Abstract
A serum-free medium (CHO-SFM) together with a fed-batch process was developed for the cultivation of a recombinant GS-CHO cell line producing TNFR-Fc. According to the metabolic characteristics of GS-CHO cell, a basal medium was prepared by supplementing DMEM:F12:RPMI1640 (2:1:1) with amino acids, insulin, transferrin, Pluronic F68 and some other ingredients. Statistical optimization approaches based on Plackett–Burman and central composite designs were then adopted to identify additional positive determinants and determine their optimal concentrations, which resulted in the final CHO-SFM medium formulations. The maximum antibody titer reached was 90.95 mg/l in the developed CHO-SFM, which was a 18 % and 10 fold higher than that observed in the commercial EX-CELL™ 302 medium (76.95 mg/l) and basal medium (8.28 mg/l), respectively. Subsequently, a reliable, reproducible and robust fed-batch strategy was designed according to the offline measurement of glucose, giving a final antibody yield of 378 mg/l, which was a threefold improvement over that in conventional batch culture (122 mg/l) using CHO-SFM. In conclusion, the use of design of experiment (DoE) method facilitated the development of CHO-SFM medium and fed-batch process for the production of recombinant antibody using GS-CHO cells.
Keywords: GS-CHO cell, Recombinant antibody, SFM, Design of experiment (DoE), Fed-batch process
Introduction
Etanercept (Enbrel) is a tumor necrosis factor receptor linked to the Fc portion of human immunoglobulin IgG1 (TNFR-Fc fusion protein) to treat rheumatoid arthritis (RA) (Bathon et al. 2000), juvenile RA (Lovell et al. 2000), and psoriatic arthritis (Mease et al. 2000). There has been an ever-increasing demand for TNFR-Fc over the past decade due to its good curative effect to RA (Chen et al. 2006; Klareskog et al. 2011). However, the contradiction between large doses for clinical trials and low capacity to express antibody fusion protein by animal cells limits its therapeutic and diagnostic applications. Therefore, it has become more and more important to enhance production efficiency, reduce production time and cost of TNFR-Fc.
Recombinant Chinese hamster ovary (CHO) cells are widely applied in biopharmaceutical industries for the production of various glycoprotein therapeutics (Merten, 2006). Serum is a rich source of different components such as growth factors, proteins, vitamins, trace elements and hormones essential for the survival and growth of cells (Riebeling et al. 2011). In addition, albumin, fetuin, and other bulk proteins existing in serum can influence the physical properties of the culture system, such as pH, shear stress, viscosity, osmolarity as well as gas delivery rates (Stein 2007). Therefore, serum has been commonly accepted as a key ingredient to maintain and proliferate cells in vitro. However, the use of serum is controversial in consideration of medium safety, variability and cost of animal sera. Development of serum-free medium has become imperative because it can assure consistent quality and productivity, facilitate purification of the proteins to manufacture, give cells better physiological conditions, eliminate the risk of contamination with adventitious agents, better control cell metabolism and provide a good platform for process optimization.
There have been several commercially available serum-free media proposed for cultures of a range of recombinant CHO cell lines (Liu and Chang 2006). However, proprietary and expensive formulations always lead to little room for further improvement and optimization. Moreover, different cell lines may have specific nutritional and metabolic requirements. Consequently, it is well worth the effort to modify batch media and feeding formulas, as well as feeding strategies according to particular cell lines in a systematic methodology.
Medium development by changing one factor at a time is generally laborious, time-consuming and has the risk of neglecting the interactions among supplements (Petiot et al. 2010). Design of experiments (DoE) and statistical analysis, which can test and indentify mixtures of components simultaneously, has therefore been implemented to increase efficiency and reliability of screening and optimization for cell culture media. Various statistical methods, including Plackett–Burman, fractional factorial design combined with the steepest ascent method and response surface methodology (RSM), have been successfully applied for medium optimization and development for different animal cells (Dong et al. 2008; Gonzalez-Leal et al. 2011; Jeon et al. 2010; Lee et al. 1999; Liu et al. 2001; Liu and Wu 2007; Sandadi et al. 2006; Yao et al. 2003).
In order to avoid limited nutrient concentration or excessive metabolic by-product accumulation (such as ammonia and lactate), most attempts to enhance culture productivity have focused on development of feed formulations and feed strategies (Hu et al. 2011; Meghrous et al. 2009; Spens and Häggström, 2007; Zhang et al. 2004). Fed-batch process has become the dominant production technology for therapeutic and recombinant proteins due to its ease of operation, flexibility to be implemented and compatibility with large scale manufacturing (Huang et al. 2010). Antibody expression rate of mammalian cells is usually non-growth associated, so the final product concentration in cultures equals the specific production rate multiplied by the integral of viable cell concentration (IVCC) over culture duration. For instance, IVCC for a GS-CHO cell line increased approximately 5 times with a fed-batch process, leading to a fourfold improvement of TNFR-Fc production (Fan et al. 2009). Additionally, low culture temperature can enhance the specific productivity of TNFR-Fc by retaining cells in G1 phase and delaying the beginning of apoptosis (Kou et al. 2011).
The purpose of this study was to develop a simple and robust chemically defined batch medium as well as feed formulation for a GS-CHO cell using design of experiments (DoE) methods. A Plackett–Burman design was employed to screen active factors for cell growth and antibody production, followed by a central composite design to optimize their concentration. Finally, feeding design was performed based on stoichiometric ratio of different nutrients for the purpose of improving TNFR-Fc production.
Materials and methods
Cell lines and cell culture
The genetically-engineered GS-CHO cells used in this study were purchased from Invitrogen. These GS-CHO cells were developed by transfecting a vector pEGFP-C1 harboring both the glutamine synthetase (GS) and humanized TNFR-Fc genes to a CHO-K1 cell line. TNFR-Fc gene was coupled with GS by stepwise increments in methionine sulfoximine (MSX) level up to 40 μM. The cells were maintained in EX-CELL™ 302 serum-free medium (SFM) and passaged every 2 or 3 days. Cultures were performed at 100 rpm with constant supply of 5 % CO2 at 37 °C.
Media and reagents
All basal media including DMEM, F12 and RPMI1640 were purchased from Gibco and EX-CELL™ 302 serum-free medium were from SAFC. All chemical supplements were obtained from Sigma-Aldrich (St. Louis, MO, USA). All amino acids and vitamins were of analytical reagent quality. Lipid mixture (L5146), consisting of cholesterol 4.5 g/l, cod liver oil fatty acids 10 g/l, polyoxyethylenesorbitan monooleate 25 g/l, and D-α-tocopherol acetate 2 g/l, was purchased from Sigma-Aldrich.
Experimental design and statistical analysis
The Plackett–Burman design (Box et al. 1978) was performed to screen the most important medium components. According to the design matrix shown in Table 2, a total of 20 trials were performed at various combinations of ‘high’ (1) and ‘low’ (−1) values of the different variables. Each row represents one trial and each column represents an independent variable. The last row of (−1) elements is a basic assembly referring to the basal SFM. The effect of each variable (coefficient) on response (cell density or antibody production) is determined by subtracting the average response of the low level (−1) from that of the high level (1), and it is considered to be more significant if the coefficient is relatively large. Furthermore, the variable with positive constant is helpful to the response while the one with negative coefficient implies an inhibitory influence on the response. T and J are dummy variables, which represent imaginary nutrients in Plackett–Burman design and used as the measure of variability to estimate the experimental error. Subsequently, a central composite design (CCD) was performed to determine the optimum levels of significant variables for antibody production. The selected variables (lipid, putrescine, FAC) were investigated at 5 different concentrations (Table 4) and 6 repeats at the center point were used to calculate pure error. Therefore, 20 trials were conducted in CCD. The CCD results were analyzed by fitting a quadratic model and the model adequacy was confirmed using analysis of variance (ANOVA). Design-Expert software (version 7.0.0, Stat-Ease, Minneapolis, MN, USA) was used to experiment design and data analysis. The confidence level for significance was 95 % (p < 0.05).
Table 2.
Matrix and results of the Plackett–Burman experimental design
| A | B | C | D | E | F | G | H | J | K | L | M | N | O | P | Q | R | S | T | Cell density ± SD (106/ml) | Antibody production ± SD (mg/l) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 2.46 (0.12) | 15.87 (0.64) |
| 2 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | 2.32 (0.23) | 28.02 (0.45) |
| 3 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 2.61 (0.20) | 21.50 (0.33) |
| 4 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 2.69 (0.05) | 8.85 (0.12) |
| 5 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | 3.40 (0.13) | 33.08 (0.62) |
| 6 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | 2.82 (0.10) | 26.77 (0.60) |
| 7 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | 2.08 (0.08) | 11.59 (1.24) |
| 8 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | 2.29 (0.01) | 12.18 (0.78) |
| 9 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1.98 (0.06) | 8.70 (0.10) |
| 10 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | 1.91 (0.28) | 11.48 (0.49) |
| 11 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 2.63 (0.28) | 24.51 (0.77) |
| 12 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | 1.87 (0.43) | 11.84 (0.46) |
| 13 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 1 | 3.57 (0.01) | 26.62 (0.97) |
| 14 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 1 | 2.07 (0.20) | 9.74 (0.31) |
| 15 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 1 | 2.74 (0.27) | 25.12 (0.42) |
| 16 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | −1 | 2.53 (0.15) | 21.75 (0.11) |
| 17 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | −1 | 2.47 (0.31) | 22.39 (0.30) |
| 18 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 1 | 2.99 (0.12) | 16.02 (0.48) |
| 19 | 1 | −1 | −1 | 1 | 1 | 1 | 1 | −1 | 1 | −1 | 1 | −1 | −1 | −1 | −1 | 1 | 1 | −1 | 1 | 2.59 (0.11) | 5.61 (0.51) |
| 20 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 1.90 (0.00) | 8.28 (0.53) |
A ethanolamine, 2.5 mg/l; B Sodium selenite, 100nM; C Putrescine, 1 mg/l; D Hydrocortisone, 1 mg/l; E Lipid, 1×; F Sodium pyruvate, 110 mg/l; G Ascorbic acid, 25 mg/l; H Glutathione, 1 mg/l; J Dummy 1; K Choline chloride, 6.25 mg/l; Ld-calcium pantothenate, 2.19 mg/l; M Folic acid, 2.58 mg/l; N Niacinamide, 2.26 mg/l; O Pyridoxine-hydrochloride, 2.27 mg/l; P Riboflavin, 0.26 mg/l; Q Thiamine hydrochloride, 2.33 mg/l; R Cyanocobalamin, 1.05 mg/l; S I-inositol, 8 mg/l; T Dummy 2
1 addition; −1 no addition. Cells were cultured in basal medium supplemented with the indicated ingredients for 5 days
a Standard deviation (SD) was determined in duplicate experiments
Table 4.
Experimental design and results of the central composite design
| A Lipid (×) |
B Putrescine (mg/l) |
C FAC (mM) |
Cell density ± SD (106/ml) | Antibody production ± SD (mg/l) | |
|---|---|---|---|---|---|
| 1 | 0.5 | 0.5 | 0.25 | 3.27 (0.04) | 51.71 (0.10) |
| 2 | 1.5 | 0.5 | 0.25 | 3.54 (0.03) | 49.04 (0.07) |
| 3 | 0.5 | 1.5 | 0.25 | 3.18 (0.01) | 72.17 (0.56) |
| 4 | 1.5 | 1.5 | 0.25 | 3.21 (0.03) | 62.07 (0.01) |
| 5 | 0.5 | 0.5 | 0.75 | 3.16 (0.03) | 53.07 (0.30) |
| 6 | 1.5 | 0.5 | 0.75 | 2.79 (0.17) | 53.72 (0.02) |
| 7 | 0.5 | 1.5 | 0.75 | 3.33 (0.03) | 86.98 (0.29) |
| 8 | 1.5 | 1.5 | 0.75 | 3.53 (0.04) | 84.18 (0.50) |
| 9 | 0.16 | 1 | 0.5 | 2.93 (0.08) | 66.10 (0.24) |
| 10 | 1.84 | 1 | 0.5 | 3.4 (0.10) | 69.03 (0.17) |
| 11 | 1 | 0.16 | 0.5 | 2.53 (0.21) | 35.84 (0.18) |
| 12 | 1 | 1.84 | 0.5 | 3.22 (0.14) | 79.70 (0.04) |
| 13 | 1 | 1 | 0.08 | 3.63 (0.08) | 49.86 (0.34) |
| 14 | 1 | 1 | 0.92 | 3.48 (0.00) | 68.60 (0.10) |
| 15 | 1 | 1 | 0.5 | 3.18 (0.04) | 69.66 (0.10) |
| 16 | 1 | 1 | 0.5 | 3.44 (0.15) | 69.77 (0.09) |
| 17 | 1 | 1 | 0.5 | 3.57 (0.11) | 68.86 (0.66) |
| 18 | 1 | 1 | 0.5 | 3.43 (0.15) | 65.40 (0.76) |
| 19 | 1 | 1 | 0.5 | 3.54 (0.09) | 67.59 (0.01) |
| 20 | 1 | 1 | 0.5 | 3.59 (0.15) | 61.80 (0.32) |
Standard deviation (SD) was determined in duplicate experiments
Experiments were performed in 125 ml Erlenmeyer flasks (Corning, Corning, NY, USA) in duplicate according to the design matrix. The culture was inoculated at a concentration of 2–3 × 105 cells/ml and harvested after 120 h of cultivation. Cell concentration and viability were determined by trypan blue exclusion method with a hemacytometer. Samples were centrifuged and stored at −20 °C for further analysis.
Feed medium, bioreactor protocol and equipment
The feed medium was prepared in a manner similar to that described by Wlaschin and Hu (2006). The feed rates of amino acids and phosphorus were determined by calculating the uptake rates of previous batch processes. For immeasurable substances including lipids, sodium selenite, ethanolamine, putrescine, vitamins and some trace elements existing in CHO-SFM, they were supposed to be depleted at the time when an increase in the death rate was observed (Spens and Häggström 2007). Then an approximately supplementing rate of each compound can be determined in the subsequent fed-batch. Overall, this feeding solution consisted of 41 ingredients as summarized in Table 6.
Table 6.
Supplementations made to the feed solution
| Feed supplementation | Concentration (mg/l) | Feed supplementation | Concentration (mg/l) |
|---|---|---|---|
| Glucose | 100000.00 | Glutamine | 4140.00 |
| Amino acids | Vitamins | ||
| l-aspartic acid | 3904.27 | Biotin | 1.44 |
| l-threonine | 3411.33 | Choline chloride | 520.83 |
| l-serine | 1569.17 | d-calcium pantothenate | 116.39 |
| l-cysteine | 1575.30 | Folic acid | 106.39 |
| l-valine | 1937.00 | Niacinamide | 62.75 |
| l-methionine | 1754.89 | Pyridoxine hydrochloride | 62.92 |
| l-isoleucine | 1790.33 | Riboflavin | 7.20 |
| l-leucine | 3187.67 | Thiamine hydrochloride | 64.58 |
| l-tyrosine | 1137.47 | Vitamin B12 | 38.89 |
| l-phenylalanine | 2007.50 | i-Inositol | 500.00 |
| l-lysine | 2144.12 | Inorganic salts | |
| l-cysteine | 853.33 | Sodium selenite | 0.56 |
| l-arginine | 2196.86 | ZnSO4·7H2O | 22.22 |
| l-proline | 1929.44 | CuSO4·5H2O | 0.07 |
| l-tryptophan | 1541.33 | Na2HPO4 | 6200.56 |
| l-glutamate | 4083.33 | Other additives | |
| l-glycine | 520.83 | Ethanolamine | 69.44 |
| Lipid | Putrescine | 41.67 | |
| Cholesterol | 62.505 | Insulin | 138.89 |
| Cod liver oil fatty acids | 138.9 | Glutathione | 27.78 |
| Tween 80 | 347.25 | ||
| d-α-tocopherol acetate | 27.78 | ||
The feed solution was based on the CHO-SFM medium to which the components listed were added. Supplemental substances were composed of amino acids, vitamins, lipids, inorganic salts and other substances
The feeding protocol was based on maintaining an expected post-feed glucose level during the culture. Sampling and feeding were performed twice a day with an approximate time interval of 8 h. Glucose analysis was performed immediately after sampling and the elapsed time of feed medium addition was less than 30 min. The volume of feed medium (Vfeed) was calculated by the following equation:
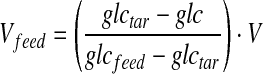 |
where glctar is the post-feed target glucose concentration, glc is the glucose concentration prior to feeding, glcfeed is glucose concentration in the feed medium, and V is the culture volume before addition. Post-feed target glucose concentration was typically controlled at a concentration of 2 g/l (11 mmol/l). In order to determine the appropriate time to add the immeasurable substances, samples were collected every 4 h during the cultivation period and the cell concentration was immediately analyzed. If an obvious cell death rate with a value exceeding 50 % was observed, the immeasurable substances were then added.
Batch and fed-batch cultures were carried out in 2-l round-bottomed bioreactors (Electrolab Ltd., Tewkesbury, UK) with a starting volume of 1 l. Exponentially growing cells were inoculated in suspension at 2–3 × 105 cells/ml. The culture set points were pH of 7.0, DO of 40 % air saturation, temperature of 37 °C and agitation of 100 rpm.
Analytical methods
The glucose, lactate, glutamine, glutamate and ammonium concentrations in the culture supernatant were determined with a BioProfile 400 analyzer (NOVA Biomedical, Waltham, MA, USA). Amino acids were analyzed by reverse phase HPLC according to AccQ Tag method following manufacturer’s instructions (Waters, Milford, MA, USA). Phosphorus was measured with molybdophosphoric acid analysis method (deZengotita et al. 2000). The antibody concentration was determined by a sandwich enzyme linked immunosorbent assay (Huang et al. 2007). Osmolality was measured on the auto freezing-point osmometer.
Results
Basal medium
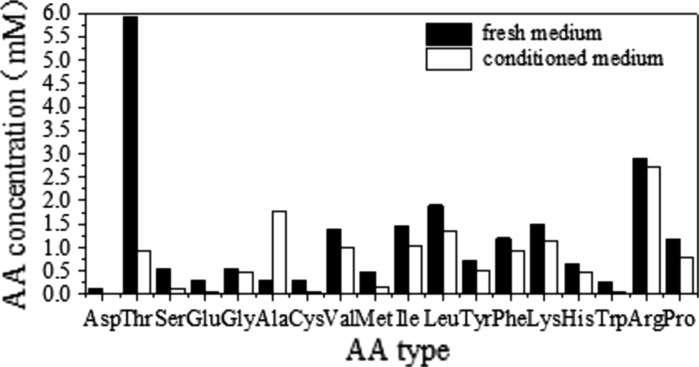
According to the literature survey and results of preliminary experiments, a basal SFM was formulated on the basis of DMEM:F12:RPMI1640 (2:1:1) with various supplements (Table 1). It is believed that amino acid utilization is specific for each cell type, culture condition and biological product. Therefore, analysis of condition medium can offer many advantages for developing a serum-free medium. As shown in Fig. 1, it is clearly demonstrated that several nutrients including l-asp, l-thr, l-ser, l-glu, l-cys, l-met, l-trp were significantly consumed after the cultivation for 5 days, while l-ala exhibited a net increase. According to the amino acid consumption profiles, the amino acids listed in Table 1 were employed as components added to the basal medium.
Table 1.
Composition of the basal SFM
| Components | Concentration (mg/l) | Components | Concentration (mg/l) |
|---|---|---|---|
| DMEM/F12/RPMI1640 = 2:1:1 supplemented with | |||
| Insulin | 5 | l-Aspartic acid | 20 |
| Transferrin | 5 | l-Threonine | 180 |
| NaHCO3 | 16.00 | l-Serine | 25 |
| Pluronic F68 | 1.000 | l-Glutamate | 40 |
| Glucose | 2.000 | l-Cystine | 30 |
| l-Glutamine | 219 | l-Valine | 50 |
| KCl | 223.5 | l-Methionine | 50 |
| CuSO4·5H2O | 0.0025 | l-Isoleucine | 40 |
| ZnSO4·7H2O | 0.8 | l-Leucine | 60 |
| Dextran sulfate | 50 | l-Tyrosine | 40 |
| Adenosine | 7 | l-Phenylalanine | 60 |
| Guanosine | 7 | l-Lysine | 50 |
| Thymidine | 0.24 | l-Histidine | 30 |
| Cytidine | 7 | l-Tryptophan | 30 |
| Uridine | 7 | l-Proline | 70 |
Fig. 1.
Amino acid utilization profiles of recombinant CHO cells grown in EX-CELL™ 302 medium. CHO cells were seeded at 2–3 × 105 cells/ml and conditioned medium was sampled after 5 days of incubation
Additives screening
A Plackett–Burman design was applied in order to identify the important medium components from a long list of candidate factors. Ethanolamine, sodium selenite, putrescine, hydrocortisone, lipid mixture, sodium pyruvate, glutathione and 10 water-soluble B vitamins were selected to evaluate their effects on CHO cells based on their potential growth promoting abilities to various cells. The treatment combinations and observed responses are presented in Table 2. Statistical analysis of the responses is shown in Table 3. The model F values for cell growth and antibody production are 203.4 and 14.9, implying that both of the two models are significant. Moreover, the values of p < 0.05 indicate that model terms are significant. Both positive and negative variables were found in this analysis. Ethanolamine, putrescine, lipid mixture, choline chloride, d-calcium pantothenate and sodium pyruvate were indentified as having stimulatory effects on cell growth. Among these positive variables, putrescine (p < 0.01) was important for antibody production. However, other nutrients, such as sodium selenite, ascorbic acid, glutathione, riboflavin and inositol, were supposed to inhibit the cell growth (Table 3). Only positive variables having stimulatory effects on cell growth and/or protein production were considered for further optimization. The effect of the dummy variables should ideally be zero. Effect of T and J (Table 3) is assumed to be experimental inaccuracy and analytical error in measuring the response, and it could also reflect the interaction between variables, which is not accounted for by Plackett–Burman analysis (Castro et al. 1992). Finally, the components added to the basal medium were determined as follows: ethanolamine (2.5 mg/l), putrescine (1 mg/l), lipid mixture (1×), sodium pyruvate (110 mg/l), choline chloride (6.25 mg/l), d-calcium pantothenate (2.19 mg/l).
Table 3.
Statistical analysis results for the Plackett–Burman experiment
| Factor | Cell density (106/ml) | Antibody production (mg/l) | ||||
|---|---|---|---|---|---|---|
| Coefficient | F value | Prob > F | Coefficient | F value | Prob > F | |
| Intercept | 2.71E + 00 | 203.40 | 0.0049 | 1.75E + 01 | 14.90 | 0.0647 |
| Ethanolamine | 9.90E − 02 | 135.19 | 0.0073 | −6.68E − 01 | 1.81 | 0.3111 |
| Sodium selenite | −5.50E − 02 | 41.72 | 0.0231 | 1.32E + 00 | 7.02 | 0.1178 |
| Putrescine | 3.39E − 01 | 1585.12 | 0.0006 | 7.08E + 00 | 202.73 | 0.0049 |
| Hydrocortisone | −2.70E − 02 | 10.06 | 0.0867 | −1.89E + 00 | 14.45 | 0.0628 |
| Lipid | 2.52E − 01 | 875.92 | 0.0011 | 2.37E − 01 | 0.23 | 0.6808 |
| Sodium pyruvate | 5.30E − 02 | 38.74 | 0.0249 | 2.79E − 01 | 0.31 | 0.6316 |
| Ascorbic acid | −1.07E − 01 | 157.92 | 0.0063 | 2.09E − 01 | 0.18 | 0.7146 |
| Glutathione | −4.90E − 02 | 33.12 | 0.0289 | −3.18E − 01 | 0.41 | 0.5880 |
| Dummy (J) | −2.2E − 02 | 3.44E − 01 | ||||
| Choline chloride | 1.45E − 01 | 290.00 | 0.0034 | 5.77E − 01 | 1.35 | 0.3657 |
| d-calcium pantothenate | 1.22E − 01 | 205.30 | 0.0048 | 1.13E + 00 | 5.16 | 0.1510 |
| Folic acid | 1.00E − 03 | 0.01 | 0.9172 | 1.72E + 00 | 12.01 | 0.0741 |
| Niacinamide | −9.00E − 03 | 1.12 | 0.4013 | 6.48E − 01 | 1.70 | 0.3224 |
| Pyridoxine hydrochloride | −5.00E − 03 | 0.34 | 0.6165 | 4.07E − 01 | 0.67 | 0.4992 |
| Riboflavin | −4.10E − 02 | 23.19 | 0.0405 | −9.21E − 01 | 3.43 | 0.2052 |
| Thiamine hydrochloride | −2.50E − 02 | 8.62 | 0.0991 | −5.26E − 01 | 1.12 | 0.4012 |
| Cyanocobalamin | 1.90E − 02 | 4.98 | 0.1553 | 4.07E − 01 | 0.67 | 0.4991 |
| I-inositol | −5.80E − 02 | 46.40 | 0.0209 | 3.44E − 02 | 0.00 | 0.9512 |
| Dummy (T) | 0 | −1.37E + 00 | ||||
Variables with positive effects are shown in bold
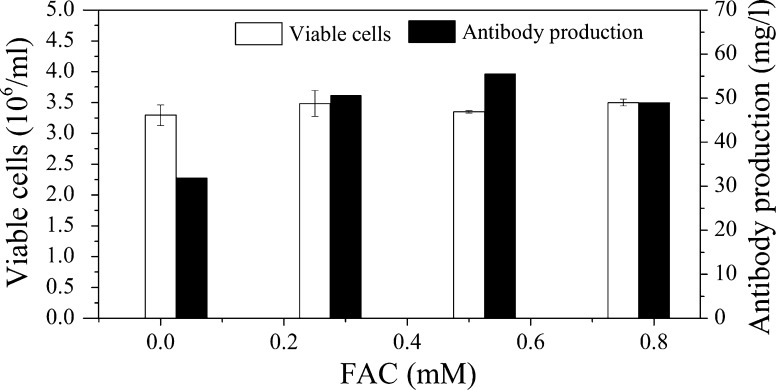
Effects of iron on cell growth and expression
Iron is crucial to maintain the growth of healthy cells. Therefore, the effects of ammonium ferric citrate (FAC) on the proliferation of GS-CHO cells was investigated at 120 h and the results are shown in Fig. 2. From Fig. 2, we concluded that FAC had little effect on cell growth, but significantly increased protein expression. When the concentration of FAC was fixed at 0.5 mM, the antibody yield reached a maximum with a value of 52.8 mg/l, which was similar to that (54 mg/l) in medium with the addition of transferrin and 82% higher than that (29 mg/l) in the reference medium without the addition of FAC. Therefore, FAC can be used as an alternative to instead transferrin and was selected for further study due to its positive effect on antibody production.
Fig. 2.
Effects of FAC on the growth and productivity of CHO cells. The basal medium was the formulation obtained from Plackett–Burman design
Optimization experiments
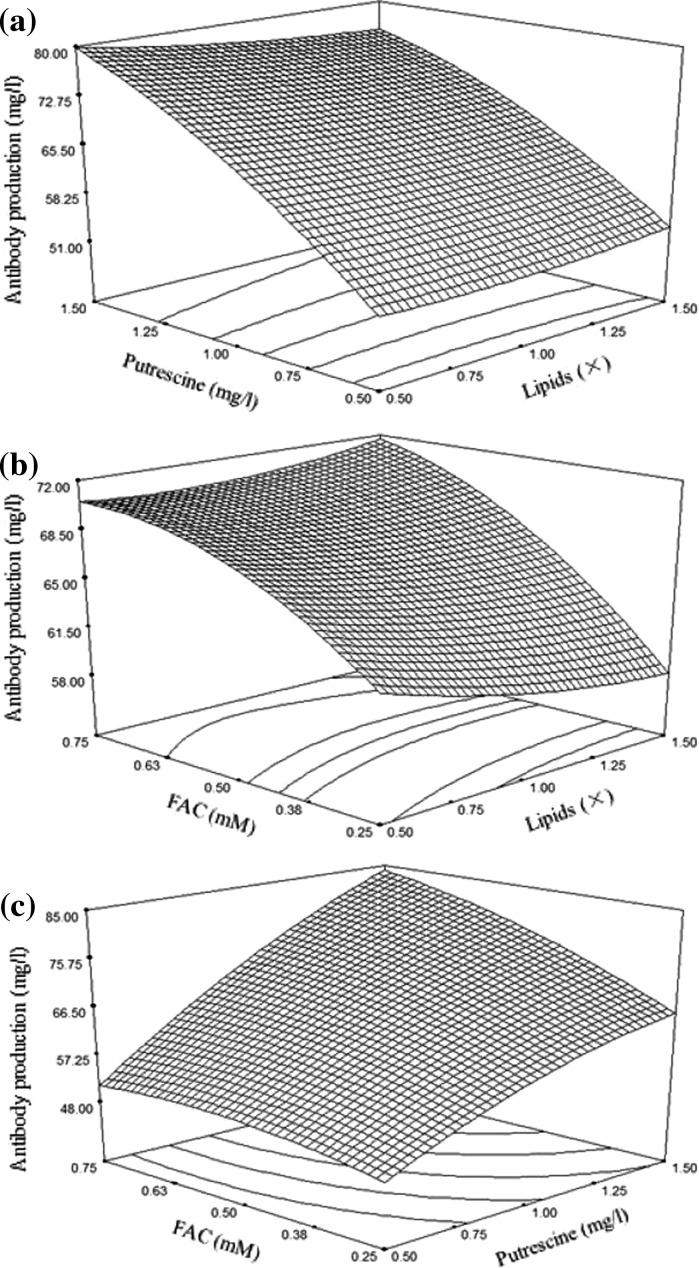
From the results of screening experiments, lipid, putrescine and FAC were selected as significant factors and their optimal concentrations for antibody production were obtained using the central composite design. The design matrix and the experimental results are given in Table 4. The second-order polynomial model for antibody production obtained by the regression analysis using Design-Expert 7.0.0 software is described as follows:
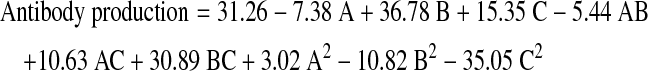 |
where A is lipid, B is putrescine, and C is FAC. The significance of this model (Table 5) was analyzed using ANOVA. The null hypothesis of the F test was zero. The p value of the F test can determine whether the null hypothesis will be rejected. The smaller the p value (less than 0.05), the stronger the evidence against the null hypothesis. The F value for the quadratic equation is 30.8, indicating that the second-order response surface model was significant at 0.01 % level (Table 5). The determination coefficient (R2) for the quadratic equation is 0.9652, which represents a very good fitness between the experimental results and the theoretical values predicted by the model. Besides, the lack of fit value of 1.18 implies that the model was not influenced by the error of lack of fit (p > 0.05). As denoted in Table 5, the lipid (B) and FAC (C) had significant linear effects and quadratic effects (B2 and C2), while interaction between B and C was also strong for antibody production. According to the model, the maximum antibody production was predicted to be 85.59 mg/l when the concentrations of lipid mixture, putrescine and FAC were 0.5×, 1.5 mg/l and 0.75 mM, respectively. The influence of lipid, putrescine and FAC on antibody production was visualized by virtue of three-dimensional response surface curves (Fig. 3). In order to confirm the accuracy of the model, three additional shake flask experiments using the predicted medium concentrations were performed. The mean value of antibody production was 90.46 ± 4.71 mg/l, which was close to the theoretically predicted value (85.59 mg/l), indicating that the model was adequate for obtaining the optimal value in the range of studied parameters.
Table 5.
Analysis of variance for the second-order regression model of antibody production
| Source | Sum of squares | df | Mean square | F value | Prob > F |
|---|---|---|---|---|---|
| Model | 2899.11 | 9 | 322.12 | 30.80 | <0.0001 |
| A | 7.31 | 1 | 7.31 | 0.70 | 0.4226 |
| B | 2157.07 | 1 | 2157.07 | 206.22 | <0.0001 |
| C | 406.21 | 1 | 406.21 | 38.83 | <0.0001 |
| AB | 14.80 | 1 | 14.80 | 1.42 | 0.2617 |
| AC | 14.12 | 1 | 14.12 | 1.35 | 0.2722 |
| BC | 119.24 | 1 | 119.24 | 11.40 | 0.0070 |
| A2 | 8.23 | 1 | 8.23 | 0.79 | 0.3959 |
| B2 | 105.54 | 1 | 105.54 | 10.09 | 0.0099 |
| C2 | 69.14 | 1 | 69.14 | 6.61 | 0.0278 |
| Residual | 104.60 | 10 | 10.46 | ||
| Lack of fit | 56.65 | 5 | 11.33 | 1.18 | 0.4296 |
| Pure error | 47.95 | 5 | 9.59 | ||
| Cor total | 3003.71 | 19 |
Model, error and total are three sources of variation in the regression analysis. Total is the total sum of squares corrected for the mean, and it is the sum of model and error. Mean square is the sum of squares divided by its degrees of freedom. The F value is the ratio of the mean square for the model to that for error
Fig. 3.
Response surface plot showing the effects of 2 different variables and their interaction on the response (Antibody production) while the third variable was held at zero level
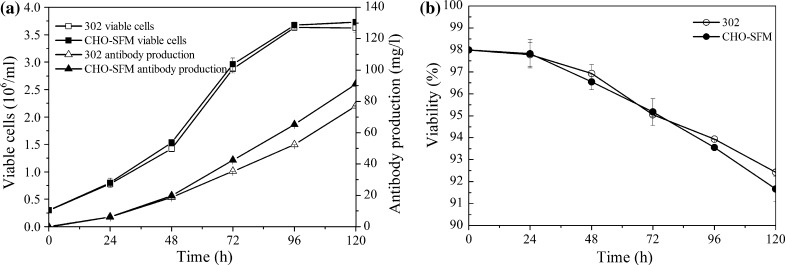
In order to make a comparison between the optimized CHO-SFM and EX-CELL™ 302 medium in regard to cell growth and antibody production, CHO cells were cultivated in both media in shake flasks for 5 days, respectively (Fig. 4). The maximum viable cell concentration obtained in the CHO-SFM medium was 3.73 × 106 cells/ml, which is similar to that in EX-CELL™ 302 medium (3.64 × 106 cells/ml). However, antibody production in the CHO-SFM medium was 90.95 mg/l, which is approximately 18 % higher than that in EX-CELL™ 302 medium (76.95 mg/l). This superiority is a consequence of higher antibody productivity per cell in CHO-SFM medium.
Fig. 4.
Comparison between the optimized CHO-SFM and EX-CELL™ 302 medium in shake flasks for a 5 day batch culture. a Cell growth and TNFR-Fc production; b cell viability
Functional assays of cells cultures in the developed SFM
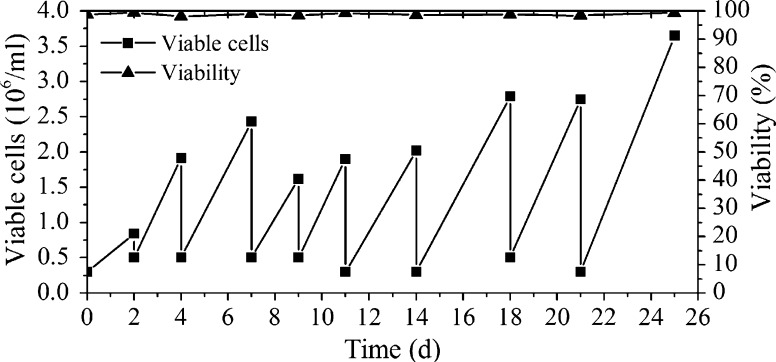
A valuable medium can not only support high cell density and protein production, but also has to possess the ability to propagate cells repeatedly and maintain cell characteristics in the long term. A common approach to characterize adaptability of a cell line is to observe its stability after subculture in the designed medium for several days. Therefore, cells producing TNFR-Fc were inoculated with a density of 3 × 105 cells/ml in a 125 ml Erlenmeyer flask with 30 ml of the developed CHO-SFM and subcultured every few days. As shown in Fig. 5, cell viability was maintained at approximately 98 % and cell specific growth rate was around 0.025 day−1. Additionally, antibody specific production rate was about 8 mg/109 cells/day. These results clearly suggested that the cells can continuously grow and maintain high cell viability in the developed CHO-SFM.
Fig. 5.
Functional assays of cells cultured in the developed SFM. Cells were inoculated at a density of 3 × 105 cells/ml and subcultured every few days
Batch culture in bioreactor
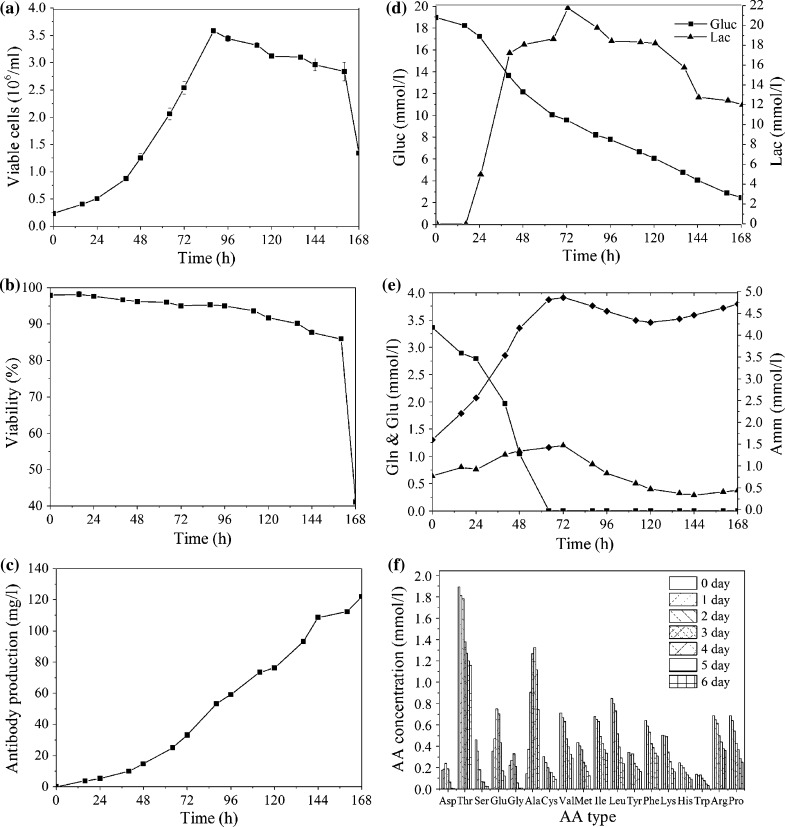
A representative bioreactor batch cultivation of GS-CHO cells in a 2 l scale using CHO-SFM was conducted to provide a performance baseline (Fig. 6). The number of viable cells increased gradually and reached the maximum of 3.6 × 106 cells/ml with a viability of 95 % at 88 h, which immediately dropped to approximately 40 % at the end of the culture (Fig. 6a, b). The final antibody concentration was around 122 mg/l (Fig. 6c). Cell died quickly at the end of the culture due to the deficiency of glucose or other substrates. Lactate concentration increased in the exponential phase and turned to utilization when glucose was consumed to a limiting concentration of about 9 mM (Fig. 6d). Glutamine was completely exhausted prior to cessation of proliferation (Fig. 6e). Ammonia concentration increased to 4.86 mM during cell culture and began to reduce at the later stage of cell growth due to the role of glutamine synthetase (Fig. 6e). The data on the concentration over time for 18 amino acids are presented in Fig. 5f, which clearly showed that l-asp, l-ser, l-cys, l-met, l-leu and l-trp were consumed at high rates (>70 %). On the contrary, alanine was accumulated during the culture period. Moreover, the amino acids with similar properties, such as leucine, phenylalanine and glutamine, may require higher amounts because they share the same amino acid transporter in the cell membrane. Although the nonessential amino acids can be synthesized using other amino acids in the medium as precursors, the addition of nonessential amino acids can reduce energy requirement for cell growth and antibody production. Hence, the balanced supplementation of amino acids to match their consumption and other needs appeared considerably important.
Fig. 6.
Batch culture. GS-CHO cells were cultured using CHO-SFM in a 2 L bioreactor with an inoculation of 2.3 × 105 cells/ml. a Cell growth; b cell viability; c antibody production; d metabolism of glucose and lactate; e concentration profile of glutamine, glutamic acid and ammonia; f concentrations of amino acids at 0, 24, 48, 72, 96, 120 and 144 h
Fed-batch culture in bioreactor
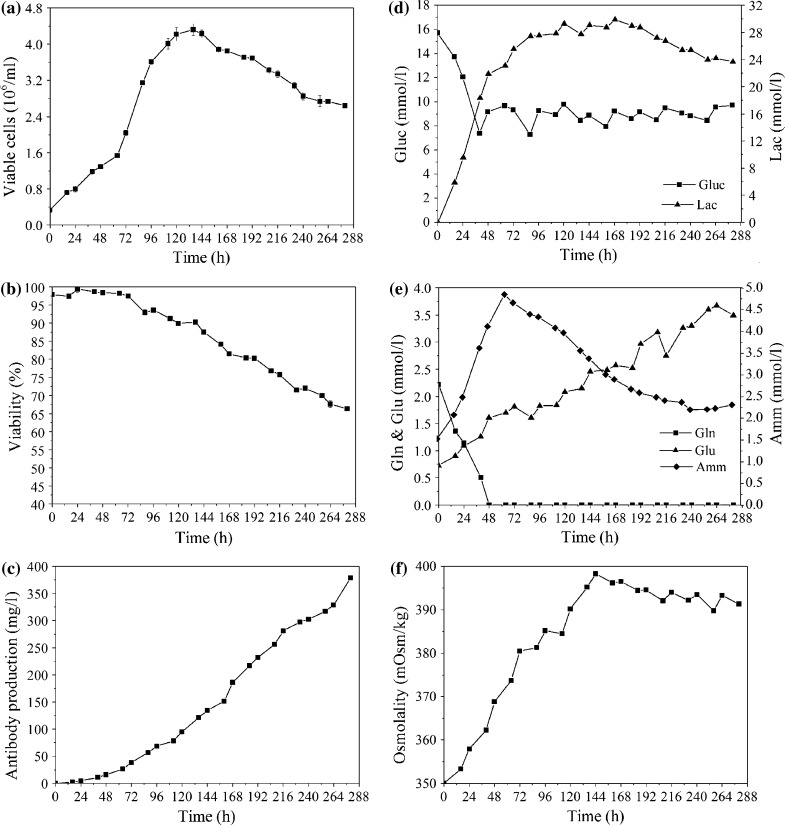
Sufficient substrate level and reduced byproduct accumulation can prolong cell culture period, increase cell density and enhance antibody expression. Therefore, a simple feeding protocol, which linked the addition of feed medium to the culture’s glucose concentration, was designed. Based on the conclusion obtained from the batch cultures (data not shown), the concentrations of residual glucose should be maintained about 11 mmol/l (2 g/l). The rational designed feeding components comprised of amino acids, vitamins, lipids, inorganic salts and some other chemicals are listed in Table 6. The maximal viable cell density reached 4.3 × 106 cells/ml, and the accumulated antibody concentration was 378 mg/l at the 11.5 days (Fig. 7a, c), which was a threefold improvement relative to that of 122 mg/l in batch culture. The waste products such as lactic acid and ammonia accumulated in the fed-batch cultures reached a maximum concentration of 29 mmol/l and 4.85 mmol/l, respectively (Fig. 7d, e). The final ammonia concentration was 2.31 mmol, which was lower than that (4.71 mmol) in batch cultures. The final osmolality of 391 Osm/kg implied a good control of nutrient supplements and lactate production (Fig. 7f). Product quality was also proven to be fine according to sialic acid content and glycosylation profile (data not shown). These results indicated that the nutritional environment was well controlled under this feeding strategy.
Fig. 7.
Fed-batch culture. GS-CHO cells were cultured in a 2-L bioreactor using the designed CHO-SFM and feed medium. a Cell growth; b cell viability; c antibody production; d metabolism of glucose and lactate; e concentration profile of glutamine, glutamic acid, and ammonia; f osmolality
Discussion
Serum-free formulations have evolved from classical basal media, such as DMEM, DMEM/F12, RPMI or IMDM, by supplementing with various beneficial factors. In order to develop a serum-free medium used for the recombinant CHO cells to produce antibody, a basal SFM was adopted and its detailed components are listed in Table 1. Insulin can stimulate cell growth and cell cycle progression, regulate metabolism of glucose and lipid, as well as increase biosynthesis of fatty acids and nucleic acids (Abdeen et al. 2011). Pluronic F68, an important medium additive for protecting suspension cells against shear damage, was elaborated in detail for its beneficial effects on CHO cell growth, metabolism, production and glycosylation of human recombinant IFN-c (Clincke et al. 2011). Dextran sulfate is an effective dispersing agent for blocking aggregation and inducing single cell suspension under serum-free condition. Therefore, insulin and Pluronic F68 were selected as important additives added to basal SFM. It is known that dextran sulfate is an effective dispersing agent for blocking aggregation and inducing single cell suspension under serum-free condition. Our results demonstrated that cell clumps were significantly prevented with the addition of dextran sulfate 5000 at 50 mg/l. However, cell viability and antibody concentration slightly decreased when the concentration of dextran sulfate exceeded 50 mg/l. It is likely that excessive supplementation of dextran sulfate was toxic to the cells, which is in agreement with previous reports (Kim et al. 2006). Moreover, the toxicity of dextran sulfate the increase of the molecular weight (Dee et al. 1997). Amino acids are considered as the most important factors for both cell growth and production because they can protect cells from nutrient depletion, elevated osmolarity, elevated pCO2 (Parampalli et al. 2007) and act as buffers of intracellular pH. Therefore, amino acids were usually added to the medium as critical factor. For example, it has been reported that balanced amino acid supplements could support immediate, stable and good growth of microencapsulated recombinant CHO cells (Lv et al. 2008).
Plackett–Burman statistical design enabled an initial screening of numerous possible influencing factors, minimizing the number of required runs. The resulting data allowed a clear ranking of medium component effects. Among 20 candidates, ethanolamine, putrescine, lipid mixture, sodium pyruvate, choline chloride and d-calcium pantothenate were selected as active factors for further optimization. Ethanolamine, a structural component of phospholipids in mammalian cell membrane and a precursor for numerous enzymes and cofactors (Spens and Häggström 2007), has been shown to play a positive role in promoting cell growth (Table 3), which is consistent with the previous report that ethanolamine is an essential component for hybridoma cell culture in serum-free medium (Murakami et al. 1982). It was demonstrated that sodium pyruvate could increase cell density in this study. Supplementing media with pyruvate can supply substrates directly to the tricarboxylic acid (TCA) cycle to provide enough energy for cell growth and help the cells to bypass glycolysis. Subsequently, the reduced glycolytic flux led to a decreased production rate of toxic lactate. As the rate of lactate production diminished, delayed or reduced inhibitory effect of lactate on cell growth could result in prolonged cell growth and then lead to a higher cell density. For instance, it has been reported that addition of sodium pyruvate successfully increased CHO cell density by reducing glucose consumption and lactate accumulation (Chen et al. 2000). Additionally, both choline chloride and d-calcium pantothenate were indentified as enhancers of cell growth. Vitamins are involved in carbohydrate, lipid, protein and nucleic acid metabolism as cofactors and coenzymes related enzyme systems. Previous researchers have reported that vitamin depletion can limit cell growth while modification of vitamin concentration can enhance cell growth and production (Kim et al. 2005). Choline is a constituent of phospholipids and the deficiency of external choline chloride may have impaired membrane lipid composition. Pantothenic acid is involved in the biosynthesis of coenzyme A cofactor incorporated in enzymatic reactions, which is responsible for establishing the crucial connection between glycolysis and the TCA cycle (Ishaque and Al-Rubeai 2002).
Response surface methodology (RSM) is an effective experimental strategy for seeking the optimum conditions in a multivariable system. Lipid, putrescine and FAC were selected for further optimization by CCD. As shown in Table 5, putrescine and FAC had significant effects (p < 0.0001) while lipid was found to be non-significant for antibody production. These results were consistent with that in screening experiment. Putrescine is one of the polyamines which are cellular constituents shown to be essential for a variety of cell processes related to growth and differentiation, such as DNA replication, transcription and translation (Janne et al. 2004). It has been widely utilized as an additive in the development in serum-free media and various literature references have reported its prominent effect on cell growth and protein expression (Jeon et al. 2010; Kim et al. 2006). In addition, there have been reports that polyamines enhance IgM productivity of the hybridoma cells in spite of their growth suppression activity (Sugahara et al. 2008). It may be that amino groups in polyamines contribute to enhancement of antibody production of CHO cells, which need to be further investigated. Exogenous lipids or their precursors are required for cell proliferation and product synthesis, because they serve as energy stores and structural constituents of cellular membranes belonging to transport and signaling systems (van der Valk et al. 2010). There have been detailed reports about effects of lipids (cholesterol, cod liver oil, vitamin E and Tween-80) on insect cell growth and expression of recombinant proteins in serum-free medium (Gilbert et al. 1996). In our study, lipids were identified as the most important factor for cell growth, however, it has non-significant effect on the antibody production. Iron plays a very important role in supporting cell growth. The limitation of iron leads to poor cell growth and eventual cell death, while excessive iron may have potential cytotoxicity. Transferrin, a widely used but expensive growth factor, plays an important role in ferric ion delivery and also acts as an extracellular antioxidant (Knöspel et al. 2010). It can be substituted by other synthetic iron chelator and chemically defined small molecule delivery systems, such as tropolone and FAC, selenite, ferric citrate and vitamin C and other iron agents (Rourou et al. 2009; Zhang and Robinson, 2005; Zhang et al. 2006). Our results demonstrated that the iron compound FAC was an important factor for the increased antibody titer. Recently, it has been reported that FAC supplementation of the culture medium can increase monoclonal antibody production of CHO cell (Bai et al. 2011). We speculate that better cell morphology and larger cell size observed under microscope contributed to the enhanced titer.
The CHO-SFM was developed based on the responses (cell density and antibody production) of Plackett–Burman and CCD, which were measured at 120 h of cultivation. In order to validate the effectiveness of CHO-SFM, process duration in shake flask batch culture was 120 h. While in bioreactor, cells were cultured for 168 h until cell viability decreased dramatically.
The simple feeding strategy using the concentrated nutrient solutions and the daily adjustment of the feeding amounts has been shown to be effective to increase cell density and extend the culture lifespan, leading to a threefold improvement in antibody production for GS-CHO cells. In cell culture, high ammonium levels may deplete cell energy, induce apoptosis and alter glycosylation patterns of the recombinant proteins (Spens and Häggström, 2007). GS-engineered cell lines can synthesize glutamine from glutamate and ammonia, resulting in a reduction of ammonia concentration. Therefore, development of a fed-batch process in our study was aimed at providing sufficient nutritional supplements and minimizing lactate accumulation, which in turn restrict osmolality increase. Different glucose levels in the broth resulted in different glucose uptake rates, which can significantly affect cell growth and antibody production. In our previous experiments, we have found that cell growth and antibody production were limited when the concentration of glucose was lower than 5 mmol/l, while increased glucose level (over 15 mmol/l) resulted in significant lactate production and high overall lactate/glucose yield coefficients. When glucose was controlled at 2 g/l (11 mmol/l), not only the specific growth rate stayed constant but also lactate production was relatively low. In our study, the improvement in the final antibody concentration was due to an increase not only in IVCC but also specific antibody production rate, which was 11.44 mg/109 cell/day, 47 % higher compared to batch culture (7.78 mg/109 cell/day).
Screening of new small molecule compounds (such as DMSO, sodium butyrate) as medium additives has also been reported to increase titer and productivity (Ma et al. 2008; Rodrigues Goulart and Arthuso 2010). In addition, proliferation control strategies, including temperature shift to conditions of mild hypothermia, the use of apoptosis inhibitors and cell engineering-based approach to inhibit apoptosis, were also investigated to achieve improved culture longevity, and thus, enhanced productivity (Huang et al. 2010).
Amino acids, vitamins, lipids, hormones, antioxidants and other components have been provided sufficiently in the fed-batch culture. It is not likely that ammonia, lactate and osmolality were the factors causing limitation or inhibition. The actual cause of the cessation for cell growth or death may be mutifactorial and/or unique to each process, which need to be characterized further.
Conclusion
A costeffective serum-free medium has been developed according to standard procedure of DoE and it has been proved to support immediate, stable, good growth and production of GS-CHO cell. Subsequently, a simple and reliable feeding protocol was designed based on stoichiometric ratio of different nutrients, which yielded volumetric productivities ranging from 120 to 380 mg/l. In conclusion, these results demonstrate the importance of rational and systematic design for CHO cell media and process developments.
Acknowledgments
We are grateful for the financial supports from the National Key Project for Basic Research (No. 2010CB1126102).
References
- Abdeen SH, Abdeen AM, EI-Enshasy HA, Shereef AAE (2011) HeLa-S3 cell growth conditions in serum-free medium and adaptability for proliferation in suspension culture. J Biol Sci 11:124–134
- Bai Y, Wu C, Zhao J, Liu YH, Ding W, Ling WLW. Role of iron and sodium citrate in animal protein-free CHO cell culture medium on cell growth and monoclonal antibody production. Biotechnol Prog. 2011;27:209–219. doi: 10.1002/btpr.513. [DOI] [PubMed] [Google Scholar]
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, Genovese MC, Wasko MC, Moreland LW, Weaver AL, Markenson J, Finck BK. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- Box GEP, Hunter WG, Hunter JS. Fractional factorial design at two levels. In: Statistics for experimenters. New York: Wiley; 1978. pp. 374–418. [Google Scholar]
- Castro PML, Hayter PM, Ison AP, Bull AT. Application of a statistical design to the optimization of culture medium for recombinant interferon-gamma production by Chinese hamster ovary cells. Appl Microbiol Biot. 1992;38:84–90. doi: 10.1007/BF00169424. [DOI] [PubMed] [Google Scholar]
- Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, FrySmith A, Burls A. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10:1–229. doi: 10.3310/hta10420. [DOI] [PubMed] [Google Scholar]
- Chen Z, Iding K, Lütkemeyer D, Lehmann J. A low-cost chemically defined protein free medium for a recombinant CHO cell line producing prothrombin. Biotechnol Lett. 2000;22:837–841. doi: 10.1023/A:1005665530028. [DOI] [Google Scholar]
- Clincke MF, Guedon E, Yen FT, Ogier V, Roitel O, Goergen JL. Effect of surfactant pluronic F-68 on CHO cell growth, metabolism, production, and glycosylation of human recombinant IFN-gamma in mild operating conditions. Biotechnol Prog. 2011;27:181–190. doi: 10.1002/btpr.503. [DOI] [PubMed] [Google Scholar]
- Dee KU, Shuler ML, Wood HA. Inducing single-cell suspension of BTI-TN5B1-4 insect cells: I. The use of sulfated polyanions to prevent cell aggregation and enhance recombinant protein production. Biotechnol Bioeng. 1997;54:191–205. doi: 10.1002/(SICI)1097-0290(19970505)54:3<191::AID-BIT1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- deZengotita VM, Miller WM, Aunins JG, Zhou WC. Phosphate feeding improves high-cell-concentration NS0 myeloma culture performance for monoclonal antibody production. Biotechnol Bioeng. 2000;69:566–576. doi: 10.1002/1097-0290(20000905)69:5<566::AID-BIT11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dong J, Mandenius CF, Lübberstedt M, Urbaniak T, Nüssler AK, Knobeloch D, Gerlach JC, Zeilinger K (2008) Evaluation and optimization of hepatocyte culture media factors by design of experiments (DoE) methodology. Cytotechnology 57:251–261 [DOI] [PMC free article] [PubMed]
- Fan L, Zhao L, Sun Y, Kou T, Zhou Y, Tan WS. A high-yielding, generic fed-batch process for recombinant antibody production of GS-engineered cell lines. J Microbiol Biotechnol. 2009;19:1695–1702. doi: 10.4014/jmb.0904.04054. [DOI] [PubMed] [Google Scholar]
- Gilbert RS, Nagano Y, Yokota T, Hwan SF, Fletcher T, Lydersen K. Effect of lipids on insect cell growth and expression of recombinant proteins in serum-free medium. Cytotechnology. 1996;22:211–216. doi: 10.1007/BF00353941. [DOI] [PubMed] [Google Scholar]
- González-Leal IJ, Carrillo-Cocom LM, Ramírez-Medrano A, López-Pacheco F, Bulnes-Abundis D, Webb-Vargas Y, Alvarez MM (2011) Use of a plackett–burman statistical design to determine the effect of selected amino acids on monoclonal antibody production in CHO cells. Biotechnol Prog 27:1709–1717 [DOI] [PubMed]
- Hu S, Deng L, Wang H, Zhuang Y, Chu J, Zhang S, Li Z, Guo M. Bioprocess development for the production of mouse-human chimeric anti-epidermal growth factor receptor vIII antibody C12 by suspension culture of recombinant Chinese hamster ovary cells. Cytotechnology. 2011;63:247–258. doi: 10.1007/s10616-011-9336-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EP, Marquis CP, Gray PP. Development of super-CHO protein-free medium based on a statistical design. J Chem Technol Biot. 2007;82:431–441. doi: 10.1002/jctb.1670. [DOI] [Google Scholar]
- Huang YM, Hu W, Rustandi E, Chang K, Yusuf-Makagiansar H, Ryll T (2010) Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol Prog 26:1400–1410 [DOI] [PubMed]
- Ishaque A, Al-Rubeai M. Role of vitamins in determining apoptosis and extent of suppression by suppression by bcl-2 during hybridoma cell culture. Apoptosis. 2002;7:231–239. doi: 10.1023/A:1015343616059. [DOI] [PubMed] [Google Scholar]
- Janne J, Alhonen L, Pietila M, Keinanen TA. Genetic approaches to the cellular functions of polyamines in mammals. Eur J Biochem. 2004;271:877–894. doi: 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- Jeon M, Lim JB, Lee G. Development of a serum-free medium for in vitro expansion of human cytotoxic T lymphocytes using a statistical design. BMC Biotechnol. 2010;10:70. doi: 10.1186/1472-6750-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Lee JC, Chang HN, Oh DJ. Effects of supplementation of various medium components on Chinese hamster ovary cell cultures producing recombinant antibody. Cytotechnology. 2005;47:37–49. doi: 10.1007/s10616-005-3775-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Lee JC, Chang HN, Oh DJ. Development of serum-free media for a recombinant CHO cell line producing recombinant antibody. Enzyme Microb Tech. 2006;39:426–433. doi: 10.1016/j.enzmictec.2005.11.047. [DOI] [Google Scholar]
- Klareskog L, Gaubitz M, Rodríguez-Valverde V, Malaise M, Dougados M, Wajdula J; Etanercept Study 301 Investigators (2011) Assessment of long-term safety and efficacy of etanercept in a 5-year extension study in patients with rheumatoid arthritis. Clin Exp Rheumatol 29:238–247 [PubMed]
- Knöspel F, Schindler RK, Lübberstedt M, Petzolt S, Gerlach JC, Zeilinger K. Optimization of a serum-free culture medium for mouse embryonic stem cells using design of experiments (DoE) methodology. Cytotechnology. 2010;62:557–571. doi: 10.1007/s10616-010-9307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou TC, Fan L, Zhou Y, Ye ZY, Zhao L, Tan WS. Increasing the productivity of TNFR-Fc in GS-CHO cells at reduced culture temperatures. Biotechnol Bioproc E. 2011;16:136–143. doi: 10.1007/s12257-010-0157-1. [DOI] [Google Scholar]
- Lee GM, Kim EJ, Kim NS, Yoon SK, Ahn YH, Song JY. Development of a serum-free medium for the production of erythropoietin by suspension culture of recombinant Chinese hamster ovary cells using a statistical design. J Biotechnol. 1999;69:85–93. doi: 10.1016/S0168-1656(99)00004-8. [DOI] [PubMed] [Google Scholar]
- Liu CH, Chang TY. Rational development of serum-free medium for Chinese hamster ovary cells. Process Biochem. 2006;41:2314–2319. doi: 10.1016/j.procbio.2006.06.008. [DOI] [Google Scholar]
- Liu C, Chu I, Hwang S. Factorial designs combined with the steepest ascent method to optimize serum-free media for CHO cells. Enzyme Microb Technol. 2001;28:314–321. doi: 10.1016/S0141-0229(00)00346-X. [DOI] [PubMed] [Google Scholar]
- Liu CH, Wu PS. Optimization of adenoviral production in human embryonic kidney cells using response surface methodology. J Biosci Bioeng. 2007;103:406–411. doi: 10.1263/jbb.103.406. [DOI] [PubMed] [Google Scholar]
- Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, Stein LD, Gedalia A, Ilowite NT, Wallace CA, Whitmore J, Finck BK. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342:763–769. doi: 10.1056/NEJM200003163421103. [DOI] [PubMed] [Google Scholar]
- Lv G, Sun Z, Li N, Li S, Zhang Y, Xie Y, Yu W, Wang W, Ma X. Design a chemically defined/medically approved medium for cell transplantation according to the metabolic characteristics of microencapsulated cells and the process of encapsulation. Biochem Eng J. 2008;39:157–163. doi: 10.1016/j.bej.2007.08.025. [DOI] [Google Scholar]
- Ma ZY, Yi XP, Zhang YX. Enhanced intracellular accumulation of recombinant HBsAg in CHO cells by dimethyl sulfoxide. Process Biochem. 2008;43:690–695. doi: 10.1016/j.procbio.2008.02.012. [DOI] [Google Scholar]
- Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis:a randomised trial. Lancet. 2000;356:385–390. doi: 10.1016/S0140-6736(00)02530-7. [DOI] [PubMed] [Google Scholar]
- Meghrous J, Mahmoud W, Jacob D, Chubet R, Cox M, Kamen AA. Development of a simple and high-yielding fed-batch process for the production of influenza vaccines. Vaccine. 2009;28:309–316. doi: 10.1016/j.vaccine.2009.10.048. [DOI] [PubMed] [Google Scholar]
- Merten O-W (2006) Introduction to animal cell culture technology—past, present and future. Cytotechnology 50:1–7 [DOI] [PMC free article] [PubMed]
- Murakami H, Masui H, Sato GH, Sueoka N, Ghow TP, Kano-Sueoka T. Growth of hybridoma cells in serum-free medium: ethanolamine is an essential component. Proc Natl Acad Sci USA. 1982;79:1158–1162. doi: 10.1073/pnas.79.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parampalli A, Eskridge K, Smith L, Meagher M, Mowry M, Subramanian A. Development of serum-free media in CHO-DG44 cells using a central composite statistical design. Cytotechnology. 2007;54:57–68. doi: 10.1007/s10616-007-9074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot E, Fournier F, Geny C, Pinton H, Marc A. Rapid screening of serum-free media for the growth of adherent Vero cells by using a small-scale and non-invasive tool. Appl Biochem Biotechnol. 2010;160:1600–1615. doi: 10.1007/s12010-009-8674-0. [DOI] [PubMed] [Google Scholar]
- Riebeling C, Schlechter K, Buesen R, Spielmann H, Luch A, Seiler A. Defined culture medium for stem cell differentiation: applicability of serum-free conditions in the mouse embryonic stem cell test. Toxicol in Vitro. 2011;25:914–921. doi: 10.1016/j.tiv.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Rodrigues Goulart H, Arthuso FS. Enhancement of human prolactin synthesis by sodium butyrate addition to serum-free CHO cell culture. J Biomed Biotechnol. 2010 doi: 10.1155/2010/405872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourou S, van der Ark A, van der Velden T, Kallel H. Development of an animal-component free medium for Vero cells culture. Biotechnol Prog. 2009;25:1752–1761. doi: 10.1002/btpr.279. [DOI] [PubMed] [Google Scholar]
- Sandadi S, Ensari S, Kearns B. Application of fractional factorial designs to screen active factors for antibody production by Chinese hamster ovary cells. Biotechnol Prog. 2006;22:595–600. doi: 10.1021/bp050300q. [DOI] [PubMed] [Google Scholar]
- Spens E, Häggström L. Defined protein and animal component-free NS0 fed-batch culture. Biotechnol Bioeng. 2007;98:1183–1194. doi: 10.1002/bit.21509. [DOI] [PubMed] [Google Scholar]
- Stein A. Decreasing variability in your cell culture. Biotechniques. 2007;43:228–229. doi: 10.2144/000112561. [DOI] [PubMed] [Google Scholar]
- Sugahara T, Nishimoto S, Miyazaki Y. Effects of polyamines on proliferation and IgM productivity of human–human hybridoma, HB4C5 cells. Cytotechnology. 2008;57:115–122. doi: 10.1007/s10616-007-9115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Valk J, Brunner D, De Smet K, Fex Svenningsen A, Honegger P, Knudsen LE, Lindl T, Noraberg J, Price A, Scarino ML, Gstraunthaler G. Optimization of chemically defined cell culture media–replacing fetal bovine serum in mammalian in vitro methods. Toxicol in Vitro. 2010;24:1053–1063. doi: 10.1016/j.tiv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Wlaschin KF, Hu WS. Fedbatch culture and dynamic nutrient feeding. Adv Biochem Eng Biotechnol. 2006;101:43–74. doi: 10.1007/10_015. [DOI] [PubMed] [Google Scholar]
- Yao CL, Liu CH, Chu IM, Hsieh TB, Hwang SM. Factorial designs combined with the steepest ascent method to optimize serum-free media for ex vivo expansion of human hematopoietic progenitor cells. Enzyme Microb Tech. 2003;33:343–352. doi: 10.1016/S0141-0229(03)00144-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Robinson D. Development of animal-free, protein-free and chemically-defined media for NS0 cell culture. Cytotechnology. 2005;48:59–74. doi: 10.1007/s10616-005-3563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Robinson D, Salmon P. A novel function for selenium in biological system: selenite as a highly effective iron carrier for Chinese hamster ovary cell growth and monoclonal antibody production. Biotechnol Bioeng. 2006;95:1188–1197. doi: 10.1002/bit.21081. [DOI] [PubMed] [Google Scholar]
- Zhang L, Shen H, Zhang Y. Fed-batch culture of hybridoma cells in serum-free medium using an optimized feeding strategy. J Chem Technol Biot. 2004;79:171–181. doi: 10.1002/jctb.940. [DOI] [Google Scholar]