Abstract
Purpose.
To characterize ocular surface discomfort and tear film parameters in a veteran population.
Methods.
Male patients seen in the Miami Veterans Affairs eye clinic aged 50 years or older were recruited to participate in the study. All patients had normal eyelid, corneal, and conjunctival anatomy. Patients filled out the Dry Eye Questionnaire 5 (DEQ5) and underwent measurement of tear film parameters. The main outcome measures were the frequency of ocular surface symptoms and the correlation between symptoms and global, aqueous, and meibomian gland parameters.
Results.
A total of 263 men participated in the study; 48% had DES based on the presence of severe symptoms. Many men had objective abnormalities in tear function measurements. Using Schirmer information, lid plugging, and meibomian quality to define objective DES, 176 patients (68%) had one or more abnormalities. Of these, 22 (8%) had aqueous tear deficiency, 124 (47%) lipid tear deficiency, and 30 (11%) a mixed pattern. When examining associations between individual clinical parameters and DEQ5 score, the only significant, but weak, correlations were with the global parameters conjunctival and corneal staining (r = 0.16) and TBUT (r = −0.15). Neither specific aqueous nor meibomian gland measurements were significantly correlated with the presence of symptoms. When considering all measured parameters in a regression model, 8% of the variability in symptoms was explained by the tear parameters.
Conclusions.
We found that ocular surface symptoms were prevalent in our population. Measurement of standard tear film parameters could not explain the degree of symptoms. This study highlights the need for future research regarding the mechanisms behind ocular surface discomfort in patients with tear film disturbances.
Almost 50% of male veterans complained of ocular surface symptoms and many had objective abnormalities in tear function measurements. When considered individually or in combination, poor correlation was found between symptoms and measured tear parameters.
Introduction
Dry eye syndrome (DES) is a prevalent condition both in the United States and worldwide, with symptoms that negatively affect the ability to work and function.1–6 DES is a leading cause of visits to optometry and ophthalmology clinics and DES medications account for approximately $1.9 billion in US sales annually.7,8 We have previously studied the impact of dry eye symptoms on a veteran population and found that 65% patients seen at the Miami Veterans Affairs (VA) eye clinic over a 3-month period reported having mild or greater ocular surface symptoms at the time of their eye clinic visit. Twenty-seven percent reported severe symptoms.9 The population in this study consisted of all comers to the eye clinic, including those on glaucoma drops, postsurgery patients, and patients with external eyelid and corneal abnormalities. As the aforementioned population has a tendency for higher ocular surface symptoms (for reasons other than DES), the focus of the present study was to repeat the survey in a population of patients without alternative explanations for discomfort to assess the burden of ocular surface symptoms.
In addition, in the above-mentioned study, no ocular examination was performed to link symptoms to ocular findings. There is a knowledge gap with regard to the tear film parameters in older male veterans, whom as a group have demographic characteristics, exposures, and medical profiles that are different from previously studied populations.10–14 Specifically, with interest in meibomian gland dysfunction increasing in recent years,15 in the present study, we aimed to evaluate whether the symptoms found in our patients were more closely related to meibomian versus aqueous abnormalities.
Methods
Study Population
The Miami VA institutional review board reviewed and approved the prospective examination of patients for this study, which was conducted in accordance with the principles of the Declaration of Helsinki. Patients were prospectively recruited from the Miami VA Medical Center eye clinic between October 2010 and December 2011 irrespective of their tear function status. Therefore, recruited patients had either normal tear film function, mostly aqueous tear deficiency, mostly lipid tear deficiency, or a mixed pattern. Patients were seen in the eye clinic for a variety of concerns including refractive issues, cataract evaluation, and retinal pathologies. Inclusion criteria included having normal eyelid, conjunctival, and corneal anatomy. Patients were not eligible to participate if they were female; younger than 50 years; used contact lenses; used any ocular medication with the exception of artificial tears/topical cyclosporine; had HIV, sarcoidosis, graft-versus-host disease, or a collagen vascular disease; had an active external ocular process (e.g., keratitis); or if they had ocular surgery within the preceding 3 months. Patients were prescreened by various eye care practitioners and eligible subjects were informed about an opportunity to participate in a 1-day research study whose purpose was to evaluate tear film function. Potential subjects were told that the goal of the study was to understand why some individuals have tear dysfunction while others have healthy tears. This script was used in an attempt to mitigate possible selection bias and recruit patients with and without DES. Interested patients were scheduled for a research visit at which time informed consent was obtained.
Female patients were excluded from the study for two reasons: (1) we wanted to study a less well represented population in dry eye research and one previously felt not to be as affected by the disease, and (2) we plan to subsequently evaluate the relationship between androgen levels and tear film parameters in this gender-specific (male) population.
Data Collection
For each individual, demographic information, past medical history, and medication information was collected. The patient self-administered the five-item dry eye questionnaire (DEQ5)16 (a validated questionnaire) with a member of the study team in presence to ensure comprehension. The DEQ5 questionnaire was selected in this population because its score combines patient responses regarding discomfort, dryness, and tearing without considering visual function. The more commonly used Ocular Surface Disease Index (OSDI), on the other hand, considers visual function and includes questions related to difficulty with reading, driving at night, working with a computer, and watching TV. Many of our patients seek eye care treatment for visual dysfunction, and on pilot testing, many had high OSDI scores for reasons other than DES. We therefore felt that the DEQ5 score was a more accurate reflection of ocular surface symptoms in this population. DEQ5 scores of 6 or greater were considered to be mild or greater symptoms and DEQ5 scores of 12 or greater were considered to be severe symptoms.16
The ocular surface examination, in the order performed, consisted of tear osmolarity (measured once in each eye) (TearLAB, San Diego, CA), tear breakup time (TBUT) (measured twice in each eye and averaged), conjunctival and corneal staining (punctuate epithelial erosions, Oxford [Bron] staining, range 0–5),17 Schirmer's strips with anesthesia, and morphologic and qualitative eyelid and meibomian gland information. For TBUT, a fluorescein strip (Fluorets Laboratoire, Chauvin, France) was wetted with the application of one drop of nonpreserved saline to the lower one-fourth of the strip. Excess fluid was gently removed such that the saturated tip delivered approximately 3 to 5 μL of liquid NaFl. With the subject gazing up, the examiner introduced the NaFl into the lower fornix. Starting with the right eye, timing was stopped upon visualization of the first break (one or more black [dry] spots) appearing in the precorneal tear film OR after 15 seconds had elapsed. The procedure was then repeated for the left eye. Morphologic information collected included the degree of eyelid vascularity (0 none; 1 mild engorgement; 2 moderate engorgement; 3 severe engorgement)18 and the presence of inferior eyelid meibomian orifice plugging (0 none; 1 less than one-third lid involvement; 2 between one-third and two-thirds involvement; 3 more than two-thirds lid involvement). Meibum quality was graded on a scale of 0 to 4 (0 = clear; 1 = cloudy; 2 = granular; 3 = toothpaste; 4 = no meibum extracted).19 Data were entered into a standardized database.
Main Outcome Measures
The main outcome measure was the characterization of ocular surface symptoms and signs in our population and the correlation between symptoms and global, aqueous, and meibomian gland parameters. A secondary outcome measure was to evaluate risk factors associated with symptoms and signs of DES.
Statistical Analysis
All statistical analyses were performed using SPSS 19.0 (SPSS, Inc., Chicago, IL) statistical package. Descriptive statistics were applied to characterize tear film parameters in the population. Logistic regression analyses were used to assess risk factors associated with ocular surface symptoms. In the analyses, the dependent variable was the presence of severe ocular surface symptoms (DEQ5 score ≥12) and the independent variables were demographics, medical history, and medication use. The χ2 analyses were used to assess risk factors associated with objective signs of DES. This type of analysis was used as there were four outcome variables (aqueous tear deficiency [ATD], lipid tear deficiency [LTD], mixed pattern, and no DES). Pearson coefficients were calculated to evaluate the correlation between tear film parameters and ocular surface symptoms. Linear regression analysis was performed to evaluate the relationship between measured signs and ocular surface symptoms. The strength of the association between DEQ5 symptom score and tear film parameters (more severe value from each eye) was summarized with the coefficient of determination R2. R2 ranges from zero to one, and can be interpreted as the percentage of variance in the dependent variable explained by all of the independent variables. An R2 less than 10% is usually considered a weak correlation even when it is statistically significant.20
Results
Study Population
A total of 263 patients were recruited to participate in the study. Demographic characteristics of the study population can be found in Table 1.
Table 1. .
Demographic Information of the Study Population
| Number | 263 |
| Age, mean (SD) [range] | 69 (9) [50–95] |
| Race/Ethnicity, n (%) | |
| White, non-Hispanic | 117 (45) |
| White, Hispanic | 65 (25) |
| Black, non-Hispanic | 72 (27) |
| Black, Hispanic | 2 (1) |
| Asian or Pacific Islander | 1 (0.4) |
| Other | 6 (2) |
| Smoking status, n (%) | |
| Never | 71 (27) |
| Former | 148 (56) |
| Current | 43 (16) |
| Self- reported health status, n (%) | |
| Excellent | 19 (7) |
| Good | 145 (55) |
| Fair | 78 (30) |
| Poor | 19 (7) |
Mean age was 69 (SD 9); 182 patients (70%) self-identified themselves as white. Overall, 81% of the population complained of mild or greater ocular surface symptoms (DEQ5 score 6 or greater) and 48% complained of severe symptoms (DEQ5 score 12 or greater).16 Patients with poorer self-reported health status were more likely to complain of severe ocular surface symptoms than those with better health status (P = 0.001, Table 2). Age, race/ethnicity, and smoking status did not significantly predict the presence of severe symptoms. Regarding medical history, having a diagnosis of osteoarthritis increased the risk of reporting severe symptoms by 1.8 (Table 2). A diagnosis of diabetes mellitus (DM), hypertension (HTN), thyroid disease, benign prostatic hyperplasia (BPH), and gout did not significantly affect the risk of reporting severe symptoms. Antihistamine use increased the risk of severe symptoms 3-fold. The use of a diuretic, on the other hand, was not significantly associated with severe symptoms.
Table 2. .
Logistic Regression Analyses Evaluating Factors Associated with Severe Ocular Surface Symptoms (DEQ5 Score 12 or Greater)
|
|
Frequencies |
Odds Ratio |
95% CI |
P
Value |
| Self-reported health status*,† | See Table 1 | 1.84 | 1.28–2.63 | 0.001 |
| Osteoarthritis* | 101 (38%) | 1.87 | 1.13–3.08 | 0.02 |
| Antihistamine use* | 58 (22%) | 3.08 | 1.65–5.73 | <0.0005 |
All three variables remained significant in a multivariable analysis (P < 0.05). CI, confidence interval.
Univariable analysis.
Analyzed as continuous variable with excellent health being the reference. The odds ratio refers to the increase in the odds of having severe ocular surface symptoms for a one unit change in self-reported health status.
Tear Film Parameters
We found that many men had objective abnormalities in their tear function measurements (Table 3).
Table 3. .
Tear Film Parameters in the Study Population
|
|
Tear Film Parameters |
|
| Global | DEQ5, mean (SD) [n] | 10.6 (5.4) [263] |
| Tear osmolarity,* mean (SD) mOsm/L [n] | 310 (14) [254] | |
| Tear osmolarity,* mild or greater disturbance %21 | 51% with value > 308 | |
| Tear osmolarity,* severe disturbance %21 | 31% with value >314 | |
| Difference in osmolarity between eyes, mean (SD) | 10.5 (8.7) | |
| Difference in osmolarity between eyes22 | 49% with difference > 8 | |
| Conjunctival and corneal staining* [n] | 27% with value >1 [262] | |
| TBUT,* mean (SD) seconds [n] | 7.3 (4.7) [263] | |
| TBUT,* severe disturbance | 41% with value <5 | |
| Aqueous | Schirmer's,* mean (SD) mm [n] | 10.9 (7.7) [263] |
| Schirmer's,* severe disturbance | 21% with value <5 | |
| MG | MG quality* [n] | 42% with value >1 [261] |
| Lid vascularity* [n] | 20% with value >1 [263] | |
| MG orifice plugging* [n] | 29% with value >1 [262] |
, number of observations; MG, meibomian gland.
More severe value of the two eyes.
Fifty-one percent had tear osmolarity values greater than 308 in the worse eye21 and 49% had an osmolarity difference of greater than 8 between eyes.22 The latter value was reported based on previous research that suggested an intereye difference greater than 8 mOsm/L was consistent with an unstable tear film.22 While 41% of patients had a TBUT less than 5, 30 patients (11%) had TBUT scores of 15 or greater.
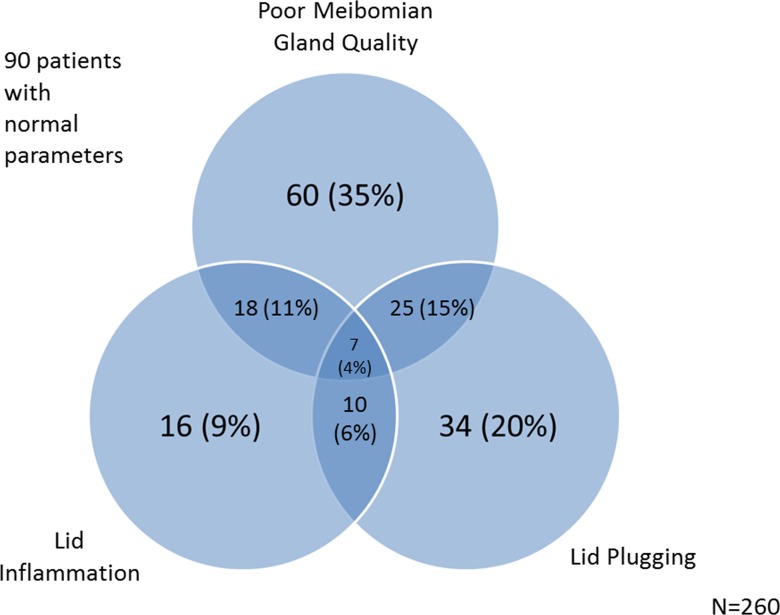
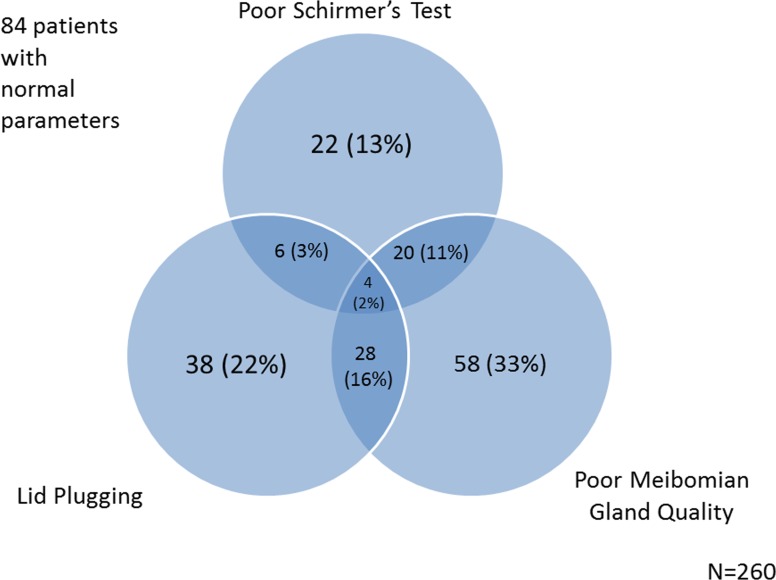
There were 170 patients with one or more indicators of lid deficiency (inflammation, plugging, poor meibomian gland quality). Of these, 16 (9%) had lid inflammation alone, 60 (35%) had poor meibomian gland quality alone, and 34 (20%) had lid plugging alone (Fig. 1). Because only 16 cases (9%) had lid inflammation alone, we included the latter two parameters in an illustration of the overlap between ATD and LTD in our population (Fig. 2). Overall, using the objective measures of Schirmer's, lid plugging, and meibomian quality as definers of DES, 176 patients (68%) had one or more abnormalities. Of these, 22 (8%) had mostly ATD (as defined by a Schirmer value <5 in the more severe eye), 124 (47%) mostly LTD (as defined by either meibomian gland quality score or lid plugging score >1 in the more severe eye), and 30 (11%) a mixed pattern.
Figure 1.
A Venn diagram modeling the overlap between the three meibomian gland parameters measured: eyelid plugging, eyelid inflammation, and meibum quality.
Figure 2.
A Venn diagram modeling the overlap between two meibomian gland parameters (eyelid inflammation and meibum quality) and a lacrimal gland parameter (Schirmer's score).
Risk Factors for Objective DES
In our population, age, race/ethnicity, self-reported health status, and smoking status did not significantly predict the presence of objective DES. Regarding medical history, patients with DM had a higher frequency of ATD (14% vs. 6%) and a lower frequency of LTD (37% vs. 53%) compared with those without DM (P value 0.04). Patients with HTN had a higher overall frequency of objective DES (71% vs. 60%) (P = 0.06), and most of these were ATD (12% vs. 1%) (P value 0.02). Thyroid disease, BPH, gout, and the use of an antihistamine or diuretic did not significantly affect the frequency of objective DES (Table 4).
Table 4. .
χ2 Analyses Evaluating Factors Associated with Objective DES
|
Variable |
Normal, n (%) |
ATD, n (%) |
LTD, n (%) |
Mixed, n (%) |
P Value |
| DM | |||||
| Yes | 30 (35) | 12 (14) | 32 (37) | 12 (14) | 0.037 |
| No | 54 (31) | 10 (6) | 92 (53) | 18 (10) | |
| HTN | |||||
| Yes | 51 (29) | 21 (12) | 84 (47) | 22 (12) | 0.018 |
| No | 33 (40) | 1 (1) | 40 (49) | 22 (12) |
Correlation between Symptoms and Signs of DES
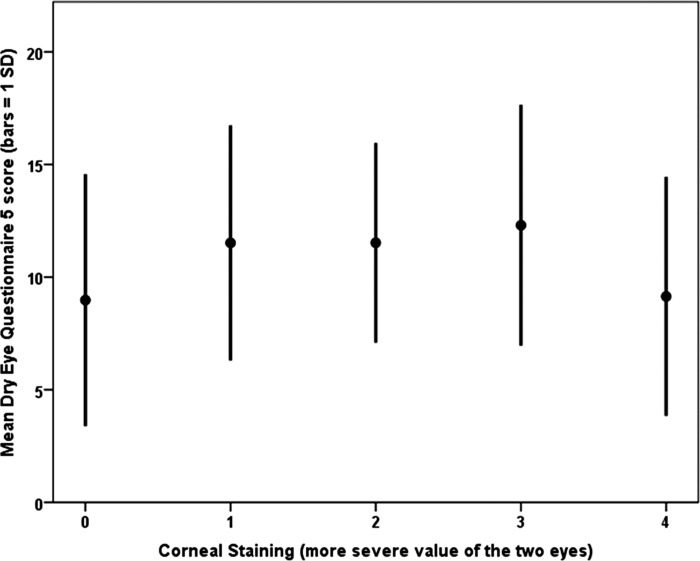
When considering the tear film parameters and DEQ5 score as continuous variables, a weak correlation was found between the global parameters of conjunctival and corneal staining (r = 0.16, P = 0.009) and TBUT (r = −0.15, P = 0.01) with DEQ5 score (Table 5, Figs. 3 and 4). A plot of DEQ5 versus conjunctival and corneal staining revealed a quadratic relationship demonstrating a stronger correlation (r = −0.23, P < 0.001) than the linear component. The negative sign indicates that discomfort, as assessed with the DEQ5, decreased with maximal conjunctival and corneal staining and was greater at intermediate staining levels (Fig. 3).
Table 5. .
Correlation between Tear Film Parameters and Presence of Ocular Surface Symptoms (Assessed by DEQ5 Score)
|
|
Tear Film Parameters* |
Pearson (r) |
R
2 |
P Value |
| Global | Tear osmolarity† | 0.03 | 0.0009 | 0.69 |
| Difference in osmolarity between eyes | 0.02 | 0.0004 | 0.75 | |
| Conjunctival and corneal staining linear component† | 0.16 | 0.026 | 0.009 | |
| Conjunctival and corneal staining quadratic component† | −0.23 | 0.053 | <0.001 | |
| TBUT† | −0.15 | 0.023 | 0.01 | |
| Aqueous | Schirmer's† | −0.11 | 0.012 | 0.07 |
| MG | MG quality† | 0.06 | 0.004 | 0.34 |
| Lid vascularity† | −0.07 | 0.005 | 0.25 | |
| MG orifice plugging† | −0.07 | 0.005 | 0.26 |
All tear parameters modeled as continuous variables.
More severe value of the two eyes.
Figure 3. .
A graph demonstrating a quadratic relationship between corneal staining and ocular surface symptoms.
Figure 4. .
A scatter plot demonstrating a weak but significant negative correlation between tear breakup time and ocular surface symptoms.
Neither specific aqueous nor meibomian gland measurements were significantly correlated with the presence of symptoms. When considering all measured tear parameters in Table 3 in a multivariable linear regression model, only the quadratic component of conjunctival and corneal staining was statistically significant (P = 0.002, R2 allvariables = 0.075). Thus, despite the impressive statistical significance of conjunctival and corneal staining, all objective parameters together accounted for less than 10% of the variability in DEQ5 score and constitute at best a weak correlation. Plots of the regression residuals demonstrated a near Gaussian distribution, which is an important assumption of the model and no nonlinear effects or variance heterogeneity were present in the model. Examining the three domains of the DEQ5 separately (questions about discomfort, dryness, and tearing) did not improve the ability of measured tear film parameters to explain symptoms with R2 measurements ranging from 0.029 (tearing) to 0.072 (discomfort). Examining correlations between the various objective measures revealed that only two had an R2 greater than 5%: TBUT with Schirmer test (r = 0.38) and TBUT with conjunctival and corneal staining (r = −0.27).
Discussion
The goal of this study was to characterize ocular surface discomfort and tear film parameters in an older, male veteran population with normal external anatomy and no confounding factors, such as topical medication use and postsurgical status. Furthermore, a secondary goal was to evaluate whether symptoms were correlated with meibomian versus aqueous abnormalities. We found that a high proportion of men in our population complained of ocular surface symptoms, with almost 50% suffering from severe symptoms. This number suggests a higher burden of symptoms in our hospital-based population compared with previously reported population-based studies in Salisbury, Maryland (where 13% of men had ≥1 symptoms often or all the time)14 and Shihpai, Taiwan (where 30% of men had ≥1 symptoms often or all the time).11 Our numbers were more in line with that of Lu et al.,12 who found that 52% of 1031 Tibetans living in Zeku, China, reported one or more symptoms often or all the time.
Using cutoff definitions for various tear parameters, we also found many objective abnormalities in various tear function measurements, with most patients displaying a lipid tear deficiency or a mixed pattern. These findings have been supported by previous hospital and population-based studies11–14,23–26 (Table 6).
Table 6. .
Literature Review of Tear Film parameters and the Correlation between DES Signs and Symptoms
|
|
Author |
n (Men) |
Location |
Symptoms |
TBUT |
Schirmer's Score |
Ocular Surface Staining |
Correlation Signs and Symptoms |
| US | Galor | 263 (263) | Miami VA hospital-based population | 48% men with severe symptoms (by DEQ5) | 67% < 10, 41% < 5 | Mean 11, 21% <5 | 27% >1, scale 0–5 | Weak correlation for TBUT and ocular surface staining with symptoms |
| Schein14 | 2420 (1016) | Salisbury, Maryland | 13% men ≥ 1 symptoms often or all the time | Mean 13 (men), 12% ≤ 5 (all) | 41% ≥1 (men), scale 0–9 | No correlation for Schirmer's, weak correlation for corneal staining with symptoms | ||
| Outside US | Lin11 | 2045 (822) | Shihpai, Taiwan | 30% men ≥ 1 symptoms often or all the time | 79% ≤ 10 (men) | 55% ≤5 (men) | 32% ≥1 (men), scale 0–3 | |
| Lu12 | 1840 (1031) | Tibetans living in Zeku, China | 52% men ≥ 1 symptoms often or all the time | 33% ≤ 10 (men) | 23% ≤5 (men) | 6% ≥1 (men), scale 0–3 | Weak correlation for TBUT, corneal staining, and Schirmer's with symptoms | |
| McCarty13 | 926 (433) | Melbourne, Australia | 6% any severe symptom (all) | 9% <8 (all) | 6% < 5 (all) | 11% >3 (all), scale 0–9 | Corneal staining most discriminating |
When considering the parameters in combination or individually, we could not find good correlation between measured signs and symptoms, despite the inclusion of newer tear film variables, such as osmolarity and meibomian gland parameters. Specifically, neither aqueous nor meibomian tear film parameters were significantly correlated with symptoms. This is supported by previous work that examined the relationship between Schirmer's score and symptoms,27,28 and by a recent article that showed that many patients with objective meibomian gland dysfunction were asymptomatic.29
This finding is problematic given that the main source of morbidity in DES is the symptomatology. DES symptoms, which include irritation, foreign body sensation, and pain, interfere with the ability to work and carry out daily functions.2–4 When treating patients with DES, it is important to understand which objective measures can best predict symptoms, as this can assist in monitoring response to treatment. Furthermore, to have a DES medication approved by the US Food and Drug Administration (FDA), the drug must show that it improves one symptom and one sign of DES over placebo (unpublished FDA mandate). The lack of correlation between currently measured signs and symptoms has limited the ability of innovators to test their products and has therefore hindered new DES therapeutic agents from entering the market.
There are several potential explanations as to why our measured parameters so poorly reflected symptomatology. The first possibility is that measuring tear film parameters at one time point may not be enough to get an overall sense of tear film function. This finding has been suggested by other researchers who also evaluated the presence of symptoms and signs of tear dysfunction on the same day.12,27 Another potential explanation is that our measurement techniques may not have been ideal. For example, with regard to tear osmolarity, studies have theorized that osmolarity levels in the central cornea reach much higher concentrations than those measured in the inferior tear meniscus, and that it is these concentrations that drive discomfort.30,31 Unfortunately, it is very difficult to measure osmolarity levels in the central cornea and there is no commercially available method in which to do so.
Finally, it is possible that there are unmeasured variables that more closely relate to the pathophysiology of ocular discomfort in the setting of tear dysfunction. Corneal nerve activity is one such potential variable. The current data on corneal sensitivity is confusing, however, with a few studies reporting that DES patients have lower sensitivities to mechanical, chemical, and thermal stimuli,32,33 and a few reporting higher mechanical sensitivity.34,35 These studies have limited patient numbers, which may explain the lack of conclusive data. The study of corneal nerve sensitivity is also limited by the lack of a commercially available measurement device.
As with all studies, this work has limitations that need to be considered when interpreting the study results. This study evaluated the symptoms and tear parameters in a population of older US veterans seeking eye care services and, as such, the findings may not be generalizable to other US-based male populations. Moreover, tear film parameters were measured at one time point and stability of measurements cannot be assessed in this study. Our measurements were also obtained using specific scales and techniques and our findings may have been altered if different measures were used. Based on our recruitment criteria, we do not have information on how many patients were screened but declined the invitation to participate in our study. Although this may have affected the frequency of tear film abnormalities in our population, it would not have affected our results on the correlation between symptoms and signs of disease. Furthermore, whereas we collected information on several risk factors associated with DES, we did not have information on others, including occupation and educational attainment.
With these limitations in mind, this study confirms that severe ocular surface symptoms are prevalent in an older, male veteran population. Measurement of standard tear film parameters could not explain the degree of measured symptoms. The study highlights the need for future research regarding the mechanisms behind ocular surface discomfort in patients with tear film disturbances. Only through a better understanding of these mechanisms will we be able to improve treatment outcomes in this chronic, debilitating disease.
Acknowledgments
We thank Bozorgmehr Pouyeh, Gail Lewis, and Ashley Katsikos for their assistance with the implementation of this study.
Footnotes
Supported by a grant from the Veterans Affairs Medical Center (AG), unrestricted funds from Research to Prevent Blindness, New York, New York, and a National Institutes of Health Center Core Grant P30EY014801 (institutional grant).
Disclosure: A. Galor, None; W. Feuer, None; D.J. Lee, None; H. Florez, None; V.D. Venincasa, None; V.L. Perez, None
References
- 1. Petris R. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop ( 2007). Ocul Surf. 2007; 5: 93–107 [DOI] [PubMed] [Google Scholar]
- 2. Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients' lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005; 46: 46–50 [DOI] [PubMed] [Google Scholar]
- 3. Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007; 143: 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003; 110: 1412–1419 [DOI] [PubMed] [Google Scholar]
- 5. Rajagopalan K, Abetz L, Mertzanis P, et al. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health. 2005; 8: 168–174 [DOI] [PubMed] [Google Scholar]
- 6. Lee AJ, Lee J, Saw SM, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002; 86: 1347–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petris R. Data dry eye market forecast to 2017. Available at: http://www.dryeyezone.com/talk/entry.php?291-GlobalData-dry-eye-market-forcast-to-2017. Accessed April 6, 2012 [Google Scholar]
- 8. Newsblurb: Size of the dry eye market. Available at: http://dryeyedigest.blogspot.com/2010/06/newsblurb-size-of-dry-eye-market.html. Accessed April 6, 2012 [Google Scholar]
- 9. Pouyeh B, Viteri E, Feuer W, et al. Impact of ocular surface symptoms on quality of life in a United States Veterans Affairs population. Am J Ophthalmol. 2012; 153: 1061–1066 e1063 [DOI] [PubMed] [Google Scholar]
- 10. Jamaliah R, Fathilah J. Prevalence of dry eye in University Malaya Medical Centre. Med J Malaysia. 2002; 57: 390–397 [PubMed] [Google Scholar]
- 11. Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003; 110: 1096–1101 [DOI] [PubMed] [Google Scholar]
- 12. Lu P, Chen X, Liu X, et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea. 2008; 27: 545–551 [DOI] [PubMed] [Google Scholar]
- 13. McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR. The epidemiology of dry eye in Melbourne, Australia. Ophthalmology. 1998; 105: 1114–1119 [DOI] [PubMed] [Google Scholar]
- 14. Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997; 124: 723–728 [DOI] [PubMed] [Google Scholar]
- 15. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012; 31: 472–478 [DOI] [PubMed] [Google Scholar]
- 16. Chalmers RL, Begley CG, Caffery B. Validation of the 5-item Dry Eye Questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010; 33: 55–60 [DOI] [PubMed] [Google Scholar]
- 17. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22: 640–650 [DOI] [PubMed] [Google Scholar]
- 18. Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf. 2003; 1: 107–126 [DOI] [PubMed] [Google Scholar]
- 19. Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011; 52: 2006–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parrish RK 2nd, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol. 1997; 115: 1447–1455 [DOI] [PubMed] [Google Scholar]
- 21. Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011; 151: 792–798 e791 [DOI] [PubMed] [Google Scholar]
- 22. Sullivan BD, Crews LA, Sonmez B, et al. Clinical utility of objective tests for dry eye disease: variability over time and implications for clinical trials and disease management. Cornea. 2012; 31: 1000–1008 [DOI] [PubMed] [Google Scholar]
- 23. Gupta N, Prasad I, Himashree G, D'Souza P. Prevalence of dry eye at high altitude: a case controlled comparative study. High Alt Med Biol. 2008; 9: 327–334 [DOI] [PubMed] [Google Scholar]
- 24. Gupta N, Prasad I, Jain R, D'Souza P. Estimating the prevalence of dry eye among Indian patients attending a tertiary ophthalmology clinic. Ann Trop Med Parasitol. 2010; 104: 247–255 [DOI] [PubMed] [Google Scholar]
- 25. Sahai A, Malik P. Dry eye: prevalence and attributable risk factors in a hospital-based population. Indian J Ophthalmol. 2005; 53: 87–91 [DOI] [PubMed] [Google Scholar]
- 26. Viso E, Rodriguez-Ares MT, Gude F. Prevalence of and associated factors for dry eye in a Spanish adult population (the Salnes Eye Study). Ophthalmic Epidemiol. 2009; 16: 15–21 [DOI] [PubMed] [Google Scholar]
- 27. Schein OD, Tielsch JM, Munoz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997; 104: 1395–1401 [DOI] [PubMed] [Google Scholar]
- 28. Sullivan BD, Bron AJ, Baudouin C, et al. Correlation of commonly used tests for the assessment of severity of dry eye disease. Paper presented at: Association of Vision and Research in Opthalmology annual meeting; May 6, 2012; Ft. Lauderdale, FL: [Google Scholar]
- 29. Viso E, Rodriguez-Ares MT, Abelenda D, Oubina B, Gude F. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci. 2012; 53: 2601–2606 [DOI] [PubMed] [Google Scholar]
- 30. Gaffney EA, Tiffany JM, Yokoi N, Bron AJ. A mass and solute balance model for tear volume and osmolarity in the normal and the dry eye. Prog Retin Eye Res. 2010; 29: 59–78 [DOI] [PubMed] [Google Scholar]
- 31. Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009; 50: 3671–3679 [DOI] [PubMed] [Google Scholar]
- 32. Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005; 46: 2341–2345 [DOI] [PubMed] [Google Scholar]
- 33. Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, et al. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007; 48: 173–181 [DOI] [PubMed] [Google Scholar]
- 34. Situ P, Simpson TL, Fonn D, Jones LW. Conjunctival and corneal pneumatic sensitivity is associated with signs and symptoms of ocular dryness. Invest Ophthalmol Vis Sci. 2008; 49: 2971–2976 [DOI] [PubMed] [Google Scholar]
- 35. De Paiva CS, Pflugfelder SC. Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol. 2004; 137: 109–115 [DOI] [PubMed] [Google Scholar]