC assembly. J Leukoc Biol. 2009;85:469–480. doi: 10.1189/jlb.1107790. [DOI] [PubMed] [Google Scholar]
- Gao F, Tang Q, Yang P, Fang Y, Li W, Wu Y. Apoptosis inducing and differentiation enhancement effect of oridonin on the all-trans-retinoic acid-sensitive and -resistant acute promyelocytic leukemia cells. Int J Lab Hematol. 2010;32:e114–e122. doi: 10.1111/j.1751-553X.2009.01147.x. [DOI] [PubMed] [Google Scholar]
- González-Sancho JM, Larriba MJ, Ordóñez-Morán P, Pálmer HG, Muñoz A. Effects of 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Anticancer Res. 2006;26:2669–2681. [PubMed] [Google Scholar]
- Guzey M, Kitada S, Reed JC. Apoptosis induction by 1 alpha, 25-dihydroxyvitamin D3 in prostate cancer. Mol Cancer Ther. 2002;1:667–677. [PubMed] [Google Scholar]
- Hou G, Wang QY, Wang XQ, Kang J, Yu RJ. Effects of vitamin A on the proliferation and apoptosis of alveolar cells of experimental emphysema in rats. Zhonghua Jie He He Hu Xi Za Zhi. 2008;31:209–212. [PubMed] [Google Scholar]
- James SY, Williams MA, Kelsey SM, Newland AC, Colston KW. The role of vitamin D derivatives and retinoids in the differentiation of human leukaemia cells. Biochem Pharmacol. 1997;54:625–634. doi: 10.1016/S0006-2952(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Luo P, Lin M, Lin M, Chen Y, Yang B, He Q. Function of retinoid acid receptor alpha and p21 in all-trans-retinoic acid-induced acute T-lymphoblastic leukemia apoptosis. Leuk Lymphoma. 2009;50:1183–1189. doi: 10.1080/10428190902934936. [DOI] [PubMed] [Google Scholar]
- McGrath J. Does ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders? Med Hypotheses. 2001;56:367–371. doi: 10.1054/mehy.2000.1226. [DOI] [PubMed] [Google Scholar]
- Muto A, Kizaki M, Yamato K, Kawai Y, Kamata-Matsushita M, Ueno H, Ohguchi M, Nishihara T, Koeffler HP, Ikeda Y. 1,25-Dihydroxyvitamin D3 induces differentiation of a retinoic acid-resistant acute promyelocytic leukemia cell line (UF-1) associated with expression of p21(WAF1/CIP1) and p27(KIP1) Blood. 1999;93:2225–2233. [PubMed] [Google Scholar]
- Ng KY, Ma MT, Leung KK, Leung PS (2010) Vitamin D and vitamin A receptor expression and the proliferative effects of ligand activation of these receptors on the development of pancreatic progenitor cells derived from human fetal pancreas. Stem Cell Rev. (Epub ahead of print) [DOI] [PubMed]
- Papi A, Rocchi P, Ferreri AM, Guerra F, Orlandi M. Enhanced effects of PPAR gamma ligands and RXR selective retinoids in combination to inhibit migration and invasiveness in cancer cells. Oncol Rep. 2009;21:1083–1089. doi: 10.3892/or_00000327. [DOI] [PubMed] [Google Scholar]
- Park JS, Koh YS, Bang JY, Jeong YI, Lee JJ. Antitumor effect of all-trans retinoic acid-encapsulated nanoparticles of methoxy poly(ethylene glycol)-conjugated chitosan against CT-26 colon carcinoma in vitro. J Pharm Sci. 2008;97:4011–4019. doi: 10.1002/jps.21221. [DOI] [PubMed] [Google Scholar]
- Pérez-López FR, Chedraui P, Haya J. Review article: vitamin D acquisition and breast cancer risk. Reprod Sci. 2009;16:7–19. doi: 10.1177/1933719108327595. [DOI] [PubMed] [Google Scholar]
- Pettersson F, Colston KW, Dalgleish AG. Differential and antagonistic effects of 9-cis-retinoic acid and vitamin D analogues on pancreatic cancer cells in vitro. Br J Cancer. 2000;83:239–245. doi: 10.1054/bjoc.2000.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popadic S, Ramic Z, Medenica L, Mostarica Stojkovic M, Trajković V, Popadic D. Antiproliferative effect of vitamin A and D analogues on adult human keratinocytes in vitro. Skin Pharmacol Physiol. 2008;21:227–234. doi: 10.1159/000135639. [DOI] [PubMed] [Google Scholar]
- Sadikoglou E, Magoulas G, Theodoropoulou C, Athanassopoulos CM, Giannopoulou E, Theodorakopoulou O, Drainas D, Papaioannou D, Papadimitriou E. Effect of conjugates of all-trans-retinoic acid and shorter polyene chain analogues with amino acids on prostate cancer cell growth. Eur J Med Chem. 2009;44:3175–3187. doi: 10.1016/j.ejmech.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Spina CS, Tangpricha V, Uskokovic M, Adorinic L, Maehr H, Holick MF. Vitamin D and cancer. Anticancer Res. 2006;26:2515–2524. [PubMed] [Google Scholar]
- Stefanska B, Salamé P, Bednarek A, Fabianowska-Majewska K. Comparative effects of retinoic acid, vitamin D and resveratrol alone and in combination with adenosine analogues on methylation and expression of phosphatase and tensin homologue tumour suppressor gene in breast cancer cells. Br J Nutr. 2012;107:781–790. doi: 10.1017/S0007114511003631. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Chen Z (2008) Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111:2505–2515 [DOI] [PubMed]
- Wang S, Hu CP, Jiang DJ, Peng J, Zhou Z, Yuan Q, Nie SD, Jiang JL, Li YJ, Huang KL. All-trans retinoic acid inhibits cobalt chloride-induced apoptosis in PC12 cells: role of the dimethylarginine dimethylaminohydrolase/asymmetric dimethylarginine pathway. J Neurosci Res. 2009;87:1938–1946. doi: 10.1002/jnr.21999. [DOI] [PubMed] [Google Scholar]
- Zhang C, Baudino TA, Dowd DR, Tokumaru H, Wang W, MacDonald PN. Ternary complexes and cooperative interplay between NCoA-62/Ski-interacting protein and steroid receptor coactivators in vitamin D receptor-mediated transcription. J Biol Chem. 2001;276:40614–40620. doi: 10.1074/jbc.M106263200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chalmers MJ, Stayrook KR, Burris LL, Wang Y, Busby SA, Pascal BD, Garcia-Ordonez RD, Bruning JB, Istrate MA, Kojetin DJ, Dodge JA, Burris TP, Griffin PR. DNA binding alters coactivator interaction surfaces of the intact VDR-RXR complex. Nat Struct Mol Biol. 2011;18:556–563. doi: 10.1038/nsmb.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser GM, McEleney K, Welsh J. Characterization of mammary tumor cell lines from wild type and vitamin D3 receptor knockout mice. Mol Cell Endocrinol. 2003;200:67–80. doi: 10.1016/S0303-7207(02)00416-1. [DOI] [PubMed] [Google Scholar]