Abstract
The aim of this study was to evaluate the diagnostic utility of lectin microarrays in pleural effusions of patients with lung cancer. A lectin microarray, LTL, PSA, LCA, UEA-1, AAL, MAL-I, MAL-II, SNA, WGA, ECL, DSA, STL, SWGA, HPA, ConA, GNA, HHL, BPL, EEL, Jacalin, WFA, ACL, MPL, DBA, SBA, was used to determine the glycoprotein profile of cells in pleural effusions from patients with lung cancer (54 cases), and with benign lung disease (54 cases). The A549 cell line, used as an experimental control, was positive for AAL, MAL-I, WGA, STL, Jacalin and ACL binding. Adenocarcinoma cells in pleural effusions were positive for ECL, DSA, AAL, MAL-I, WGA, STL, Jacalin, and ACL binding. AAL, WGA, and ACL positive binding was the most common, found in 54, 48, and 38 samples, respectively. ECL and DSA binding was positive in only 4 samples. In comparison, reactive mesothelial cells displayed positive binding for all markers in the microarray panel. SNA and AAL positive binding was detected in the majority of samples; 50/54 and 48/54 samples, respectively. Positive binding of DBA, MAL-II and EEL was present in only 2, 4 and 4 samples, respectively. SNA binding had the highest sensitivity (92.6 %), specificity (100 %), and accuracy (96.3 %). SNA may be used as a biomarker to distinguish reactive mesothelial cells from adenocarcinoma cells. The lectin microarrays proved able to distinguish carcinoma cells from reactive mesothelial cells in pleural effusions.
Keywords: Cytopathology; Effusion, pleural; Lectin microarray; Lung cancer
Introduction
The cytological diagnosis of pleural effusions can be difficult, especially in specimens containing abundant reactive mesothelial cells. The detection rate for malignant pleural effusions is approximately 50–60 % (Ghayumi et al. 2005), and distinguishing the carcinoma cells from reactive mesothelial cells is particularly challenging when there are relatively few carcinoma cells (Afify et al. 2005). Additional techniques, such as immunocytochemistry and ELISA, aid in the differential diagnosis (Wu et al. 2008; Zhou et al. 2009). Many tumour markers directed against specific cellular antigens have been analysed in pleural effusions to enhance the cytological diagnosis, with varying degrees of efficacy (Afify et al. 2002; Queiroz et al. 2001), but an optimum panel of tumour markers is yet to be determined.
Glycosylation is a major form of posttranslational modification (Lehle et al. 2006), and variations in glycosylation are associated with cell differentiation and malignant transformation (Dennis et al. 1999; Hakomori 2002). This makes it a potentially valuable diagnostic indicator of cellular diversity and differentiation (Brooks and Leathem 1991). Specific surface glycosylation patterns are characteristic features of certain cell types, such as embryonic stem cells (Muramatsu 2002). Recently, a growing body of evidence has indicated that changes in the expression of sugar-bearing molecules may actively direct a variety of cell biological processes (Haltiwanger and Lowe 2004; Van Dyken et al. 2007). The combinatorial possibilities inherent in a glycan structure far exceed DNA- and peptide-based structural diversity (Gabius et al. 2004). This enormous structural diversity coupled with accessibility at the cell surface makes glycosylation an important mechanism for regulating cellular interactions with the environment, neighbouring cells, and pathogens (Tao et al. 2008).
The purpose of this study was to determine the diagnostic capacity of a lectin microarray in pleural effusions. We demonstrated that the lectin microarray can be used to reveal surface glycan signatures of carcinoma cells and reactive mesothelial cells in pleural effusions. We also evaluated the value of the lectin microarray in differentiating the pleural effusions of patients with lung cancer from those of patients with benign lung disease.
Materials and methods
Patients
This study was conducted in accordance with the regulations of the institutional review boards at China Medical University and, was performed at The First Affiliated Hospital, China Medical University (Shenyang, China) between February and August in 2010. Internal review board approval for this study and/or informed consent of the patients was obtained. A total of 108 pleural samples were collected from the patients at the Laboratory of Cytopathology of the First Affiliated Hospital of China Medical University for inclusion in this study. There were 51 males (47.2 %) and 57 females (52.8 %).
From a total of 108 effusions, 54 were defined as reactive mesothelial cells (Tuberculous, n = 22 and parapneumonic, n = 32) and 54 adenocarcinoma cells. Of the 54 patients with benign effusion, 33 were men (61.1 %) and 21 were women (38.9 %), with a mean age of 59.9 years (range 18–87). Of the 54 patients with malignant effusion, 18 were men (33.3 %) and 36 were women (66.7 %), with a mean age of 63 years (range 30–84). The effusions were considered malignant if malignant cells were found on cytological examination or in a biopsy specimen. Only specimens histologically diagnosed as primary malignancies of lung or pleura were considered; malignancies of any other cause were excluded.
Tuberculous pleurisy was diagnosed if one of the following criteria was met: identification of bacillus in pleural fluid or biopsy specimen cultures; the presence of caseous granulomas in pleural biopsy tissue; radiological and clinical evidence of tuberculous pleurisy with acid-fast bacillipositive sputum, followed by response to anti-tuberculous therapy.
Parapneumonic pleurisy was determined by the presence of an acute febrile illness with purulent sputum, pulmonary infiltrate and responsiveness to antibiotic treatment; or identification of the microorganism in the pleural effusion in the absence of any other cause associated with pleural effusions.
Preparation of cells from pleural effusions
All specimens were received as fresh effusion, with a volume range of 20–2,000 mL. The specimens were centrifuged for 30 min at 2,000 rpm at 4 °C. The resulting pellet was used for the preparation of two cytological smears (alcohol fixed Papanicolau stain), and the rest of the pellet was stored at −70 °C until being used for lectin array hybridisation.
Cell culture
The human lung cancer cell line, A549 (adenocarcinoma), was from American Type Culture Collection (Manassas, VA, USA). The cells were maintained in Dulbecco’s Modified Eagle Medium (GIBCO, Inc., Grand Island, NY) supplemented with 10 % foetal bovine serum (GIBCO, Inc.), 100 units/mL streptomycin (Sigma, St. Louis, MO, USA), and 100 units/mL penicillin (Sigma). Cells were cultured at 37 °C in a humidified 5 % v/v CO2 atmosphere to 75 % confluence.
Lectin microarray
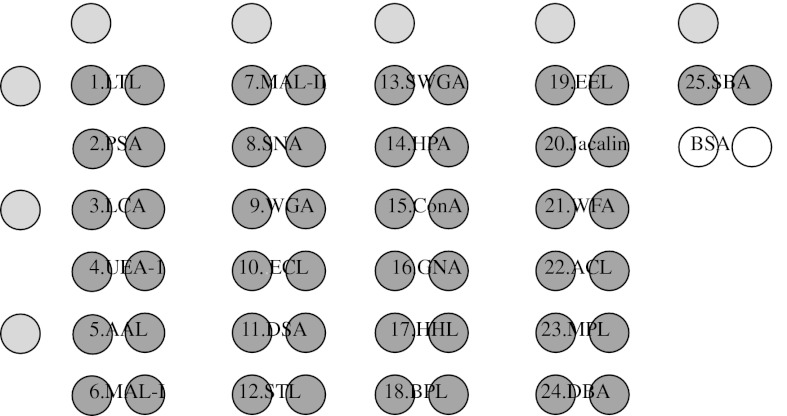
To immobilise lectins on a glass slide, we used 3-glycidoxypropyltrimethoxysilane as a silan coupling reagent to prepare epoxy-coated glass slides (Zhu et al. 2000). Lectins (Vector Laboratories, Inc., Burlingame, CA, USA) (Table 1) were dissolved in spotting solution at a concentration of 1 mg/mL (SNA, MAL-I and MAL-II) or 0.5 mg/mL (the other lectins), and spotted in duplicate (Fig. 1) onto a 3-glycidoxypropyltrimethoxysilane glass slide using a microarray printing robot (MicroGridII, BioRobotics Ltd., Cambridge, UK). After spotting, excess non-immobilised lectins were removed by washing with 10 mM Tris-buffered saline (TBS) pH7.4, containing 1 % (v/v) Triton X-100. The glass slides were incubated in a chamber (>80 % humidity) at 25 °C for 24 h for lectin immobilisation. After incubation, the slides were blocked with 1 % BSA in PBS at 20 °C for 1 h. Glycan-binding specificities of the lectins (Tao et al. 2008; Wearne et al. 2006) are listed in Table 1.
Table 1.
Lecting-binding signatures for the A549 cell line, adenocarcinoma cells and reactive mesothelial cells in pleural effusions
| No. | Lectin names | A549 | AC | RMC |
|---|---|---|---|---|
| 1 | Lotus tetragonolobus lectin, LTL | − | 0 | 18 |
| 2 | Pisum sativum agglutinin, PSA | − | 0 | 24 |
| 3 | Lens culinaris agglutinin, LCA | − | 0 | 28 |
| 4 | Ulex europaeus lectin type 1, UEA-1 | − | 0 | 10 |
| 5 | Aurentia lectin, AAL | + | 54 | 48 |
| 6 | Maackia amurensis lectin I, MAL-I | + | 16 | 28 |
| 7 | Maackia amurensis lectin II, MAL-II | − | 0 | 4 |
| 8 | Sambucus nigra, SNA | − | 0 | 50 |
| 9 | Wheat germ agglutinin, WGA | + | 48 | 40 |
| 10 | Erythrina cristagalli lectin, ECL | − | 4 | 30 |
| 11 | Datura stramonium agglutinin, DSA | − | 4 | 38 |
| 12 | Solanum tuberosum lectin, STL | + | 28 | 26 |
| 13 | Succinylated WGA, SWGA | − | 0 | 12 |
| 14 | Helix pomatia agglutinin, HPA | − | 0 | 20 |
| 15 | Canavalia ensiformis,ConA | − | 0 | 32 |
| 16 | Galanthus nivalis agglutinin, GNA | − | 0 | 22 |
| 17 | Hippeastrum hybrid lectin, HHL | − | 0 | 10 |
| 18 | Bauhinia purpurea lectin, BPL | − | 0 | 14 |
| 19 | Euonymus europaeus lectin, EEL | − | 0 | 4 |
| 20 | Jackfruit lectin, Jacalin | + | 10 | 28 |
| 21 | Wisteria floribunda lectin, WFA | − | 0 | 16 |
| 22 | Amaranthus caudatus lectin, ACL | + | 38 | 38 |
| 23 | Maclura pomifera lectin, MPL | − | 0 | 18 |
| 24 | Dolichos biflorus agglutinin, DBA | − | 0 | 2 |
| 25 | Soybean agglutinin, SBA | − | 0 | 14 |
AC adenocarcinoma cells, RMC reactive mesothelial cells
Fig. 1.
A lectin spot format used in this study. The couple spots of each lectin were spotted next to each other on glass slides. The yellow spots represented the signs, gray spots represented the lectins and white spots represented negative control, respectively. (Color figure online)
Lectin array hybridisation
Cells suspended in PBS were allowed to bind on lectin microarrays at room temperature for 30 min and unbound cells were removed by gravity in cold PBS. Bound cells immobilised on the glass slides were stained with Papanicolaou’s stain and analysed by two cytopathologists. The analysis was performed without knowledge of the patients’ clinical status.
Statistical analysis
SPSS11.0 software was used for the statistical analysis. Cells captured in different spots of lectin microarrays were compared using the χ2 test or Fisher exact test when theoretical effective was insufficient. Lectin spots were assessed as “positive” if only reactive mesothelial cells were captured and “negative” if adenocarcinoma cells, or together with reactive mesothelial cells, were captured. P values less than 0.05 were considered statistically significant. The utility of each lectin spot was assessed for sensitivity, specificity and accuracy and was compared with the cytological results. Sensitivity (%) mean binding reactive mesothelial cell samples percentage in 54 reactive mesothelial cells, sensitivity (%) = positive cases (binding reactive mesothelial cell samples)/total reactive mesothelial cell samples (54); specificity (%) mean not binding carcinoma cell samples percentage in 54 carcinoma cells, specificity (%) = negative cases (not binding carcinoma cell samples)/total carcinoma cell samples (54); accuracy (%) = positive cases + negative cases/total cases = binding reactive mesothelial cell samples + not binding carcinoma cell samples/total reactive mesothelial cell samples (54) + total carcinoma cell samples (54).
Results
A lectin-binding signature for the A549 cell line
The lectin-binding signature of the A549 cell line was determined to test the efficacy of the method (Table 1; Fig. 2A). Statistically significant lectin binding was observed for the AAL, MAL-I, WGA, STL, Jacalin and ACL markers, and no binding was observed in the remaining markers. Therefore, the A549 cell line possesses a specific surface glycan signature that can be captured by the lectin microarray described.
Fig. 2.
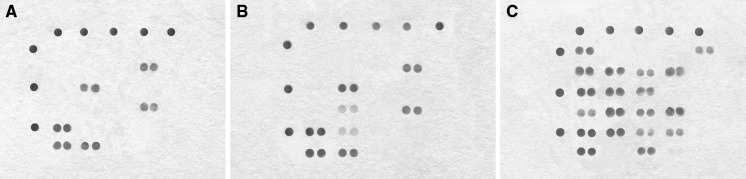
A Lectin-binding scanning figure for A549 cell line was observed in the spots of AAL, MAL-I, WGA, STL, Jacalin and ACL. B In addition to the marker profile of A549 cell line, lectin-binding scanning figure for adenocarcinoma cell was observed in the spots of ECL and DSA, but the signal intensity of them was weak. C Lectin-binding scanning figure for reactive mesothelial cells was observed in all spots except for MAL-II, WGA, EEL, WFA, STL and DBA
A lectin-binding signature for adenocarcinoma cells in pleural effusion
To demonstrate the capability of the lectin microarrays to characterise the cell surface glycan repertoires of adenocarcinoma cells in pleural effusions of patients with lung cancer, 54 malignant samples were tested (Table 1; Fig. 2B). The cell binding maps were determined using microscopy (Fig. 3A–D). In addition to the marker profile of the A549 cell line, adenocarcinoma samples showed significant binding of ECL and DSA lectins. AAL, WGA, and ACL binding was present in 54, 48, and 38 samples, respectively, whereas ECL and DSA were present in only 4/54 samples.
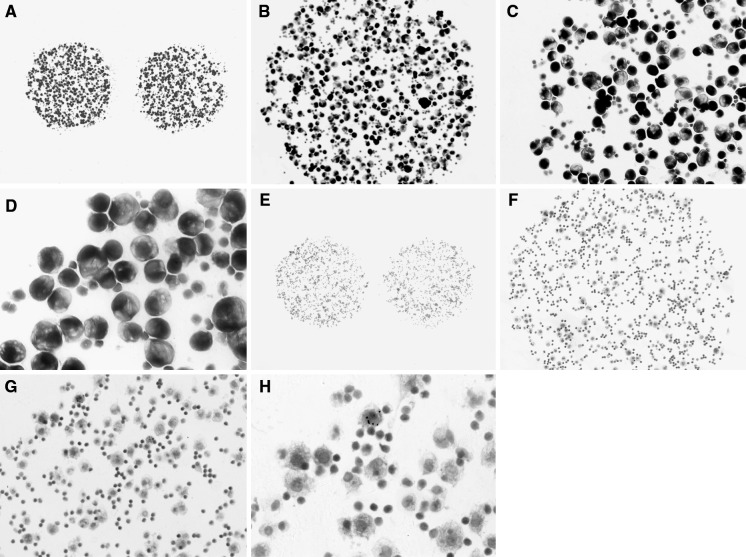
Fig. 3.
A lectin binding figure for adenocarcinoma cells in pleural effusion. The adenocarcinoma cells showed an large abnormal nucleus displaced to the periphery by a mucus vacuole, the chromatin was a coarse irregular pattern. Papanicolaou stain: A–D were ×40, ×100, ×200 and ×400, respectively. A lectin binding figure for reactive mesothelial cells in pleural effusion. The reactive mesothelial cells showed small spherical nuclei locate in the center of the cell, the chromatin net was delicate and rather inconspicuous, lymphocytes accompanied in background. Papanicolaou stain: E–H were ×40, ×100, ×200 and ×400, respectively
A lectin-binding signature for reactive mesothelial cells in pleural effusion
Characterisation of the cell surface glycan repertoire of reactive mesothelial cells in pleural effusion is detailed in Table 1 and Fig. 2C. The cell binding maps of 54 reactive mesothelial cells samples are shown in Fig. 3E–H. While all lectin markers in the microarray displayed positive binding to reactive mesothelial cells. SNA and AAL binding was present in the majority of samples (50/54 and 48/54, respectively), while MAL-II, EEL, and DBA binding was present in 4/54, 4/54, and 2/54 samples, respectively.
A lectin binding signature for distinguishing carcinoma cells from reactive mesothelial cells in pleural effusions
Reactive mesothelial cells had the most diverse surface glycoprotein profile, binding to all lectin markers in the microarray. In contrast, adenocarcinoma cells bound to a lower number of lectins. Seventeen lectins LTL, PSA, LCA, UEA-1, MAL-II, SNA, SWGA, HPA, ConA, GNA, HHL, BPL, EEL, WFA, MPL, DBA, and SBA bound only to reactive mesothelial cells, and not to adenocarcinoma cells. There was also significant diversity with respect to glycan profile within this sample group.
Table 2 shows sensitivity, specificity, and accuracy of each lectin in the microarray for distinguishing reactive mesothelial cells in pleural effusions of patients with and without lung cancer. The presence of bound reactive mesothelial cells was assessed as a ‘positive’ result for calculating sensitivity, specificity and accuracy. When evaluating diagnostic efficacy for differentiating adenocarcinoma cells and reactive mesothelial cells, SNA had the highest sensitivity (92.6 %), specificity (100 %) and accuracy (96.3 %) (Table 2).
Table 2.
Sensitivity, specificity and accuracy for distinguishing reactive mesothelial cells with lectin microarrays in pleural effusions
| Lectin spot | AC | RMC | χ2 | P value | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| LTL | 0 | 18 | 21.600 | <0.01 | 33.3 | 100 | 66.7 |
| PSA | 0 | 24 | 30.857 | <0.01 | 44.4 | 100 | 72.2 |
| LCA | 0 | 28 | 37.800 | <0.01 | 51.9 | 100 | 75.9 |
| UEA-1 | 0 | 10 | 11.020 | <0.01 | 18.5 | 100 | 59.3 |
| AAL | 54 | 48 | 4.412 | <0.05 | 88.9 | 0 | 44.4 |
| MAL-I | 16 | 28 | 5.523 | <0.05 | 51.9 | 70.4 | 61.1 |
| MAL-II | 0 | 4 | 2.337 | >0.05 | 7.4 | 100 | 53.7 |
| SNA | 0 | 50 | 93.103 | <0.01 | 92.6 | 100 | 96.3 |
| WGA | 48 | 40 | 3.927 | <0.05 | 74.1 | 11.1 | 42.6 |
| ECL | 4 | 30 | 29.017 | <0.01 | 55.6 | 92.6 | 74.1 |
| DSA | 4 | 38 | 45.039 | <0.01 | 70.4 | 92.6 | 81.5 |
| STL | 28 | 26 | 0.148 | >0.05 | 48.1 | 48.1 | 48.1 |
| SWGA | 0 | 12 | 13.500 | <0.01 | 22.2 | 100 | 61.1 |
| HPA | 0 | 20 | 24.545 | <0.01 | 37.0 | 100 | 68.5 |
| ConA | 0 | 32 | 45.474 | <0.01 | 59.3 | 100 | 79.6 |
| GNA | 0 | 22 | 27.628 | <0.01 | 40.7 | 100 | 70.4 |
| HHL | 0 | 10 | 11.020 | <0.01 | 18.5 | 100 | 59.3 |
| BPL | 0 | 14 | 16.085 | <0.01 | 25.9 | 100 | 63.0 |
| EEL | 0 | 4 | 2.337 | >0.05 | 7.4 | 100 | 53.7 |
| Jacalin | 10 | 28 | 13.155 | <0.01 | 51.9 | 81.5 | 66.7 |
| WFA | 0 | 16 | 18.783 | <0.01 | 29.6 | 100 | 64.8 |
| ACL | 38 | 38 | 0.000 | >0.05 | 70.4 | 29.6 | 50.0 |
| MPL | 0 | 18 | 21.600 | <0.01 | 33.3 | 100 | 66.7 |
| DBL | 0 | 2 | 0.509 | >0.05 | 3.7 | 100 | 51.9 |
| SBA | 0 | 14 | 16.085 | <0.01 | 25.9 | 100 | 63.0 |
AC adenocarcinoma cells, RMC reactive mesothelial cells
Biomarker discovery based on glycan signatures
To examine the power of the microarray platform for biomarker discovery, we analyzed lectin-binding by comparing the cell surface glycan signatures. The most significant biomarker was SNA, it effectively captured 50 of 54 samples of reactive mesothelial cells and did not capture any adenocarcinoma cells. This indicates that the SNA lectin may be used a biomarker to distinguish adenocarcinoma cells from reactive mesothelial cells.
Discussion
Morphological differentiation of reactive mesothelial cells from carcinoma cells in pleural effusions can be a diagnostic challenge. Adenocarcinoma metastatic to the pleural membrane is often associated with prominent mesothelial hyperplasia and may result in diagnostic confusion. The difficulty is even greater when neoplastic cells show only slight atypia, or when they are scarce in the effusion (Afify et al. 2002). False negative results from the cytological examination of pleural effusion are a serious problem. Such errors in diagnosis are usually caused by misinterpretation of adenocarcinoma cells as reactive mesothelial cells (Afify et al. 2005). The rate of false positive diagnoses is also significant and often caused by over-interpretation of reactive mesothelial cells as malignant cells (Politi et al. 2005). Glycobiologists have long believed that glycosylation features differ significantly depending on cell types and states of differentiation (Tateno et al. 2007). Recently, Rosenfeld et al. (2007) developed a novel lectin microarray with 24 immobilised lectins. Specific binding of glycoprotein was observed using fluorescent-labelled cells detected with an evanescent-field fluorescence scanner. The net intensity for each spot was calculated by subtracting background from signal intensity. However, this method of detection can only determine the signal intensity for each lectin spot and not types of cells, and therefore accurate discrimination between benign and malignant of cells is not possible.
We created a lectin microarray that does not need metabolic fluorescent labelling, and exploits the ability of lectins to bind to the accessible cell surface glycans. We found a unique cell-binding signature for adenocarcinoma cells and reactive mesothelial cells. Therefore, we did not only obtain the signal intensity for each lectin spot, but were also able to distinguish different types of cells binding to each specific lectin by microscopy, and even detect a single cell on each lectin spot. This is more sensitive and accurate than the method described by Rosenfeld et al. (2007). We showed a broader diversity of glycan structures on the surface of mesothelial cells than adenocarcinoma cells. The adenocarcinoma cells showed positive binding to only 8 of the 25 lectins present in the array (Table 1; Fig. 3A–D). This result may reflect that there are fewer types of glycan structures on the surface of adenocarcinoma cells, but the glycan profile is more uniform on the surface of adenocarcinoma cells than mesothelial cells. Adenocarcinoma cells were positive for binding to ECL and DSA, in addition to AAL, MAL-I, WGA, STL, Jacalin and ACL binding profile seen with the A549 cell line. AAL, WGA, and ACL binding was present in 54, 48, and 38 samples, respectively, whereas ECL and DSA were present in only 4/54 samples. The 54 reactive mesothelial cell samples displayed positive binding to all lectin biomarkers in the microarray, but there was no uniformity in the binding profiles.
However, SNA and AAL binding was detected in the majority of samples, 50/54 and 48/54, respectively. MAL-II, EEL and DBA binding was found in only 4/54, 4/54 and 2/54 samples, respectively. Therefore, we do not only present a high-throughput method to profile the accessible surface glycans of adenocarcinoma cells and reactive mesothelial cells in pleural effusion, but also demonstrate a potential clinical tumour marker applications where we can distinguish carcinoma cells from reactive mesothelial cells.
A major goal of high-throughput analyses is the identification of novel tumour markers that may have diagnostic value. Tumour markers that may be useful in distinguishing carcinoma cells from reactive mesothelial cells have been reported (Wu et al. 2008; Jiang et al. 2008; Sun et al. 2009). However, there are few markers for reactive mesothelial cells (HBME-1 and D2-40) and the diagnostic efficiency of them are suboptimal (Wu et al. 2008; Bassarova et al. 2006). In the present study, SNA effectively captured 50 of 54 cases of reactive mesothelial cells and did not capture any adenocarcinoma cells. It had the highest sensitivity (92.6 %), specificity (100 %) and accuracy (96.3 %) all lectin spots. This indicates that SNA may be a novel biomarker to distinguish adenocarcinoma cells from reactive mesothelial cells in pleural effusion.
The results reported here demonstrate the enormous potential of lectin microarray technology for identifying cell surface markers in benign and malignant pleural effusions. This method may also be used diagnostically to differentiate between carcinoma cells and reactive mesothelial cells in pleural effusions and improve the detection of malignancy.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (to Guang-Ping Wu, Grant No.81171650 and to Qun He, Grant No.20672144).
Contributor Information
Yu-Qing Shi, Email: shiyuqing101@sina.com.
Qun He, Email: hequn@mail.cmu.edu.cn.
Yu-Jie Zhao, Email: yjzhao@mail.cmu.edu.cn.
En-Hua Wang, Email: wangeh@hotmail.com.
Guang-Ping Wu, Phone: +86-24-83282177, FAX: +86-24-83282177, Email: wug_ping@sina.com.
References
- Afify AM, Al-Khafaji BM, Paulino AF, Davila RM. Diagnostic use of muscle markers in the cytologic evaluation of serous fluids. Appl Immunohistochem Mol Morphol. 2002;10:178–182. doi: 10.1097/00022744-200206000-00014. [DOI] [PubMed] [Google Scholar]
- Afify AM, Stern R, Michael CW. Differentiation of mesothelioma from adenocarcinoma in serous effusions: the role of hyaluronic acid and CD44 localization. Diagn Cytopathol. 2005;32:145–150. doi: 10.1002/dc.20201. [DOI] [PubMed] [Google Scholar]
- Bassarova AV, Nesland JM, Davidson B. D2-40 is not a specific marker for cells of mesothelial origin in serous effusions. Am J Surg Pathol. 2006;30:878–882. doi: 10.1097/01.pas.0000208280.29291.34. [DOI] [PubMed] [Google Scholar]
- Brooks SA, Leathem AJ. Prediction of lymph node involvement in breast cancer by detection of altered glycosylation in the primary tumour. Lancet. 1991;338:71–74. doi: 10.1016/0140-6736(91)90071-V. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta. 1999;1473:21–34. doi: 10.1016/S0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- Gabius HJ, SiebertHC AndreS, Jimenez-Barbero J, Rudiger H. Chemical biology of the sugar code. ChembioChem. 2004;5:740–764. doi: 10.1002/cbic.200300753. [DOI] [PubMed] [Google Scholar]
- Ghayumi SM, Mehrabi S, Doroudchi M, Ghaderi A. Diagnostic value of tumor markers for differentiating malignant and benign pleural effusions of Iranian patients. Pathol Oncol Res. 2005;11:236–241. doi: 10.1007/BF02893857. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- Jiang B, Wu GP, Zhao YJ, Wang SC. Transcription expression and clinical significance of TTF-1 mRNA in pleural effusion of patients with lung cancer. Diagn Cytopathol. 2008;36:849–854. doi: 10.1002/dc.20926. [DOI] [PubMed] [Google Scholar]
- Lehle L, Strahl S, Tanner W. Protein glycosylation, conserved from yeast to man: a model organism helps elucidate congenital human diseases. Angew Chem Int Ed Engl. 2006;45:6802–6818. doi: 10.1002/anie.200601645. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Development. Carbohydrate recognition in spermatogenesis. Science. 2002;295:53–54. doi: 10.1126/science.1068156. [DOI] [PubMed] [Google Scholar]
- Politi E, Kandaraki C, Apostolopoulou C, Kyritsi T, Koutselini H. Immunocytochemical panel for distinguishing between carcinoma and reactive mesothelial cells in body cavity fluids. Diagn Cytopathol. 2005;32:151–155. doi: 10.1002/dc.20203. [DOI] [PubMed] [Google Scholar]
- Queiroz C, Barral-Netto M, Bacchi CE. Characterizing subpopulations of neoplastic cells in serous effusions. The role of immunocytochemistry. Acta Cytol. 2001;45:18–22. doi: 10.1159/000327182. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R, Bangio H, Gerwig GJ, Rosenberg R, Aloni R, Cohen Y, Amor Y, Plaschkes I, Kamerling JP, Maya RB. A lectin array-based methodology for the analysis of protein glycosylation. J Biochem Biophys Meth. 2007;70:415–426. doi: 10.1016/j.jbbm.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wu GP, Fang CQ, Liu SL. Diagnostic utility of MOC-31, HBME-1 and MOC-31mRNA in distinguishing between carcinoma cells and reactive mesothelial cells in pleural effusions. Acta Cytol. 2009;53:619–624. doi: 10.1159/000325401. [DOI] [PubMed] [Google Scholar]
- Tao SC, Li Y, Zhou J, Qian J, Schnaar RL, Zhang Y, Goldstein IJ, Zhu H, Schneck JP. Lectin microarrays identify cell-specific and functionally significant cell surface glycan markers. Glycobiology. 2008;18:761–769. doi: 10.1093/glycob/cwn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno H, Uchiyama N, Kuno A, Togayachi A, Sato T, Narimatsu H, Hirabayashi J. A novel strategy for mammalian cell surface glycome profiling using lectin microarray. Glycobiology. 2007;17:1138–1146. doi: 10.1093/glycob/cwm084. [DOI] [PubMed] [Google Scholar]
- Van Dyken SJ, Green RS, Marth JD. Structural and mechanistic features of protein O glycosylation linked to CD8 + T-cell apoptosis. Mol Cell Biol. 2007;27:1096–1111. doi: 10.1128/MCB.01750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearne KA, Winter HC, O’Shea K, Goldstein IJ. Use of lectins for probing differentiated human embryonic stem cells for carbohydrates. Glycobiology. 2006;16:981–990. doi: 10.1093/glycob/cwl019. [DOI] [PubMed] [Google Scholar]
- Wu GP, Zhang SS, Fang CQ, Liu SL, Wang EH. Immunocytochemical panel for distinguishing carcinoma cells from reactive mesothelial cells in pleural effusions. Cytopathology. 2008;19:212–217. doi: 10.1111/j.1365-2303.2008.00559.x. [DOI] [PubMed] [Google Scholar]
- Zhou WB, Bai M, Jin Y. Diagnostic value of vascular endothelial growth factor and endostatin in malignant pleural effusions. Int J Tuberc Lung Dis. 2009;13:381–386. [PubMed] [Google Scholar]
- Zhu H, Klemic JF, Chang S, Bertone P, Casamayor A, Klemic KG, Smith D, Gerstein M, Reed MA, Snyder M. Analysis of yeast protein kinases using protein chips. Nat Genet. 2000;26:283–289. doi: 10.1038/81576. [DOI] [PubMed] [Google Scholar]