Abstract
The nasal pathway represents an alternative route for non-invasive systemic administration of drugs. The main advantages of nasal drug delivery are the rapid onset of action, the avoidance of the first-pass metabolism in the liver and the easy applicability. In vitro cell culture systems offer an opportunity to model biological barriers. Our aim was to develop and characterize an in vitro model based on confluent layers of the human RPMI 2650 cell line. Retinoic acid, hydrocortisone and cyclic adenosine monophosphate, which influence cell attachment, growth and differentiation have been investigated on the barrier formation and function of the nasal epithelial cell layers. Real-time cell microelectronic sensing, a novel label-free technique was used for dynamic monitoring of cell growth and barrier properties of RPMI 2650 cells. Treatments enhanced the formation of adherens and tight intercellular junctions visualized by electron microscopy, the presence and localization of junctional proteins ZO-1 and β-catenin demonstrated by fluorescent immunohistochemistry, and the barrier function of nasal epithelial cell layers. The transepithelial resistance of the RPMI 2650 cell model reached 50 to 200 Ω × cm2, the permeability coefficient for 4.4 kDa FITC-dextran was 9.3 to 17 × 10−6 cm/s, in agreement with values measured on nasal mucosa from in vivo and ex vivo experiments. Based on these results human RPMI 2650 cells seem to be a suitable nasal epithelial model to test different pharmaceutical excipients and various novel formulations, such as nanoparticles for toxicity and permeability.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-012-9493-7) contains supplementary material, which is available to authorized users.
Keywords: RPMI 2650, Human nasal epithelial cell, Retinoic acid, Hydrocortisone, Cell microelectronic sensing, Paracellular permeability
Introduction
Exploring different alternative routes for systemic drug delivery is a great challenge in pharmaceutical development. Besides conventional topical treatments, the nasal route can be also exploited for systemic, non-invasive delivery of drugs (Illum 2003). Intranasal administration can be especially effective in crisis treatment like in migraine or epilepsy, due to the rapid absorption of drugs through the highly vascularised nasal mucosa. The nasal route is also favourable for the delivery of peptides such as calcitonin, buserelin, due to the avoidance of the metabolism in the gastrointestinal tract. The most relevant anatomical region in the nasal cavity concerning systemic nasal drug delivery is the respiratory epithelium lining the middle and inferior turbinate (Schmidt et al. 1998). For successful formulation of a nasal delivery system, testing on reliably established in vitro cell culture, ex vivo tissue and in vivo animal models are crucial (Chien et al. 1992).
The physiological conditions of the human nose, temperature of 30 °C and pH 5.6, can be mimicked by in vitro conditions. These conditions alter the extent of dissolution of an active agent in a pharmaceutical formulation and they need to be taken into consideration in nasal preparations (Kürti et al. 2011). Ex vivo excised animal tissue models are frequently utilized for nasal drug absorption studies (Wadell et al. 1999, 2003; Schmidt et al. 2000). Several in vivo models of rat, rabbit, dog, sheep, and monkey were reported to deliver pharmacons via the nasal route (Chien et al. 1992; Costantino et al. 2007; Horvát et al. 2009). However, there are several disadvantages of the ex vivo tissue and in vivo animal models, including differences between the species in enzyme activities or in cell type distribution, and specialities in many anatomical and physiological features in various animal nasal cavities, compared with those of the human (Chien et al. 1992).
In vitro cell culture models of the human nasal epithelium based on primary culture technologies have proven to be useful for studies of nasal epithelial permeability and drug absorption (Lin et al. 2005). However limiting factors hinder the widespread usage and usefulness of in vitro primary nasal cell culture models. The shortage of human nasal tissue and the low reproducibility have prompted to seek an alternative to primary cultures of nasal epithelial cells, that is, the use of nasal epithelial cell lines. Cultured immortalized nasal epithelial cells are widely used models for drug toxicity and metabolism studies, since they are known to express important biological features like intercellular tight and adherens junctions, mucin secretion, cilia, and various transporters, resembling those found in in vivo systems (Schmidt et al. 1998). The use of an immortalized cell line has the advantages of ease of culture, lower cost, genetic homogeneity and reproducibility of the results.
RPMI 2650 is the only human nasal epithelial cell line derived from a spontaneously formed tumour. Although this cell line originates from an anaplastic nasal septum tumour, its properties are closely related to normal human nasal epithelium concerning its karyotype (Moorhead 1965), its cytokeratin polypeptide pattern (Moll et al. 1983), and the presence of mucoid material on the cell surface (Moore and Sandberg 1964). This particular cell line has been mostly used for nasal metabolism studies and toxicity assays (Kürti et al. 2012) and less for permeability studies, since there are conflicting reports with regard to the ability of these cells to form monolayer. Some experts claim that RPMI 2650 nasal epithelial cells do form monolayer (Bai et al. 2008) while others described that they grow in clumps rather than in monolayer (De Fraissinette et al. 1995). Poor differentiation and lack of polarization have also been reported for this cell line (Werner and Kissel 1996). Furthermore, there are inconclusive data on the ability of RPMI 2650 cells to form tight junctions, though there is evidence that they can form perijunctional F-actin rings (Werner and Kissel 1996). Due to these reasons RPMI 2650 human nasal epithelial cell line is rarely used for nasal transport studies (Bai et al. 2008; Wengst and Reichel 2010).
The criteria for transport experiments with RPMI 2650 human nasal epithelial cell culture model are (1) the formation of confluent cell layers, (2) the expression and correct localization of intercellular junctional proteins, (3) the functional barrier properties measured by transepithelial electric resistance and permeability coefficients for drug or marker molecules. The paracellular permeability, one of the most important determinants of drug transport, of various epithelial tissues and barriers differs greatly (Deli 2009). Transepithelial resistance, measuring paracellular ion flux and reflecting the tightness of the intercellular junctional complex, is low in the nasal mucosa, which is considered as a leaky epithelial tissue. The tight junctions in the epithelium of the small intestine, the stomach and the colon are of intermediate tightness, while endothelial cells of brain capillaries and skin epithelial cells form very tight paracellular barriers (Deli 2009).
Cell proliferation and differentiation during culturing can be affected by many factors, including surface coating, cell seeding density, interface composition, concentration of serum in the cell culture medium and application of growth and differentiating factors. Retinoic acid, a metabolite of vitamin A, hydrocortisone, a glucocorticoid hormone and cyclic adenosine monophosphate (cAMP), a second messenger, which influence barrier formation and function of epithelial and endothelial cell layers were selected for the study (Yoon et al. 2000; Deli et al. 2005).
There is a need for in vitro systems to test the epithelial toxicity and permeability of nasal pharmaceutical formulations. The aim of our work was to characterize and improve RPMI 2650 model for pharmaceutical screening purposes. The effects of growth and differentiating factors on the barrier function of the nasal epithelial cell layers were tested by both morphological and functional methods. Real-time cell electronic sensing (RT-CES) was used for dynamic monitoring of the barrier properties of nasal epithelial cells for the first time.
Materials and methods
All reagents were purchased from Sigma–Aldrich Ltd., Hungary, unless otherwise indicated.
Cell culture
RPMI 2650 (ATCC cat. no. CCL 30) cells were grown in Eagle’s minimal essential medium (MEM) supplemented with 10 % foetal bovine serum (FBS) and 50 μg/mL gentamicin in a humidified 37 °C incubator with 5 % CO2. The cells were seeded on rat tail collagen (0.05 v/v%) coated culture dishes at a density of 5 × 105 cells/cm2 and the medium was changed every 2 days. When RPMI 2650 cells reached approximately 80–90 % confluency in the dish they were trypsinized with 0.05 % trypsin–EDTA solution. For the cell viability assays cells were passaged to 96-well plates, for immunostaining cells were cultured on glass coverslips. For permeability studies cells were cultured on Transwell filter inserts (polycarbonate membrane, 0.4 μm pore size, 1.1 cm2 surface area, Corning Costar Co., MA, USA). All surfaces were coated with 0.05 v/v% rat tail collagen before cell seeding. Cell growth and morphology were monitored using a Nikon Eclipse TE2000 microscope (Nikon, Japan).
Electron microscopy
RPMI 2650 cells grown on Transwell filter membrane were fixed with 3 % paraformaldehyde in 0.05 M cacodylate buffer (pH 7.5) for 30 min at 4 °C. After washing with cacodylate buffer several times, the membranes of the culture inserts with the cells were removed from their support and placed into 24-well chamber slide and were postfixed in 1 % OsO4 for 30 min. Finally the membranes of the culture inserts with the cells were removed from their support and embedded in Taab 812 (Taab; Aldermaston, UK). Following polymerisation at 60 °C for 12 h, ultrathin sections were cut perpendicularly for the membrane using a Leica UCT ultramicrotome (Leica Microsystems, Milton Keynes, UK) and examined using a Hitachi 7100 transmission electron microscope (Hitachi Ltd., Japan). Electron micrographs were made by Megaview II (lower resolution, Soft Imaging System, Germany). Brightness and contrast were adjusted if necessary using Adobe Photoshop CS3 (CA, USA).
Immunohistochemistry
To stain for junctional proteins RPMI 2650 cells cultured on rat tail collagen coated glass coverslips were washed in phosphate buffered saline (PBS) and fixed with 4 % paraformaldehyde—PBS for 30 min. After washing with PBS cells were blocked with 3 % bovine serum albumin (BSA) in PBS and incubated with primary antibodies anti-ZO-1 and anti-β-catenin (both from Invitrogen, CA, USA) for 1 h and 30 min. Incubation with secondary antibody Cy3-labeled anti-rabbit IgG and Hoechst dye 33342 to stain cell nuclei lasted for 1 h. Between and after incubations cells were washed three times with PBS. Coverslips were mounted in Gel Mount (Biomeda, USA) and staining was examined by Olympus Fluoview FV1000 confocal laser scanning microscope.
Treatments
The effect of retinoic acid (RA) was tested in three different concentrations 0.01, 100, 300 μg/mL. Hydrocortisone (HC) was examined at 500 nM; 8-(4-chlorophenylthio) adenosine 3′,5′-cyclic monophosphate sodium salt (CPT-cAMP) at 250 μM in the presence of 17.5 μM 4-(3-Butoxy-4-methoxybenzyl)-2-imidazolidinone (RO20-1724, Calbiochem, Germany).
Cell viability assays
Lactate dehydrogenase (LDH) release, the indicator of cell membrane damage and necrotic cell death, was determined from culture supernatants by a commercially available kit (Cytotoxicity detection kit LDH, Roche, Switzerland). For LDH release assay RPMI 2650 cells were cultured in 96-well plates. After 24-h treatments with growth and differentiating factors 50 μL samples from culture supernatants were incubated with equal amounts of reaction mixture for 15 min. The enzyme reaction was stopped by 0.1 M HCl. Absorbance was measured at a wavelength of 450 nm with a microplate reader (Fluostar Optima, BMG Labtechnologies, Germany). Cytotoxicity was calculated as percentage of the total LDH release from cells treated with 1 % Triton X-100 detergent.
Living cells convert the yellow dye 3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to purple, insoluble formazan crystals. RPMI 2650 cells were cultured in 96-well plates. After treatments the cells were incubated with 0.5 mg/mL MTT solution for 3 h in CO2 incubator. The amount of formazan crystals was dissolved in dimethyl-sulfoxide and determined by measuring absorbance at 570 nm with a microplate reader (Fluostar Optima, BMG Labtechnologies, Germany).
Real-time monitoring the biological status of the human RPMI 2650 nasal epithelial cells
Real-time cell electronic sensing (RT-CES) is a label-free technique for dynamic monitoring of living cells (Xia et al. 2008; Muendoerfer et al. 2010). The xCELLigence system (Roche, Switzerland) utilizes an automatical and continuous electronic readout called impedance to non-invasively quantify adherent cell proliferation and viability in real-time. A special 96-well E-plate (Roche, Hungary) contains gold microelectronic sensor arrays. The interaction between cells and electrode generates impedance response that correlates linearly with cell index reflecting cell number, adherence and cell growth (Ózsvári et al. 2010). The E-plate was coated with 0.2 % gelatine in PBS solution for 20 min at 37 °C. Culture medium (80 μL) was added to each well for background readings, then 80 μL cell suspension was dispensed at the density of 6 × 103 cells/well. A cells were kept in incubator at 37 °C for 30 h and monitored every 5 min. The cell index at each time point was defined as (Rn − Rb)/15, where Rn is the cell-electrode impedance of the well when it contains cells and Rb is the background impedance of the well with the medium alone.
Measurement of transepithelial electrical resistance
Transepithelial electrical resistance (TEER), representing the permeability of tight junctions for sodium ions in culture conditions, was measured by an EVOM resistance meter (World Precision Instruments Inc., USA) using STX-2 electrodes, and it was expressed relative to the surface area of epithelial layers (Ω × cm2). The TEER of nasal epithelial cell layers varied between 50 and 200 Ω × cm2. RT-CES made it also possible to monitor the TEER changes after treatments. TEER values (Rn) were calculated from cell index and background impedance by the equation given in the previous section.
Permeability experiments
To measure the flux of fluorescein isothiocyanate labeled dextran (FITC-dextran, mw: 4.4 kDa) across epithelial cell layers RPMI 2650 cells were seeded onto Transwell inserts, grown for 2 days and treated with growth and differentiating factors for 24 h. They were transferred to 12-well plates containing 1.5 mL Ringer–Hepes solution (118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 5.5 mM d-glucose, 20 mM Hepes, pH 7.4) in the basolateral compartments. In the apical chambers culture medium was replaced by 500 μL Ringer–Hepes containing 100 μg/mL FITC-dextran solution. The plates were kept in a 37 °C incubator with 5 % CO2 for 1 h on a rocking platform. After incubation the concentrations of the marker molecule in samples from the apical and basolateral compartments were determined by a fluorescent microplate reader (Fluostar Optima, BMG Labtechnologies, Germany; emission: 485 nm, excitation: 520 nm). Flux across cell-free inserts was also measured. The apparent permeability coefficient (Papp) was calculated. Cleared volume was calculated from the concentration difference of the tracer in the abluminal compartment (Δ[C]A) after 1 h and luminal compartments at 0 h ([C]L), the volume of the abluminal compartment (VA; 1.5 mL) and the surface area available for permeability (A; 1.1 cm2) by the following equation (Youdim et al. 2003):
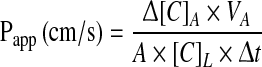 |
Statistical analysis
All data presented are mean ± SD. The values were compared using the analysis of variance followed by Dunnett tests using GraphPad Prism 5.0 software (GraphPad Software Inc., USA). Changes were considered statistically significant at P < 0.05. All experiments were repeated at least three times, the number of parallel samples varied between 4 and 12.
Results and discussion
Growth and morphology of RPMI 2650 nasal epithelial cell line
RPMI 2650 human nasal epithelial cells reached confluency in 2 days after seeding, which was confirmed by phase contrast microscopy (Kürti et al. 2012). RPMI 2650 cells grew in mono- or multilayers as in vivo as demonstrated by the electron microscopic images. A cubic and polarized cell morphology, microvilli and mucoid material on the apical surface could be observed (Fig. 1). Healthy, intact cell constituents, like mitochondria, endoplasmic reticulum and large cell nuclei with few cytoplasm were visualized. Intercellular junctions are important in the paracellular barrier function of the cell layers. Adherens junctional protein β-catenin was detected at the border of RPMI 2650 cells by immunofluorescent microscopy (Fig. 2). Faint cytoplasmic staining was also visible in agreement with the known signalling mechanism of this protein.
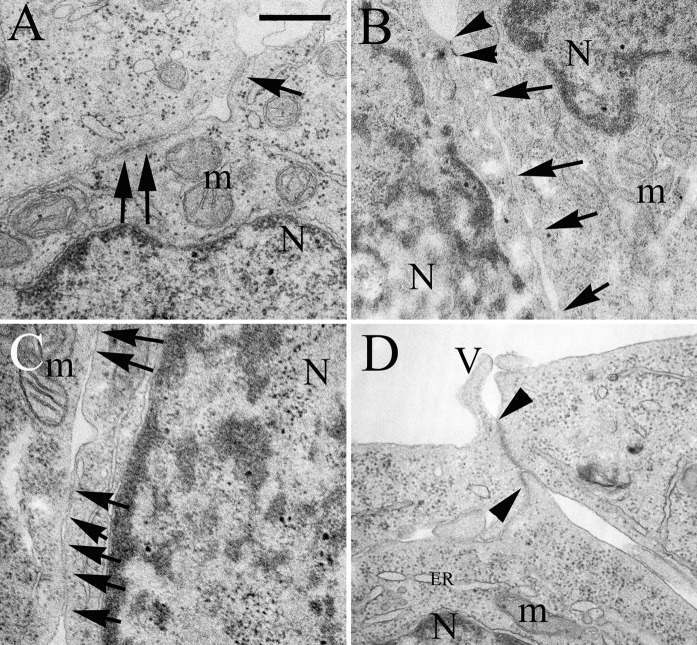
Fig. 1.
Electron micrographs on the intercellular junctions of the RPMI 2650 layers treated with culture medium (a), 300 μg/mL retinoic acid (b), 500 nM hydrocortisone (c); 250 μM 3′–5′-cyclic adenosine monophosphate (d) for 24 h. Arrows indicate intercellular junctions, arrowheads show the length of tight junctions. N Nucleus, m mitochondrion, ER endoplasmatic reticulum, V microvilli; bar 500 nm. Control conditions: RPMI 2650 cells were grown in Eagle’s minimal essential medium supplemented with 10 % foetal bovine serum and 50 μg/mL gentamicin
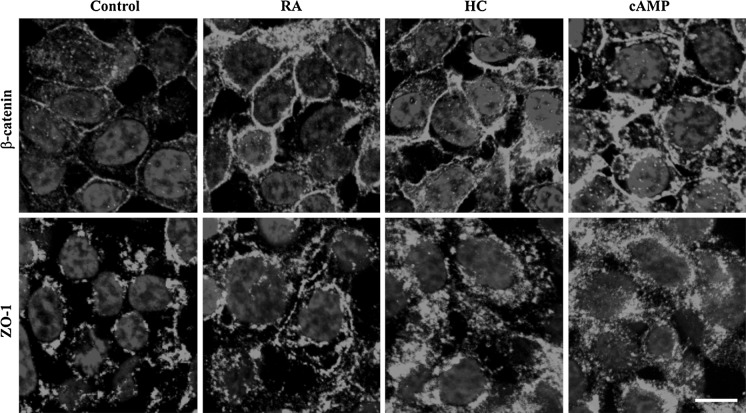
Fig. 2.
Immunohistochemical staining of RPMI 2650 cells for junctional proteins β-catenin and zona occludens-1 (ZO-1) visualized by confocal fluorescence microscopy. Cells were treated with retinoic acid (RA, 300 μg/mL), hydrocortisone (HC, 500 nM) or 3′–5′-cyclic adenosine monophosphate (cAMP, 250 μM) for 24 h; bar 10 μm. Control conditions: RPMI 2650 cells were grown in Eagle’s minimal essential medium supplemented with 10 % foetal bovine serum and 50 μg/mL gentamicin
The confluency of cell layers and the presence of intercellular junctions are two crucial points in the establishment of an in vitro system. There were contradictory data in the literature, RPMI 2650 cells did not show differentiation into goblet cells or ciliated cells and did not express tight junctions, they formed clusters with free spaces in between, instead of growing to confluence (De Fraissinette et al. 1995). In our experiments confluent cell layers and intercellular junctions appeared (Figs. 1, 2) which can be explained by the specific culture conditions as follows.
Proper surface coating is a main point in the evaluation of an in vitro model for permeability experiments. The type of the surface on which the cells grow influences the preservation of specific phenotypic characteristics (Gruenert et al. 1995) and the development of specialized epithelial functions such as barrier formation (Yankaskas and Boucher 1990). The extracellular matrix is the physiological microenvironment for the epithelial cell migration, proliferation, and differentiation; it is an essential element in the development of a successful culture of human nasal epithelial cells (De Fraissinette et al. 1995). The use of biological matrices, such as collagen, laminin, and matrigel is essential to obtain a stable, well-differentiated and reproducible nasal culture system (Agu et al. 2001). In our preliminary experiments several methods were tested, different peptides and also a collagen gel were used for surface coating. The best cell differentiation, functional polarisation, and transepithelial resistance were observed in the case of 0.05 v/v% rat tail collagen coated surfaces in agreement with previous findings (Agu et al. 2001). This collagen matrix enhanced the attachment of RPMI 2650 cells and established proper surface for the nasal epithelial cell layers. Polycarbonate membranes coated by rat tail collagen were the most suitable surface for growing nasal epithelial cells for permeability experiments. Rat tail collagen contains mainly collagen type I, which has shown the most promising results concerning other airway epithelial cell cultures as well. This could be related to the fact that the respiratory basement membrane is composed mainly of collagen fibres (Chien et al. 1992). In our experiments all surfaces were coated with rat tail collagen solution before seeding RPMI 2650 cells.
Appropriate initial cell density is also an important factor in the establishment of a cell culture model of the nasal barrier concerning drug transport (Hellinger et al. 2010). An initial cell density of 5 × 105 cells/cm2 was found to be optimal, otherwise RPMI 2650 cells do not reach confluency and form cell clusters which is typical for carcinoid cells and was also observed in previous studies (De Fraissinette et al. 1995).
Real-time monitoring of the effect of treatments on the nasal epithelial cell layers
We have used microelectric sensing to monitor the biological status of the RPMI 2650 cells in real-time and provide quantitative information on viability, adherence and permeability (Kürti et al. 2012). RT-CES measures changes in the impedance of individual microelectronic wells, containing gold microelectrodes integrated into the bottom of the culturing plate. Electric impedance correlates linearly with cell index reflecting cell number and growth, and cell–matrix and intercellular adherence (Solly et al. 2004). Dynamic monitoring of adherent cell proliferation is a unique technique to optimize culture conditions and follow the effect of different treatments. The presence or absence of serum, a major component of cell culture media and the effect of selected bioactive molecules, retinoic acid, hydrocortisone and cAMP were tested to induce better attachment, proliferation and differentiation of human nasal epithelial cells.
Cells were let to attach to the microwells of E-plate for 6 h in MEM supplemented with 10 % FBS. The cell index grew dynamically during that period (Fig. 3a). Then the medium was replaced by fresh culture medium containing 0, 5 or 10 % FBS. In the absence of serum the cell index did not increase further in the next 24 h. The presence of serum resulted in the elevation of RPMI 2650 cell index measured by RT-CES as shown on Fig. 3a. MEM supplemented with 10 % FBS induced the highest cell index, which reflected better cell attachment and growth (Fig. 3a, b). Serum is a source of adhesion promoting components, nutrients and trace minerals, transport and stabilizing proteins like transferrin and albumin, growth factors and hormones (Lechner 1984) which might have contributed to better cell growth and attachment, and higher cell index. Serum may also contain factors that regulate the formation of tight junctions in epithelial cells (Hashimoto and Shimizu 1993). In agreement with these data, the use of serum at 5–10 % as a medium supplementation was recommended for better attachment and long-term viability in primary cultures of nasal epithelial cells (Usui et al. 2000; Agu et al. 2001). Longer incubation with serum-supplemented medium also induced squamous, terminal differentiation of human airway epithelial cells (Wu et al. 1986).
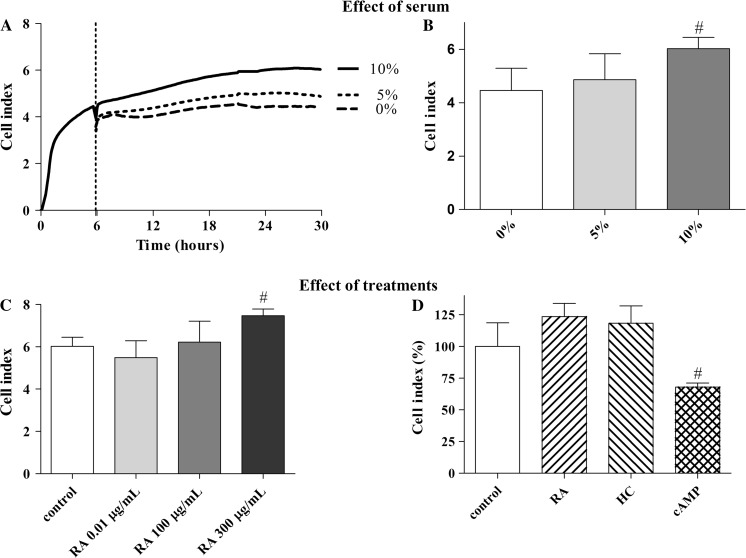
Fig. 3.
Cell index of RPMI 2650 layers treated with different concentrations of fetal bovine serum (panel a and b), and retinoic acid (RA, 0.01–300 μg/mL (c), 300 μg/mL (d)), hydrocortisone (HC, 500 nM), and 3′–5′-cyclic adenosine monophosphate (cAMP, 250 μM) for 24 h measured by real-time impedance monitoring (panel c and d). Data are presented as mean ± SD, n = 4. Cell index is expressed as an arbitrary unit and calculated from impedance measurements between cells and sensors (xCELLigence, Roche). Control conditions: RPMI 2650 cells were grown in Eagle’s minimal essential medium supplemented with 10 % foetal bovine serum and 50 μg/mL gentamicin
Retinoids regulate the growth and differentiation of normal, premalignant and malignant cell types, especially epithelial cells, mainly through interaction with nuclear retinoic acid receptors and retinoid X receptors (Bogos et al. 2008). Vitamin A deficiency induces replacement of the normal pseudostratified mucociliary epithelium by a metaplastic stratified squamous epithelium in vivo. Although retinoic acid is crucial in the development of mucosecretory phenotype of human airway epithelial cultures, its use as a medium supplementation is debated (Gray et al. 1996; Yoon et al. 2000). In the presence of retinoic acid epithelial cultures show mucociliary differentiation and cuboidal phenotype. The minimum required concentration of retinoic acid for airway cultures was suggested to be 0.3 μg/mL (Yoon et al. 2000). Well-differentiated mucociliary phenotype of human nasal epithelium cultures was also reported without retinoids (Van Scott et al. 1988; Werner and Kissel 1995; Agu et al. 2001). Retinoic acid at 0.01 μg/mL concentration had no effect on a nasal model based on RPMI 2650 cells (Wengst and Reichel 2010).
In the present experiments retinoic acid enhanced the cell index of human RPMI 2650 cells in a dose-dependent manner (Fig. 3c). The cell index of the RPMI 2650 layers was significantly increased following a 24-h retinoic acid treatment at 300 μg/mL concentration; although the other lower doses (0.01 and 100 μg/mL) had no effect. We could confirm the absence of efficacy at the lowest dose on this cell line (Wengst and Reichel 2010). The effect of retinoic acid was independent from the presence of serum and it elevated the cell index compared to the control groups at all serum concentrations tested (Fig. S1A).
Hydrocortisone in physiological concentration of 500 nM also increased the cell index of human nasal epithelial cells (Fig. S1B). This finding is in agreement with data on the favourable effect of hydrocortisone on epithelial cells described previously (Wu et al. 1986; Van Scott et al. 1988). Hydrocortisone is a potent inducer of the formation of barrier properties in cultured endothelial cells (Perrière et al. 2007; Nakagawa et al. 2009), and the increase in the cell index of RPMI 2650 layers may be also related to the formation of a tighter barrier.
Cholera toxin, which acts through cAMP as a second messenger, improves cell growth and inhibits the differentiation-inducing activity of serum (Lechner 1984; Wu et al. 1986). Several studies have shown that the levels of cAMP are inversely related to the rate of cell division, with high levels present in non-dividing cells (Otten et al. 1972; Bombik and Burger 1973). In brain endothelial cells cAMP is a strong inducer of barrier functions (Deli et al. 2005). However, in contrast to our previous findings on brain endothelial cells and to the effects of retinoic acid and hydrocortisone on RPMI 2650 cells no increase of cell index was seen in nasal epithelial cells treated with 250 μM cAMP either in the presence or absence of serum (Fig. S1C). Retinoic acid, hydrocortisone and cAMP in the tested doses did not decrease epithelial cell viability measured by MTT dye conversion assay and LDH release test (Fig. S2).
Comparing the effects of different bioactive molecules, retinoic acid was the most effective inducer of cell index in RPMI 2650 cells (Fig. 3d). The highest cell index could be observed in the case of MEM containing 10 % serum and supplemented with 300 μg/mL retinoic acid at 24 h.
The effect of treatments on the formation of intercellular junctions
All epithelia have at least one important function in common: they serve as selective permeability barriers, separating fluids on either side that have a different chemical composition. In polarized epithelia, tight and adherens junctions can be detected at the apical region of the intercellular cleft and appear as a zipper-like seal between adjacent cells. Intercellular junctions are involved in the control of epithelial paracellular permeability (Deli 2009). They also promote cell-to-cell communication and transfer signals that mediate contact inhibition of cell growth, increase resistance to apoptosis, and regulate cell shape and polarity (Bauer et al. 2011).
To differentiate between the effects of the tested treatments on cell growth or barrier formation the ultrastructural barrier properties of nasal epithelial cells were examined by transmission electron microscopy. Treatments with retinoic acid, hydrocortisone or cAMP increased the number and length of intercellular junctions and focal adhesions as shown on representative images (Fig. 1). Interestingly, cAMP treatment, which had no effect on cell index resulted in longer intercellular connections and tight junctions (Fig. 1d), an observed tendency.
The immunostaining and localization of adherens and tight junctional proteins were also changed in human RPMI 2650 cells by treatments. Immunostaining for adherens junction protein β-catenin and tight junction related protein ZO-1 were more intense and better localized to the cell periphery in RPMI 2650 cells treated with retinoic acid, hydrocortisone or cAMP then in the control group (Fig. 2).
Permeability experiments with a paracellular marker molecule
RPMI 2650 cells achieved confluency within 2 days on collagen coated polycarbonate membranes in the culture conditions described above. The presence of intercellular junctions was confirmed by electron and immunofluorescent microscopy. Besides morphological characterisation, the functional properties of RPMI 2650 nasal epithelial layers, like transepithelial electric resistance and paracellular permeability, were also investigated.
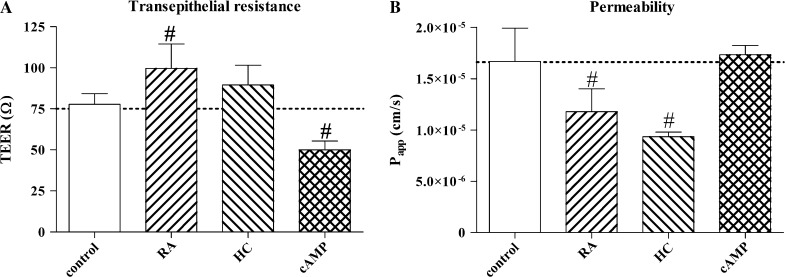
Retinoic acid and hydrocortisone significantly decreased the paracellular permeability for FITC-dextran 4.4 kDa and increased the transepithelial resistance of nasal epithelial cell layers (Fig. 4). These parallel effects indicate strengthened barrier formation in agreement with the morphological observations. While cAMP did not change the epithelial paracellular permeability, interestingly, it significantly decreased the electric resistance of RPMI 2650 cells (Fig. 4). The reason for this phenomenon might be related to the opening of ion channels, which is a known effect of cAMP (Van Scott et al. 1988).
Fig. 4.
Barrier functions measured by transepithelial resistance (a) and permeability for FITC-labelled dextran 4.4 kDa (b) of RPMI 2650 layers treated with retinoic acid (RA, 300 μg/mL), hydrocortisone (HC, 500 nM) or 3′–5′-cyclic adenosine monophosphate (cAMP, 250 μM). Papp, apparent permeability coefficient. Control conditions: RPMI 2650 cells were grown in Eagle’s minimal essential medium supplemented with 10 % foetal bovine serum and 50 μg/mL gentamicin
The flux of FITC-dextran 4.4 kDa from the apical to the basolateral compartments is around 4.5 % in the control group as compared to the amount in the apical compartment at t0 time point. Treatments with retinoic acid, hydrocortisone or cAMP resulted in 3.2, 2.5 and 4.7 % flux of the marker molecule from the donor to the acceptor compartment. As a comparison, nasal absorption of FITC-dextran 4.4 kDa in rats is 2.2 % of the administered amount (Yamamoto et al. 1993). Our data on RPMI 2650 cells treated with retinoic acid, or hydrocortisone as an in vitro nasal system are comparable to results from in vivo animal models.
The results of the permeability assays on RPMI 2650 cells with FITC-dextran 4.4 kDa could model the absorption of large molecules like peptides. The nasal pathway can be exploited for systemic peptide delivery due to the advantages of intranasal application (Kissel and Werner 1998; Costantino et al. 2007). There are marketed pharmaceutical products for nasal systemic peptide delivery such as calcitonin which is applied in the treatment of osteoporosis, and there is a growing interest for the nasal delivery of biologically active peptides (Illum 2003; Sipos et al. 2010).
Conclusions, significance
Biopharmaceutical studies including bioavailability experiments are needed to test nasal formulations of novel active agents. Before in vivo preclinical testing, it is reasonable to screen drug candidates with respect to their permeability through the nasal mucosa and epithelial toxicity by using in vitro models. RPMI 2650 human nasal epithelial cells were able to form confluent multilayers, express intercellular junctions and present some barrier properties similar to in vivo conditions. Using a real-time cell microelectronic sensing technique the kinetic of the cell growth and differentiation of RPMI 2650 cells could be followed for the first time. Treatment with retinoic acid and hydrocortisone reinforced the paracellular barrier both morphologically and functionally. The presented in vitro nasal epithelial model may be applicable to test the toxicity and permeability of pharmaceutical excipients (Kürti et al. 2012) and drug candidates.
Novel excipients for nasal formulations need to be investigated for epithelial toxicity and permeability on in vitro nasal models, similarly to the application of the widely used Caco-2 model for testing oral formulations (Szűts et al. 2011). New formulations for systemic nasal drug delivery, like nanoparticles (Kürti et al. 2011), also need to be screened for nasal cytotoxicity and epithelial permeability. RPMI 2650 cells may serve as a nasal in vitro model to test epithelial toxicity and permeability across the nasal barrier.
Electronic supplementary material
Supplementary material Cell index changes in RPMI 2650 cells treated with retinoic acid (RA, 300 μg/mL), 603 hydrocortisone (HC, 500 nM), 3′-5′-cyclic adenosine monophosphate (cAMP, 250 μM) for 604 24 h in the presence of different concentrations of fetal bovine serum. Cell index is expressed 605 as an arbitrary unit and calculated from impedance measurements between cells and sensors 606 (xCELLigence, Roche). (TIFF 1387 kb)
Supplementary material Cell viability assays on RPMI 2650 cells treated with retinoic acid (RA, 300 μg/mL), 609 hydrocortisone (HC, 500 nM), 3′-5′-cyclic adenosine monophosphate (cAMP, 250 μM). 610 Lactate dehydrogenase (LDH) release assay reflects plasma membrane damage and necrotic 611 cell death (A), MTT dye conversion test shows cell viability based on cytoplasmic and 612 mitochondrial enzyme activity (B). C, control; TX, 1 % Triton X-100. Control conditions: 613 RPMI 2650 cells were grown in Eagle’s minimal essential medium without phenol red 614 supplemented with 10 % foetal bovine serum and 50 μg/mL gentamicin. (TIFF 991 kb)
Acknowledgments
The Project named “TÁMOP-4.2.1/B-09/1/KONV-2010-0005—Creating the Center of Excellence at the University of Szeged” is supported by the European Union and co-financed by the European Regional Development Fund.
References
- Agu RU, Jorissen M, Willems T, Augustijns P, Kinget R, Verbeke N. In vitro nasal drug delivery studies: comparison of derivatised, fibrillar and polymerised collagen matrix-based human nasal primary culture systems for nasal drug delivery studies. J Pharm Pharmacol. 2001;53:1447–1456. doi: 10.1211/0022357011777981. [DOI] [PubMed] [Google Scholar]
- Bai S, Yang T, Abbruscato TJ, Ahsan F. Evaluation of human nasal RPMI 2650 cells grown at an air–liquid interface as a model for nasal drug transport studies. J Pharm Sci. 2008;97:1165–1178. doi: 10.1002/jps.21031. [DOI] [PubMed] [Google Scholar]
- Bauer HC, Traweger A, Zweimueller-Mayer J, Lehner C, Tempfer H, Krizbai I, Wilhelm I, Bauer H. New aspects of the molecular constituents of tissue barriers. J Neural Transm. 2011;118:7–21. doi: 10.1007/s00702-010-0484-6. [DOI] [PubMed] [Google Scholar]
- Bogos K, Renyi-Vamos F, Kovacs G, Tovari J, Dome B. Role of retinoic receptors in lung carcinogenesis. J Exp Clin Cancer Res. 2008;27:18–24. doi: 10.1186/1756-9966-27-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombik BM, Burger MM. cAMP and the cell cycle: inhibition of growth stimulation. Exp Cell Res. 1973;80:88–94. doi: 10.1016/0014-4827(73)90278-4. [DOI] [PubMed] [Google Scholar]
- Chien YW, Su KSE, Chang S. Nasal systemic drug delivery. New York: Informa Healthcare Inc.; 1992. [Google Scholar]
- Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337:1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- De Fraissinette A, Brun R, Felix H, Vonderscher J, Rummelt A. Evaulation of the human cell line RPMI 2650 as an in vitro nasal model. Rhinology. 1995;33:194–198. [PubMed] [Google Scholar]
- Deli MA. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta. 2009;1788:892–910. doi: 10.1016/j.bbamem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Deli MA, Ábrahám CS, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Shimizu M. Epithelial properties of human intestinal Caco-2 cells cultured in a serum-free medium. Cytotechnology. 1993;13:175–184. doi: 10.1007/BF00749813. [DOI] [PubMed] [Google Scholar]
- Hellinger É, Bakk ML, Pócza P, Tihanyi K, Vastag M. Drug penetration model of vinblastine-treated Caco-2 cultures. Eur J Pharm Sci. 2010;41:96–106. doi: 10.1016/j.ejps.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Horvát S, Fehér A, Wolburg H, Sipos P, Veszelka S, Tóth A, Kis L, Kurunczi A, Balogh G, Kürti L, Erős I, Szabó-Révész P, Deli MA. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur J Pharm Biopharm. 2009;72:252–259. doi: 10.1016/j.ejpb.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Illum L. Nasal drug delivery-possibilities, problems and solutions. J Control Release. 2003;87:187–198. doi: 10.1016/S0168-3659(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Kissel T, Werner U. Nasal delivery of peptides: an in vitro cell culture model for the investigation of transport and metabolism in human nasal epithelium. J Control Release. 1998;53:195–203. doi: 10.1016/S0168-3659(97)00253-8. [DOI] [PubMed] [Google Scholar]
- Kürti L, Kukovecz Á, Kozma G, Ambrus R, Deli MA, Szabó-Révész P. Study of the parameters influencing the co-grinding process for the production of meloxicam nanoparticles. Powder Technol. 2011;212:201–217. doi: 10.1016/j.powtec.2011.05.018. [DOI] [Google Scholar]
- Kürti L, Veszelka S, Bocsik A, Dung NTK, Ózsvári B, Puskás LG, Kittel Á, Szabó-Révész P, Deli MA. The effect of sucrose esters on a culture model of the nasal barrier. Toxicol In Vitro. 2012;26:445–454. doi: 10.1016/j.tiv.2012.01.015. [DOI] [PubMed] [Google Scholar]
- Lechner JF. Interdependent regulation of epithelial cell replication by nutrients, hormones, growth factors, and cell density. Fed Proc. 1984;43:116–120. [PubMed] [Google Scholar]
- Lin H, Yoo JW, Roh HJ, Lee MK, Chung SJ, Shim CK, Kim DD. Transport of anti-allergic drugs across the passage cultured human nasal epithelial cell monolayer. Eur J Pharm Sci. 2005;26:203–210. doi: 10.1016/j.ejps.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Moll R, Krepier R, Franke WW. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983;23:256–269. doi: 10.1111/j.1432-0436.1982.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Moore GE, Sandberg AA. Studies of a human tumor cell line with a diploid karyotype. Cancer. 1964;17:170–175. doi: 10.1002/1097-0142(196402)17:2<170::AID-CNCR2820170206>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Moorhead PS. Human tumor cell line with a quasi-diploid karyotype (RPMI 2650) Exp Cell Res. 1965;39:190–196. doi: 10.1016/0014-4827(65)90022-4. [DOI] [PubMed] [Google Scholar]
- Muendoerfer M, Schaefer UF, Koenig P, Walk JS, Loos P, Balbach S, Eichinger T, Lehr CM. Online monitoring of transepithelial electrical resistance (TEER) in an apparatus for combined dissolution and permeation testing. Int J Pharm. 2010;392:134–140. doi: 10.1016/j.ijpharm.2010.03.046. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Kawaguchi H, Shimizudani T, Shimono T, Kittel Á, Tanaka K, Niwa M. A new blood–brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem Int. 2009;54:253–263. doi: 10.1016/j.neuint.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Otten J, Johnson GS, Pastan I. Regulation of cell growth by cyclic adenosine 3′,5′-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3′,5′-monophosphate levels in fibroblasts. J Biol Chem. 1972;247:7082–7087. [PubMed] [Google Scholar]
- Ózsvári B, Puskás GL, Nagy LI, Kanizsai I, Gyuris M, Madácsi R, Fehér LZ, Gerö D, Szabó C. A cell-microelectronic sensing technique for the screening of cytoprotective compounds. Int J Mol Med. 2010;25:525–530. doi: 10.3892/ijmm_00000373. [DOI] [PubMed] [Google Scholar]
- Perrière N, Yousif S, Cazaubon S, Chaverot N, Bourasset F, Cisternino S, Declèves X, Hori S, Terasaki T, Deli MA, Scherrmann JM, Temsamani J, Roux F, Couraud PO. A functional in vitro model of rat blood–brain barrier for molecular analysis of efflux transporters. Brain Res. 2007;1150:1–13. doi: 10.1016/j.brainres.2007.02.091. [DOI] [PubMed] [Google Scholar]
- Schmidt MC, Peter H, Lang SR, Ditzinger G, Merkle HP. In vitro cell models to study nasal mucosal permeability and metabolism. Adv Drug Deliv Rev. 1998;29:51–79. doi: 10.1016/S0169-409X(97)00061-6. [DOI] [PubMed] [Google Scholar]
- Schmidt MC, Simmen D, Hilbe M, Boderke P, Ditzinger G, Sandow J, Lang S, Rubas W, Merkle HP. Validation of excised bovine nasal mucosa as in vitro model to study drug transport and metabolic pathways in nasal epithelium. J Pharm Sci. 2000;89:396–406. doi: 10.1002/(SICI)1520-6017(200003)89:3<396::AID-JPS10>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Sipos E, Kurunczi A, Fehér A, Penke Z, Fülöp L, Kasza Á, Horváth J, Horvát S, Veszelka S, Balogh G, Kürti L, Erős I, Szabó-Révész P, Párducz Á, Penke B, Deli MA. Intranasal delivery of human beta-amyloid peptide in rats: effective brain targeting. Cell Mol Neurobiol. 2010;30:405–413. doi: 10.1007/s10571-009-9463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solly K, Wang X, Xu X, Strulovici B, Zheng W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;4:363–372. doi: 10.1089/adt.2004.2.363. [DOI] [PubMed] [Google Scholar]
- Szűts A, Láng P, Ambrus R, Kiss L, Deli MA, Szabó-Révész P. Applicability of sucrose laurate as surfactant in solid dispersions prepared by melt technology. Int J Pharm. 2011;410:107–110. doi: 10.1016/j.ijpharm.2011.03.033. [DOI] [PubMed] [Google Scholar]
- Usui S, Shimizu T, Kishioka C, Fujita K, Sakakura Y. Secretory cell differentiation and mucus secretion in cultures of human nasal epithelial cells: use of a monoclonal antibody to study human nasal mucin. Ann Otol Rhinol Laryngol. 2000;109:271–277. doi: 10.1177/000348940010900307. [DOI] [PubMed] [Google Scholar]
- Van Scott MR, Lee NP, Yankaskas JR, Boucher RC. Effect of hormones on growth and function of cultured canine tracheal epithelial cells. Am J Physiol. 1988;255:C237–C245. doi: 10.1152/ajpcell.1988.255.2.C237. [DOI] [PubMed] [Google Scholar]
- Wadell C, Björk E, Camber O. Nasal drug delivery—evaluation of an in vitro model using porcine nasal mucosa. Eur J Pharm Sci. 1999;7:197–206. doi: 10.1016/S0928-0987(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Wadell C, Björk E, Camber O. Permeability of porcine nasal mucosa correlated with human nasal absorption. Eur J Pharm Sci. 2003;18:47–53. doi: 10.1016/S0928-0987(02)00240-3. [DOI] [PubMed] [Google Scholar]
- Wengst A, Reichel S. RPMI 2650 epithelial model and three-dimensional reconstructed human nasal mucosa as in vitro models for nasal permeation studies. Eur J Pharm Biopharm. 2010;74:290–297. doi: 10.1016/j.ejpb.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Werner U, Kissel T. Development of a human nasal epithelial cell culture model and its suitability for transport and metabolism studies under in vitro conditions. Pharm Res. 1995;12:565–571. doi: 10.1023/A:1016210231121. [DOI] [PubMed] [Google Scholar]
- Werner U, Kissel T. In vitro cell culture models of the nasal epithelium: a comparative histochemical investigation of their suitability for drug transport studies. Pharm Res. 1996;13:978–988. doi: 10.1023/A:1016038119909. [DOI] [PubMed] [Google Scholar]
- Wu R, Sato GH, Whitcutt MJ. Developing differentiated epithelial cell cultures: airway epithelial cells. Fundam Appl Toxicol. 1986;6:580–590. doi: 10.1016/0272-0590(86)90170-3. [DOI] [PubMed] [Google Scholar]
- Xia M, Huang R, Witt LK, Southall N, Fostel J, Cho MH, Jadhav A, Smith CS, Inglese J, Portier CJ, Tice RR, Austin CP. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ Health Perspect. 2008;116:284–291. doi: 10.1289/ehp.10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Morita T, Hashida M, Sezaki H. Effect of absorption promoters on the nasal absorption of drugs with various molecular weights. Int J Pharm. 1993;93:91–99. doi: 10.1016/0378-5173(93)90167-E. [DOI] [PubMed] [Google Scholar]
- Yankaskas JR, Boucher RC. Transformation of airway epithelial cells with persistence of cystic fibrosis or normal ion transport phenotypes. Methods Enzymol. 1990;192:565–571. doi: 10.1016/0076-6879(90)92094-T. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Kim KS, Kim SS, Lee JG, Park IY. Secretory differentiation of serially passaged normal human nasal epithelial cells by retinoic acid: expression of mucin and lysozyme. Ann Otol Rhinol Laryngol. 2000;109:594–601. doi: 10.1177/000348940010900612. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Avdeef A, Abbott NJ. In vitro trans-monolayer permeability calculations: often forgotten assumptions. Drug Discov Today. 2003;8:997–1003. doi: 10.1016/S1359-6446(03)02873-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Cell index changes in RPMI 2650 cells treated with retinoic acid (RA, 300 μg/mL), 603 hydrocortisone (HC, 500 nM), 3′-5′-cyclic adenosine monophosphate (cAMP, 250 μM) for 604 24 h in the presence of different concentrations of fetal bovine serum. Cell index is expressed 605 as an arbitrary unit and calculated from impedance measurements between cells and sensors 606 (xCELLigence, Roche). (TIFF 1387 kb)
Supplementary material Cell viability assays on RPMI 2650 cells treated with retinoic acid (RA, 300 μg/mL), 609 hydrocortisone (HC, 500 nM), 3′-5′-cyclic adenosine monophosphate (cAMP, 250 μM). 610 Lactate dehydrogenase (LDH) release assay reflects plasma membrane damage and necrotic 611 cell death (A), MTT dye conversion test shows cell viability based on cytoplasmic and 612 mitochondrial enzyme activity (B). C, control; TX, 1 % Triton X-100. Control conditions: 613 RPMI 2650 cells were grown in Eagle’s minimal essential medium without phenol red 614 supplemented with 10 % foetal bovine serum and 50 μg/mL gentamicin. (TIFF 991 kb)