Abstract
Purpose.
We evaluated quantitatively direct effects of ceramide (Cer) and free cholesterol (FC) on meibomian lipid films (MLF) using a Langmuir trough (LT) and a Brewster angle microscope (BAM).
Methods.
Meibum was obtained from healthy volunteers. A series of mixtures of meibum with Cer or FC (mixed MLF) taken in different ratios were tested. Standard rheologic parameters, such as elasticity and hysteresis of MLF, were computed. BAM was used to study the morphology of MLF.
Results.
Pure MLF were capable of withstanding multiple compression–expansion cycles with little hysteresis observed (∼1.9 J/g meibum). The films made of either pure Cer or pure FC were clearly collapsible, and had much higher rigidity and hysteresis than pure meibum. Adding progressively higher amounts of Cer or FC to meibum had a strong impact on the rigidity, stability, and morphology of the mixed MLF: their hysteresis increased many fold compared to pure meibum. A concomitant increase in the rigidity and collapsibility of the mixed MLF was observed.
Conclusions.
Cer and FC changed the surface properties of mixed MLF in a way that implied their destabilization and/or disruption. One of the mechanisms that might lead to these effects is strong aggregation of meibum lipids with FC or Cer that leads to the formation of smaller particles of meibum surrounded by a thinner layer of FC or Cer. As Cer and FC can be elevated in meibum and the tear film because of certain pathologic processes, or can be of exogenous nature, our results can explain (partially) a less stable tear film in those subjects.
Introduction
The preocular tear film (TF) is a complex aqueous structure that is enriched with lipids, proteins, carbohydrates and other molecules of biological origin. TF covers the entire ocular surface.1 The outermost layer of the tear film, also called tear film lipid layer (TFLL), is formed predominantly from a mixture of lipids called “meibum.” Meibum is produced by holocrine lipid-secreting meibomian glands (MG), which are located in the tarsal plates of the eyelids.2–4 This outer layer is believed to prevent the evaporation of the TF, lubricate the ocular surface, and avert the invasion of pathogenic microorganisms.5 Reportedly, adverse changes in the lipid composition of meibum6–12 could impact negatively the integrity and stability of the TF, compromising the health of the ocular surface, and predisposing it to the development of various ocular pathologies. One of the latter, the dry eye disease (DED), is one of the most prevalent ocular diseases. One of the forms of DED is known commonly as meibomian gland dysfunction (MGD),13 while another common condition that is associated with meibum and TFLL abnormalities is chronic posterior blepharitis (CPB).14
Comprehensive chemical analyses of normal (i.e., non-dry eye) meibum samples collected from healthy subjects have demonstrated a very complex nature of meibomian lipids. Many lipid classes were shown to be present in normal meibum, but only very small amounts of ceramides (Cer) or free cholesterol (FC) were observed.15–17 However, Nicolaides et al. found that the lipids expressible from the meibomian glands with clinically evident MGD in a rabbit model of DED had an increased ratio of epidermally associated lipids, like free sterols and Cer, compared to normal, non-dry eye controls.12 The same investigators postulated that this increase in Cer and free sterols could be explained by an abnormal keratinization of the meibomian gland ducts happening in MGD.18 Conversely, a few years later Shine and McCulley reported that FC amounted for approximately 0.27% of meibum (wt/wt) in various DE patients and normal subjects,19 though in 2003 evidence emerged that this number can be much higher in meibomian keratoconjuctivitis (or meibomianitis) patients with and without associated acne rosacia.20 In the latter study, minocycline treatment of such patients led to a profound decrease in the initially very high pretreatment levels of free fatty acids (FFA) and FC: the levels of FFA decreased almost 2-fold (from the pretreatment level of approximately 2% of all detected lipids to 1.3%), while the level of FC decreased more than 10-fold (from approximately 4.5%–0.3%). A likely mechanistic explanation of this observation is inhibition of either the activity of bacterial lipases that were found to be actively hydrolyzing meibomian-type lipids,21 and/or the activity of lipolytic enzymes that are present endogenously in ocular tissues. Regardless the source of the enzymes, activation of lipases of either type can happen due to inflammatory processes, like MGD and CPB, and could stimulate enzymatic hydrolysis of large quantities of cholesteryl esters present in meibum and tears, which in turn could increase the presence of FC and FFA in meibum and on the ocular surface.
Aside from purely physiological mechanisms through which levels of FC and Cer in TF and TFLL can be increased, it is important to consider that these compounds can be introduced inadvertently through exogenous sources, such as medical and cosmetic preparations, such as mascara, crèmes, and ointments (see Discussion).
Earlier, we demonstrated that even small, physiologically relevant amounts of FFA could disrupt meibomian lipid films (MLF) in vitro, possibly through a solubilization mechanism.22 Cer, on the other hand, are high-melting and rigid amphiphilic lipids, increased amounts of which could impede meibum melting and, due to their amphiphilic nature, spreading. FC, also a very high-melting, rigid and amphiphilic lipid, is known for its ability to intercalate lipid layers.
The goal of our current study was to evaluate, qualitatively and quantitatively, the changes in biophysical and morphologic properties of MLF after mixing with various amounts of FC and Cer. Our preliminary data on the effects of Cer and FC on MLF (Arciniega JC, et al. IOVS 2010;51:ARVO E-Abstract 6284) and (Uchiyama E, et al. IOVS 2010;51:ARVO E-Abstract 4161) demonstrated that Cer and FC, if present in the mixed MLF in sufficient quantities, had a strong impact on the TF. In this study, we provide a detailed description of these effects as well as new data on MLF formed of meibum premixed with Cer and FC.
Materials and Methods
Collection of Meibum Samples
All meibum donors gave informed consent. Meibum samples were obtained from healthy subjects following a previously described protocol.23,24 To minimize uncertainties associated with (possible) interdonor variability of samples, four healthy subjects (three male and one female subjects; average age 44 ± 6 years) with no ocular pathologies were recruited for this study. Over a period of a year, each subject donated samples three to five times. The procedures were approved by the University of Texas Southwestern Medical Center Institutional Review Board and were conducted in accordance with the Declaration of Helsinki. HIPPA regulations were followed. Spectroscopy and high pressure liquid chromatography (HPLC) grade chloroform and methanol were obtained from Burdick & Jackson (Muskegon, MI) and Sigma-Aldrich (St. Louis, MO). A dry, preweighed, 1.5 mL glass vial (Total Recovery; Waters Corp., Milford, MA) was filled with approximately 1 ml of spectroscopy-grade chloroform–methanol solvent mixture (2:1, vol/vol). Aqueous tears (AT) were collected using microcapillaries.22 Meibum was collected with a platinum spatula and then transferred into the vial. The dry weights of the samples were determined gravimetrically and varied from 0.5 to 1 mg. Then, the samples were dissolved in chloroform to make a 1 mg/mL stock solution of meibum. Before and between the analyses, the samples were stored at −80°C. The meibomian lipid samples were evaluated by high pressure liquid chromatography-mass spectrometry (HPLC-MS) as described earlier.23,24 A Waters Alliance HPLC system and an LCQ Deca XP Max ion trap mass spectrometer (Thermo Electron, Waltham, MA) were used. The repetitive MS analyses of the samples showed no deterioration of meibomian lipids during at least one year of storage in indicated conditions. The results of the HPLC-MS and other analyses of these normal samples already have been reported recently.25,26 The analyses demonstrated a low intersample variability of the meibomian lipids among healthy, non-dry eye subjects. The lipid composition of meibum samples analyzed in our current study was shown to match the average lipid composition of meibum reported previously.17,24–28 The biophysical experiments with fresh samples were conducted within a few days of collection. Bovine brain Cer and FC (>99% pure) were purchased from Sigma-Aldrich Corp.
Langmuir Surface Balance Experiments
A Tris-buffered saline solution (TBS, pH 7.4) was used as an aqueous subphase for Langmuir trough (LT) experiments. The pressure-area (π/A) measurements were performed using a thermostated miniature LT (NIMA 102 M; NIMA Technology, Ltd., Coventry, England) as described previously.27 To mimic the conditions of the eye blinking, the dynamic compression-expansion cycles were performed at a fast compression rate of 20 cm2/min, and the trough surface areas changing between 75 and 15 cm2. The surface pressure was monitored using a Wilhelmy plate (made of Whatman, Chr 1 filter paper) following the manufacturer's recommendations. The temperature of the LT was controlled by a refrigerating/heating circulating water thermostat (RTE-7; Thermo Fisher Scientific, Waltham, MA) and was monitored with a solid-state thermometer (model TMP; NIMA Technology, Ltd.). The trough was isolated from the environment using a Plexiglas cabinet (model CSL; NIMA Technology, Ltd.) to protect the samples from air drafts, dust, and so forth. MilliQ-grade deionized water (18 MΩ) was used to make the aqueous buffers.
A series of mixed solutions of meibum with Cer or FC (1 mg of total lipid mixture per 1 mL of chloroform) with varying Cer or FC-to-meibum ratios (10:0, 8:2, 6:4, 4:6, 2:8, 1:9, 0.5:9.5, and 0:10, wt/wt) was made. Before the experiments, the surface of subphase always was checked for contaminations by measuring the initial surface pressure. A vacuum probe was used to aspirate the impurities from the surface, if needed. The trough with the buffer was allowed to pre-equilibrate at 34°C for 15 minutes. By the end of the equilibration period, the trough's temperature stabilized. Then, using an HPLC microsyringe, 16 μL of the lipid mixture solution in chloroform were loaded carefully onto the surface of the TBS subphase to form pure or mixed MLF. The solvent was allowed to evaporate for 15 minutes, and then the film was compressed and decompressed at least twice to allow the MLF to form and stabilize. Then, multiple (π/A) isotherms of the lipids mixture were recorded in cycles.22,27
The rheologic parameters used in the analysis of the experimental data were initial surface pressure (πin), maximum surface pressure (πm), collapse pressure (πc), hysteresis (ΔΔG), and two-dimensional in-plane elasticity (Cs−1). The details of the approach have been described in our previous reports on the topic.22,27
Brewster Angle Microscopy Experiments
The morphologic evaluation of the air–water interface in the LT was conducted using a Brewster angle microscope (micro-BAM, Model 3; NIMA Technologies, Ltd.). The microscope was equipped with a laser (wavelength 659 nm, power output 30 mW), polarizer, analyzer, and CCD video camera with 3.6 × 4 mm field of view and a 6 μm resolution. The images used in this study show 2 × 2 mm frames. The p-polarized light beam was pointed at the air–water interface at exactly 53.1° (so-called “Incident Brewster Angle”). The reflectivity of the clean aqueous surface at this angle was almost zero and the pure surface appeared black. When lipids were present at the air–water interface, they manifested themselves as complex whitish to grayish surface structures. The whiteness of the structures depended on the degree of lipid organization: the whiter the structure, the more organized and solid the surface layers and structures. The gray coloration of the lipid films would mean that they were in an amorphous, disorganized state.
Results
Meibomian Lipid Films on the TBS Subphase
In control experiments, the TBS subphase with no added lipids was checked for the presence of surface active contaminants. When evaluated using micro-BAM, the air–water interface appeared black. When tested with a pressure sensor, the resulting (π/A) isotherm was flat with a surface pressure fluctuating around zero (not shown). As soon as meibum was added in the amount sufficient to form a monolayer (0.2 μg/cm2),27 the lipids spread quickly and formed a film. However, one or two adjustment (or preparatory) compression-decompression cycles were needed to allow lipids to distribute across the surface of the trough and assume a stable configuration. We considered these preparatory cycles to be somewhat similar to the blinking during which meibum gets loaded onto, and distributed across, the human ocular surface. A typical (π/A) isotherm demonstrated that, after the initial preparatory cycle(s), meibum formed stables films, which were capable of withstanding multiple compression-expansion cycles (Fig. 1). As it was previously described,27 at physiological temperatures human meibum was not capable of producing maximum surface pressures πm higher than approximately 25 mN/m at the highest tested loads. In our current experiments, different samples of normal meibum produced πm around 10 mN/m, with values of Cs−1 being 9.1 ± 0.2 mN/m (mean ± SD, n = 3) at the trough's surface area of 18 cm2, 6.1 ± 0.4 at 25 cm2, and 3.4 ± 0.3 at 40 cm2. The micro-BAM was used to visualize MLF and to study their surface features. Figure 1 illustrates morphologic features of the MLF under different surface pressures. At low surface pressures, MLF formed a network of bright, thick aggregates of well-organized lipid structures surrounded by darker areas of thinner lipid films, lipid monolayers, and/or lipid-free zones (Fig. 1A). As the surface pressure increased, the bright aggregates increased concomitantly in size, displaying a net-like pattern, while the dark (presumably, lipid-free or highly disorganized and diluted) areas diminished (Fig. 1B). This trend continued, and by the time the MLF reached the highest achievable surface pressure πm, the MLF transformed in a bright, compact, and presumably well organized lipids aggregates (Fig. 1C). These structures were observed for every tested sample of meibum. Interestingly, under constant pressure the net-like structures were stable and did not change over time unless the temperature had increased, in which case most of them melted and disappeared from the view. However, cooling the trough and closing the barriers restored the nets to their original form. This observation corroborated the reports of others.29
Figure 1.
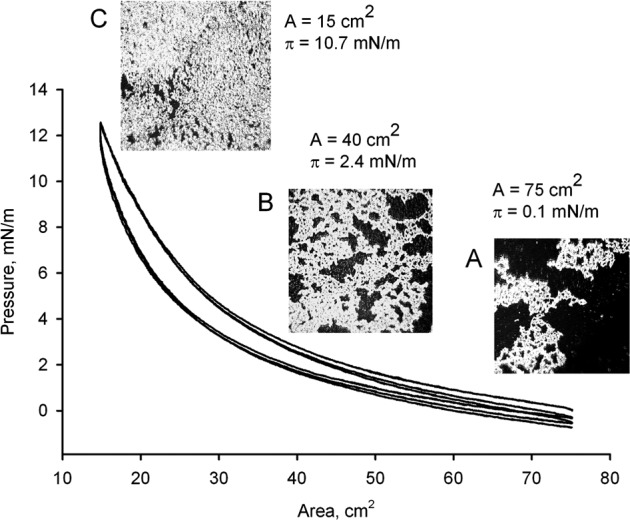
Cyclic (π/A)-isotherms of meibum at 34°C and micro-BAM photographs of MLF taken at different degrees of its compression. Meibum was loaded in the amount of 16 μg per 75 cm2 of the trough's surface area (∼0.21 μg/cm2). (A) A microphotograph of a relaxed meibomian lipid film at π = 0.1 mN/m. Meibum formed bright, net-like aggregates, which were surrounded by much darker spaces populated sparingly with disorganized lipid molecules. (B) At π = 2.4 mN/m, MLF condensed without losing its overall appearance. However, the dark areas around the lipid structures diminished considerably. (C) At π = 10.7 mN/m, MLF was transformed in a highly condensed structure with virtually no black areas.
The area enclosed between the compression and decompression curves of the same cycle – hysteresis (ΔΔG) – was computed as described previously.27 For pure meibum loaded in the initial amount of 16 μg per 75 cm2 of the trough's surface area (or ∼0.21 μg/cm2) and compressed to 14.5 cm2, the value of ΔΔG was approximately 3.1 μJ (or 1.9 J/g of meibum).
Effects of Cer on Meibomian Lipids Films
To evaluate the effects of Cer on MLF when loaded directly onto the surface of an aqueous subphase, a series of mixed chloroformic solutions of Cer and human meibum was prepared. The tested Cer-to-meibum weight ratios were 10:0, 8:2, 6:4, 4:6, 2:8, 1:9, 0.5:9.5, and 0:10, respectively. The (π/A) isotherms of these mixtures (Fig. 2) differed significantly from those of pure meibum: the films made from Cer/meibum mixtures had much higher elasticity and ΔΔG compared to pure meibum. The Cer-enriched MLF showed a considerable increase in the values of πm and elasticity (see below) even at low Cer-to-meibum ratios, such as, for example, 0.5:9.5 (wt/wt). The latter mixtures produced πm around 20 mN/m, with values of Cs−1 being 17.0 ± 1.4 mN/m (mean ± SD, n = 3) at the trough's surface area of 18 cm2, 13.9 ± 0.6 at 25 cm2, and 7.5 ± 0.2 at 40 cm2. The mixed lipid layer formed of a mixture of Cer and meibum in the ratio of 2:8 (wt/wt) or less did not show the classic collapsing pattern. However, its πm rose faster than that of pure meibum, especially at higher degrees of compression meaning higher rigidity of the Cer-enriched meibum. The mixed MLF formed from Cer and meibum taken in the ratio of 4:6 (wt/wt) or above, abruptly collapsed at ≥40 mN/m. The extent of collapsing was proportional to the ratio of Cer-to-meibum in the mixture. The morphology of the MLF is shown in Figure 3. At low surface pressures, the Cer-enriched MLF mixture (Fig. 3A) appeared to be similar to meibum, with brighter, more condensed lipid aggregates surrounded by darker areas of thinner and/or less organized lipid material. However, a significantly higher surface pressure was achieved at the areas between 40 and 15 cm2 with the Cer-meibum mixtures than with pure MLF (Figs. 3B, 3C). The morphology of the Cer-enriched MLF (2:8 wt/wt) at 15 cm2 differed dramatically from that of pure MLF. In the presence of Cer, the meibomian lipids were not able to form a uniform, homogeneous layer at higher compression ratios. Instead, a brighter, much more complex and undulating pattern was observed.
Figure 2.
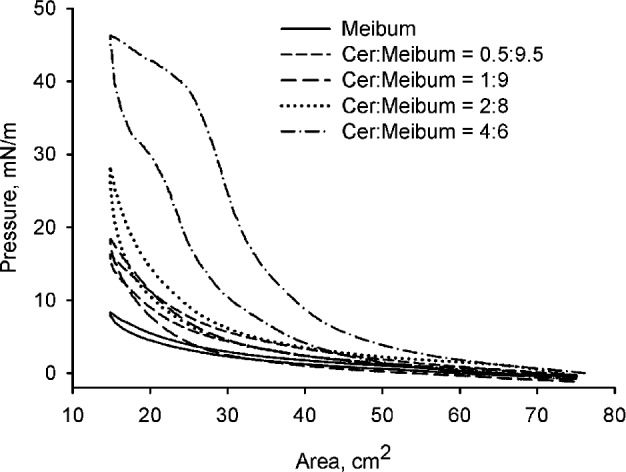
(π/A)-Isotherms of MLF and Cer-enriched mixed MLF on TBS subphase at 34°C. Increasing the concentration of Cer in the mixed MLF affected the π/A-isotherm: note the progressive increases in hysteresis and in-plane elasticity modulus of the films when using a range of lipid mixtures containing higher proportions of Cer in meibum.
Figure 3.
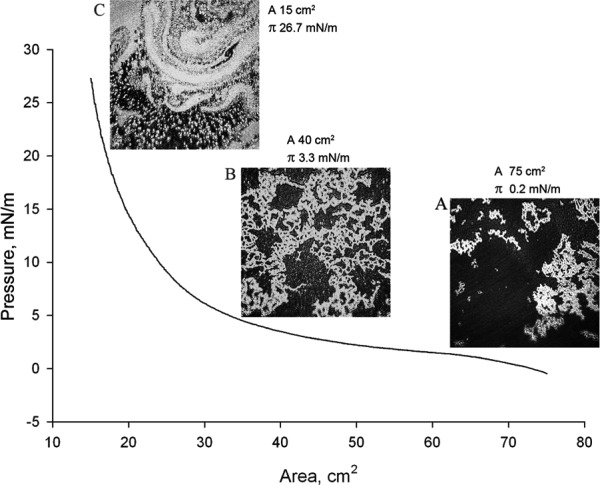
An ascending (compression) branch of a (π/A)-isotherm of Cer-enriched mixed MLF at 34°C and micro-BAM photographs of the film taken at different degrees of its compression. The (π/A)-isotherm of Cer-enriched meibum was obtained using a Cer/meibum weight ratio of 2:8 (wt/wt). (A) At a low surface pressure of 0.2 mN/m, the lipid mixture looked similar to meibum, with brighter, more condensed lipid aggregates surrounded by darker areas of a thinner and/or less organized lipid material. However, some thinning of the structures was observed. (B) Compared to pure MLF, a slightly higher surface pressure of 3.3 mN/m was achieved when the mixed MLF was compressed from the initial A = 75 cm2 to A = 40 cm2. However, the morphology of the Cer-enriched MLF (2:8, wt/wt) in these conditions resembled that of pure MLF at comparable degrees of compression. (C) When compressed to 15 cm2 and π = 26.7 mN/m, the mixed MLF differed dramatically from pure MLF, displaying a swirling pattern (astonishingly similar to the van Gogh's “The Starry Night” painting).
To quantitate the effects of Cer on meibum, the in-plane elasticity modulus Cs−1 of Cer was calculated using a method described in our previous report and illustrated in Figure 4.22,27 The MLF showed a relatively constant Cs−1 of 15 ± 5 mN/m regardless the area of compression. Adding Cer to meibum in the 0.5:9.5 (wt/wt) ratio did not lead to a dramatic change compared to meibum alone: only a moderate 5 mN/m increase was observed throughout the compression cycle. However, Cer mixed with meibum in the ratio of 2:8 (wt/wt) produced a dramatically higher Cs−1 value during the initial and final parts of the compression cycle.
Figure 4.
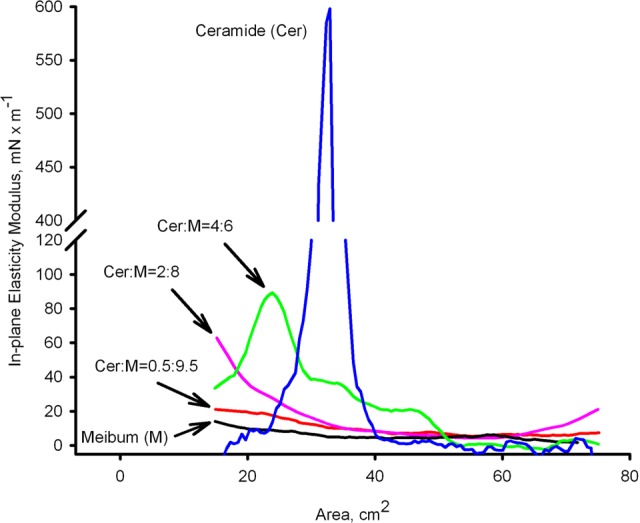
The in-plane elasticity moduli (Cs−1) of pure Cer and Cer-enriched mixed MLF at 34°C. Pure Cer produced highly rigid films with Cs−1 approaching 600 mN/m. The pure MLF showed a relatively constant Cs−1 of 15 ± 5 mN/m regardless its compression ratio. When mixed with meibum, Cer caused a ratio-dependent increase in Cs−1.
A completely different phenomenon was observed when a ratio of Cer-to-meibum of 4:6 (wt/wt) or above was tested. In these experiments, a low initial value was similar to that of pure meibum, followed by a sudden increase in the Cs−1 values approximately at 2/3 into the compression cycle (Fig. 4). This rise in the Cs−1 continued with the increase in the Cer-to-meibum ratio, reaching values as high as 600 mN/m for pure Cer. Then, an abrupt decrease in the elasticity was registered, with the Cs−1 values dropping to 30 ± 5 mN/m and below, an obvious sign of collapsing.
Even small amounts of exogenous Cer caused a noticeable increase in the hysteresis of the mixed MLF. When Cer was added to meibum in the amount of 0.5:9.5 (wt/wt, or 5%), ΔΔG of the film more than doubled from 1.9 J/g for pure meibum to 4.7 J/g for its mixture with Cer. Further increase in the Cer-to-meibum ratio led to a concentration-depended increase in ΔΔG: 5.1 J/g for the 2:8 (wt/wt) and 24.1 J/g for the 4:6 (wt/wt) mixtures. Note that ΔΔG of pure Cer was measured to be a very high 126 J/g.
Effects of FC on Meibomian Lipids Films
Though FC in a normal meibum sample amounts for on average 0.5% to 0.7% (wt/wt) of all cholesterol-containing compounds (Fig. 5A, black trace, and prior results28), in aqueous tears collected from the same normal donor it rose more than 10-fold to approximately 9% (Fig. 5A, red trace). Notably, this ratio remained high for all four participating volunteers (Fig. 5B). Also, in agreement with our earlier report24 on the increased amounts of shorter chain lipids in aqueous tears compared to meibum, noticed was an increase in the relative amount of shorter chain cholesteryl esters – their corresponding peaks for aqueous tears samples were substantially higher than their counterparts in meibum samples. The largest increase, from 0.6% to 6%, was observed for cholesteryl esters with C16 to C20 fatty acids, while the rest of the cholesteryl esters pool remained virtually unchanged.
Figure 5.
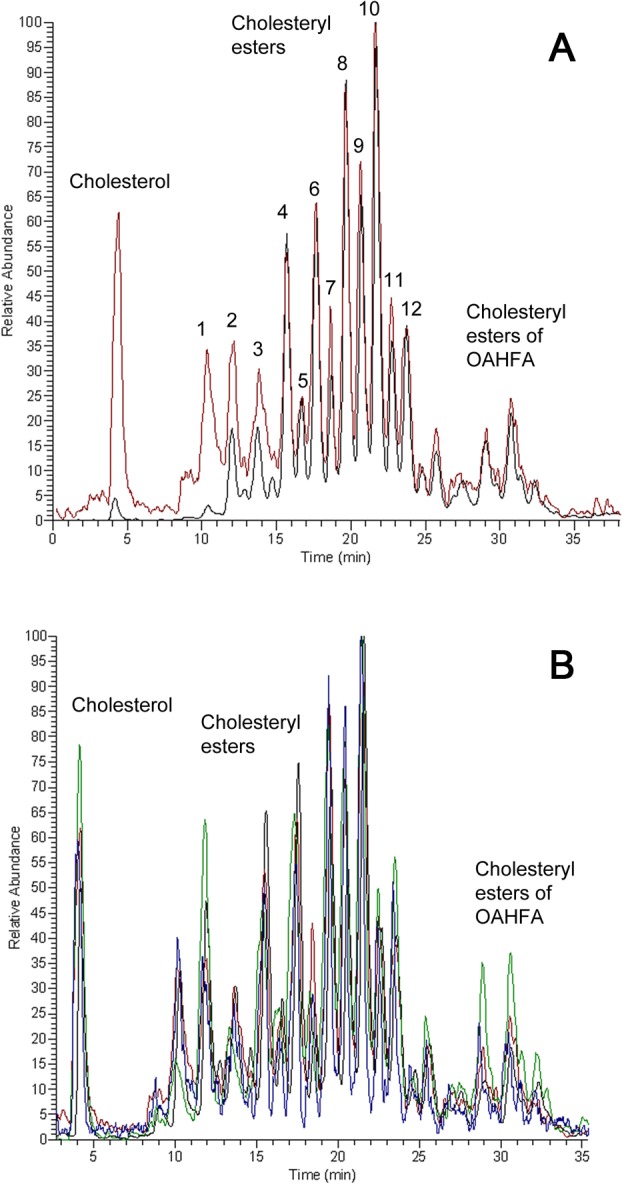
Mass spectrometric analysis of FC and other cholesteryl-containing compounds in meibum and aqueous tears samples collected from healthy, non-dry eye donors. (A) Cholesteryl-containing compounds detected in meibum (black trace) and aqueous tears (red trace) samples of a healthy, non-dry eye Caucasian male. Note a more than 10-fold difference in the signal of FC. The following cholesteryl esters (labeled hereinafter as FA-Chl) were detected: peak 1, C16:0-Chl and C18:1-Chl; peak 2, C18:0-Chl and C20:1-Chl; peak 3, C16:0-Chl and C18:1-Chl; peak 3, C19:0-Chl; peak 4, C20:0-Chl and C22:1-Chl; peak 5, C21:0-Chl; peak 6, C22:0-Chl and C24:1-Chl; peak 7, C23:0-Chl; peak 8, C24:0-Chl and C26:1-Chl; peak 9, C25:0-Chl; peak 10, C26:0-Chl and C28:1-Chl; peak 11, C27:0-Chl; peak 12, C28:0-Chl and C30:1-Chl. (B) Cholesteryl-containing compounds detected in aqueous tears samples. The analytes were detected as described earlier28 by monitoring ions m/z 369. Four samples are shown. Note the consistently high presence of FC in these samples.
To study the effects of FC on MLF, a series of mixed solutions of FC and meibum were prepared. The FC-to-meibum weight ratios were similar to those used in our previous experiments with Cer (10:0, 8:2, 6:4, 4:6, 2:8, 1:9, 0.5:9.5, and 0:10, respectively). A progressive increase in the FC-to-meibum ratio produced significant changes in the shape of (π/A) isotherms. As we reported previously,27 pure MLF at a surface concentration of approximately 0.2 μg meibum/cm2 occupied all the available surface of the trough (∼75 cm2) forming a stable, elastic layer that, after the initial adjustment, showed no signs of collapsing even after repetitive compression–decompression cycles. When FC was mixed with meibum in a ratio of 0.5:9.5 (wt/wt) or above, there was a gradual ratio-dependent increase in the values of πm and hysteresis ΔΔG (Fig. 6). Also, the latter mixtures produced πm around 20 to 25 mN/m, with values of Cs−1 being 18.2 ± 1.5mN/m (mean ± SD, n = 3) at the trough's surface area of 18 cm2, 12.0 ± 0.2 at 25 cm2, and 4.6 ± 0.3 at 40 cm2. Note that, as with Cer, FC more than quadrupled the values of achievable πm and increased ΔΔG at least 10-fold from 3.1 μJ for pure meibum to 33 μJ for the FC:meibum = 4:6 (wt/wt) mixture, or 1.9 and 20 J/g of lipid mixture, respectively. When the lowest tested amount of FC was added to meibum to make a 0.5:9.5 (wt/wt) mixture, it doubled the value of ΔΔG of the mixed MLF from 1.9 to 3.8 J/g. If present in a 1:9 (wt/wt) ratio, FC caused a 3-fold increase in ΔΔG to 5.6 J/g, while in the 2:8 and 4:6 mixtures it was even higher at 16.5 and 21 J/g, respectively. For lipid films of FC, ΔΔG was found to be approximately 67 J/g.
Figure 6.
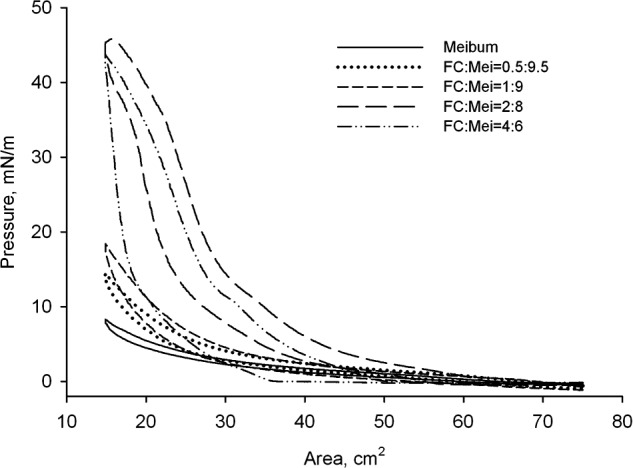
Surface pressure – area (π/A) isotherms of FC-enriched MLF at 34°C. When FC was present in a ratio of 0.5:9.5 (wt/wt) or above, there was a gradual ratio-dependent increase in the values of π and hysteresis.
Figure 7 shows a (π/A) isotherm of a FC-to-meibum mixture made in a 2:8 (wt/wt) ratio. Compared to pure meibum, the FC-to-meibum mixtures displayed a higher surface pressure throughout the compression cycle. During the initial stage of the compression, a pattern of bright scattered lipids aggregates was observed (Fig. 7A). Then, as the surface pressure rose to 5 to 10 mN/m, the lipids mixture developed a net-like pattern, where bright lipid aggregates were surrounded by much larger dark areas (Fig. 7B). These structures resembled those formed of pure meibum at similar surface pressures. However, after being compressed further to a surface area of below 30 cm2 and surface pressure of more than approximately 20 mN/m, the mixture started to differ significantly from pure meibum: the typical net-like pattern characteristic of pure human meibum disappeared being replaced with aggregates of different, and more diverse, shapes (Fig. 7C).
Figure 7.
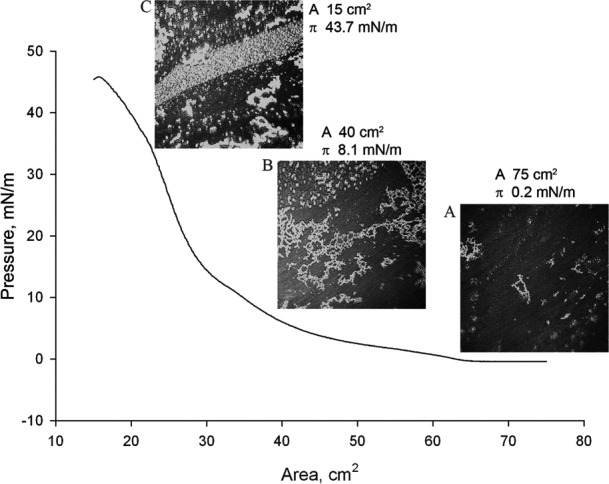
A compression branch of a (π/A)-isotherm of FC-enriched mixed MLF at 34°C and micro-BAM photographs of the film taken at different degrees of its compression. The (π/A) isotherm of FC-enriched meibum was obtained using a FC/meibum weight ratio of 2:8 (wt/wt). (A) At a low surface pressure of 0.2 mN/m, the lipid mixture looked more dispersed and scattered than pure meibum. Brighter, more condensed lipid aggregates were smaller than those of pure meibum. (B) Upon closing the trough's barriers to π = 8.1 mN/m, the FC-enriched mixed MLF re-arranged forming condensed net-like lipid structures somewhat similar to structures formed from pure meibum. Note that in the presence of FC the values of π and πm quadrupled compared to pure meibum. Still, the bright particles seemed to be more dispersed as those of pure meibum and of Cer-enriched mixed MLF. (C) When compressed to 15 cm2 and π = 43.7 mN/m, the mixed FC-enriched MLF differed dramatically from pure MLF, displaying much more heterogeneous (and, apparently, more dispersed) a pattern.
As mentioned above, the pure MLF produced a relatively stable film with in-plane elasticity modulus Cs−1 of 10 ± 5 mN/m. Incremental additions of even small amounts of FC to meibum, such as 0.5:9.5 (wt/wt) or so, led to a progressive increase in the Cs−1 values compared to pure MLF (Fig. 8, and data presented above). An increase in the FC-to-meibum ratio to 2:8 (wt/wt) or 4:6 (wt/wt), produced a rapid rise in Cs−1 values around the surface areas of approximately 30 cm2 to 80 mN/m and 100 mN/m, respectively, followed by a rapid collapsing of the film. In our hands, pure FC formed a thin, elastic (i.e., very rigid) layer capable of generating high surface pressures and reaching Cs−1 values as high as 640 mN/m before collapsing. However, this film was almost imperceptible when using the micro-BAM (see below).
Figure 8.
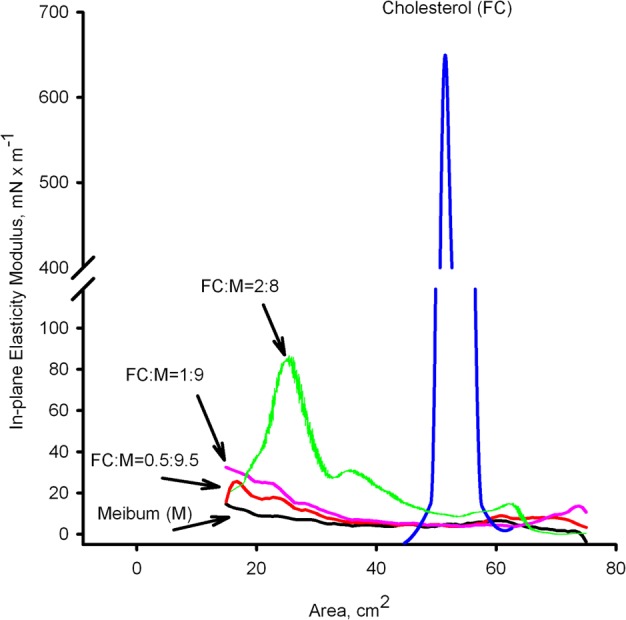
The in-plane elasticity moduli (Cs−1) of pure FC and FC-enriched mixed MLF at 34°C. Pure FC produced a highly rigid film with Cs−1 approaching 640 mN/m. The effect of FC was concentration-dependent.
A series of images obtained using the micro-BAM is shown in Figure 9. The tested concentrations of Cer and FC in their corresponding mixtures with meibum are high and not likely to be met in vivo. However, these extreme cases were chosen to depict the complex relationship of FC and Cer with human meibum, and illustrate the mechanism of their interaction with meibomian lipids. As a baseline against which the lipid mixtures were to be evaluated, TBS with no added lipids or contaminants is shown in Figure 9A, while Figure 9B shows the pure MLF at a surface concentration of 0.2 μg/cm2. MLF appears as bright net-like aggregates surrounded by darker areas. Figure 9C illustrates pure bovine brain Cer loaded on the surface at a concentration of 0.2 μg/cm2. Cer appears as a rigid, noncompressible and nonexpandable whitish-to-grayish sheet-like structure, which fractured easily upon a physical impact. A surface film made from FC appeared as fine, freely moving round grayish aggregates, only visible at high surface pressures (Fig. 9D). Note that the amount of loaded FC was similar to other tested compounds and mixtures (0.2 μg/cm2). Figure 9E represents a Cer/meibum 8:2 (wt/wt) mixture. Here, a more flexible and fluid surface film was observed. It was composed of bright, small, roundish lipid aggregates. A FC/meibum 8:2 (wt/wt) mixture is shown in Figure 9F. One can see a complex morphology of the film, which was very different from other tested samples. Several types of freely moving structures could be observed, most likely composed of meibum material as a core, surrounded by FC.
Figure 9.
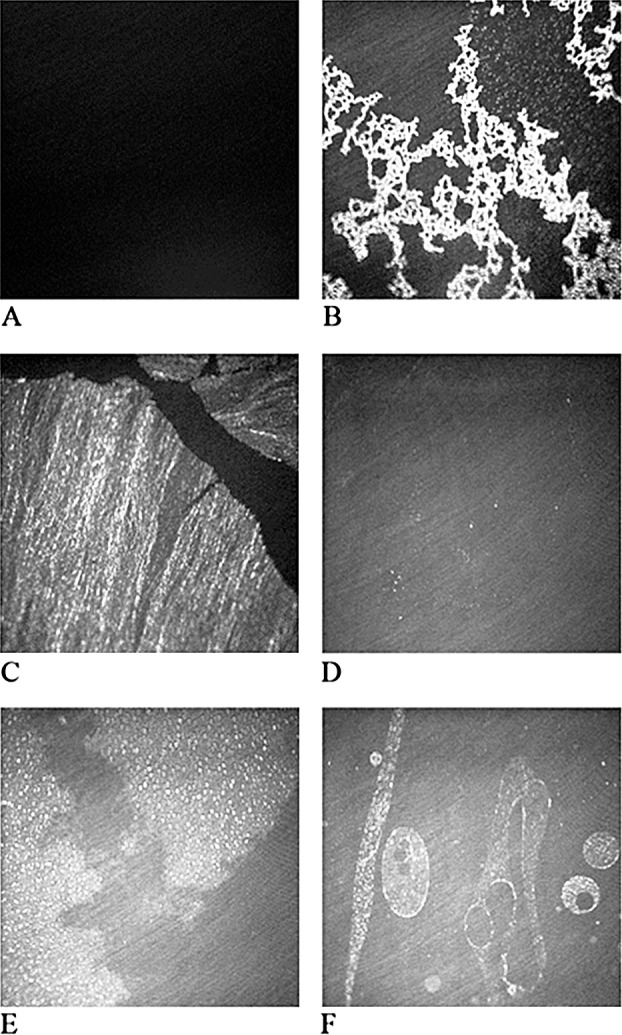
A series of images of surface films obtained using the micro-BAM. (A) TBS with no added lipids or contaminants is shown. (B) Pure meibomian lipid films (MLF) at a surface concentration of 0.21μg/cm2. (C) Pure Cer loaded on the surface at a concentration of 0.2 μg/cm2. Cer appeared as a rigid, noncompressible and nonexpandable structure, which easily fractured upon physical impact. Note black, apparently lipid-free channels (cracks) between sheets of Cer. (D) FC loaded on the surface at a concentration of 0.2 μg/cm2. FC appears as fine, freely moving round grayish aggregates, only visible at high surface pressures. (E) A Cer-enriched mixed MLF at a weight ratio of 8:2 (wt/wt) and a surface concentration of 0.2 μg/cm2. This lipid mixture was much more dispersed, dynamic, and compressible than Cer alone. (F) A FC/meibum 8:2 (wt/wt) mixture is shown. Here, freely moving roundish superstructures can be observed, most likely composed of smaller buckshot-like sub-structures made from dispersed meibum material surrounded by FC (see Fig. 9 for details).
Discussion
Abnormal keratinization of the meibomian gland ductal epithelium and plugging of the meibomian gland orifices in rabbits and humans,18,30 with a concomitant increase in Cer production in epinephrine-induced MGD of rabbits,12 have been reported as signs or complications of MGD. Obata noted similarities between the human keratinizing stratified squamous epithelium of meibomian ducts and the epidermis of skin.31 The epidermis, especially the stratified squamous layer, is producing and metabolizing Cer actively.32 In those tissues where keratinization occurs (such as stratum corneum), Cer form massive intra- and extracellular lamellar membranes and bodies that, together with keratin, FC, and free fatty acids, create a water-permeability barrier. It seems plausible that the excessive amounts of Cer overproduced by abnormal epithelial cells could mix with normal meibomian gland secretions and change its lipid balance in the process that can be exacerbated by the diminishing capacity of acinar cells to produce normal lipids.31 This idea also is supported by recent histologic studies conducted by Knop et al. whose data demonstrate the possibility of mixing of meibum with lipids produced by stratum corneum.33 The levels of FC in meibum also can be elevated because of inflammation or bacterial infection due to the enhanced activity of lipases.21,34,35 These pathologic processes may alter the chemical composition of human meibum, negatively affecting the TF. Moreover, even in normal aqueous tears the levels of FC are much higher than those in meibum (Fig. 5). Thus, our hypothesis was that Cer and FC could be responsible for the disadvantageous changes in the rheologic properties of the TF and TFLL, and the subsequent onset of DED.
To test the effects of Cer and FC on meibomian films, a series of mixed solutions with varying Cer- or FC-to-meibum ratios (10:0, 8:2, 6:4, 4:6, 2:8, 1:9, 0.5:9.5, and 0:10, wt/wt) was made. These ratios were selected primarily to elucidate and demonstrate the mechanisms through which Cer and FC could affect the meibomian films: one should not expect either of the compounds to exceed a few percentage points in vivo. However, the mass ratio of FC to other cholesteryl-containing compounds in aqueous tears is at least 10-fold higher than in meibum, which could affect the properties of MLF in vivo. Notably, in an earlier animal study,12 FC and Cer that comprised, respectively, 4.5% and approximately 8% of meibum in normal subjects, were shown to be increased by 50% to 100% in the MGD animals. With the exception of studies by Shine and McCulley,19,20 we are not aware of any attempt to measure the changes in meibomian FC in the relation to dry eye disease. Importantly, Lam et al detected a 30% increase in the levels of Cer in human dry eye patients.11 Therefore, one could not exclude a possibility that levels of FC and Cer could reach noticeably higher levels in pathologic meibum and, even more so, in pathologic aqueous tears.
Previously, we reported a remarkable ability of FFA to disrupt, or prevent the formation of, human MLF, which was studied using the LT technique.22 When added even in small, but physiologically relevant concentrations, these compounds demonstrated strong surfactant properties, and facilitated the wetting and removal of other lipids from the air–water interface.22
In our current experiments, the presence of Cer and FC had a strong impact on the physicochemical properties of MLF: the hysteresis and rigidity of the mixed MLF were considerably higher than those measured for films formed from pure meibum. For a few tested conditions (such as surface areas 18, 25, and 40 cm2; see above), there was a statistically significant increase in the in-plane elasticity moduli of the FC–meibum and Cer–meibum mixtures versus pure meibum even if FC or Cer were present in 0.5:9.5 (wt/wt) ratios (P < 0.05). However, there was no statistically significant difference observed between the effects of FC and Cer, for tested conditions (P > 0.3). An up to 10-fold higher hysteresis ΔΔG of the tested FC-to-meibum mixtures compared to pure meibum meant that the mixed MLF with exogenously added Cer or FC rearranged in such a way that prevented them from re-expanding quickly and from returning to their precompression state after they had been compressed sufficiently. The return of the compressed mixed MLF to its original, precompression state occurred only when the trough's barriers were open completely, and the surface pressure dropped to the precompression levels.27 In our experiments, hysteresis of the mixed MLF was directly proportional to the amounts of Cer and/or FC in the films: the higher the presence of these compounds, the higher the hysteresis. In vivo, the higher rigidity of FC- and/or Cer-enriched MLF, and their reduced dynamics, could, and probably do, hamper restoration of the TF and TFLL after blinking.
Meibomian lipids, which are more hydrophobic than Cer, normally do not contain noticeable amounts of Cer, and melt at approximately 32°C.24 However, Hykin and Bron reported that lid margin hyperkeratinization increased with age in both eyelids,36 which could elevate the Cer level in MLF and, consequently, disrupt the normal TFLL physiology. Thus, it was important to evaluate the effects of Cer on MLF qualitatively and quantitatively.
In the absence of Cer, and before being subjected to multiple compression–expansion cycles, pure MLF appeared as relatively uniform and whitish dot-like structures surrounded by darker areas (possibly empty or filled with disorganized lipid material). However, following just a few compression–expansion cycles, these dot-like structures rearranged quickly into much more visible and stable net-like structures, which persisted virtually indefinitely regardless the number of subsequent compression–decompression cycles.
In our hands, even small amounts of exogenously added Cer had a strong impact on the mixed MLF. When Cer were added to meibum in a ratio of 0.5:9.5 (wt/wt) or higher, the shape of π/A isotherm became distinctively different from that of pure MLF: the isotherms showed an abrupt increase in their slopes (i.e., a progressively faster increase in π). Using the micro-BAM for direct visualization of this transition, much larger aggregates that tended to self-assemble in mobile, curvilinear agglomerates were detected (Fig. 3C). At high compression ratios, their appearance and dynamics were very different from those of pure meibum. This possibly can affect meibum, TF, and/or TFLL in subjects with MGD, whose meibum was shown to have higher concentration of Cer than meibum of normal subjects.11,12 Cer species consist of a long-chain sphingosine base linked to a fatty acid via an amide bond. Cer have high melting points (typically, between 90°C and 110°C), they are amphiphilic (meaning that they have highly polar and highly hydrophobic groups in their structures), but at the same time they are extremely poorly soluble in water. At physiological temperatures, Cer have been shown to form highly condensed and rigid lipid films. These properties make Cer an important part of the skin permeability barrier.37,38
Thus, increased levels of Cer possibly can have a dual effect on meibum: Cer can increase the melting temperature of meibum, and Cer can cause changes in the way meibum spreads on the ocular surface. A typical surface structure formed by pure Cer on the TBS subphase is shown in Figure 9C. There, Cer appeared to form well-organized, solid, glass-like sheets that fractured and collapsed easily. This observation is critical due to the fact that normal human TFLL is very dynamic and recovers to its original state quickly after the blink. Figure 9E illustrates a Cer/MLF mixed at a weight ratio of 8:2 (wt/wt). This lipid mixture was more flexible, dynamic, and compressible than Cer alone. It was formed of bright (i.e., highly organized) dot (or buckshot)-like structures, enclosed by grayish, more loosely organized lipids. The increased fluidity of the film (compared to pure Cer) undoubtedly was caused by a strong Cer–meibum interaction, whose effects on π and πm were tangible even at low Cer-to-meibum ratios (Fig. 2).
FC easily forms interfacial layers, but they are not very stable. A FC molecule consists of a rigid polycyclic body, a flexible alkane tail, and an OH group,39 which makes it relatively amphiphilic. When placed at an air–water interface, FC's hydroxyl group forms hydrogen bonds with molecules of water, while the rest of the molecule either lies flat on the surface of the aqueous subphase, or is positioned at an angle to it. The degree of tilting generally depends on the surface pressure of the layer. Slotte and Mattjus observed the collapse behavior of FC films, and reported that a typical monolayer collapsed at approximately πm ∼47 to 48 mN/m.40 This correlates well with our results, which showed that a FC monolayer collapsed at 46 to 47 mN/m (not shown). The same investigators used epifluorescence microscopy to visualize the FC monolayer, and described it as formed from many bright drop-like inclusions similar to the FC and meibum aggregates shown in our Figure 9F.
All mixtures of FC and meibum exhibited higher rigidity and hysteresis than pure MLF. Interestingly, darker and, seemingly, empty spaces surrounded by brighter lipid aggregates were evident. This could mean only that those darker areas were, actually, filled with disordered lipid molecules, in this case FC–meibum mixtures. Had those dark areas been empty, the π/A isotherms would have reflected their presence by showing little or no resistance to the closing barriers of the LT until the empty spaces had closed. Figure 9D demonstrates a surface film of FC as observed using the micro-BAM. In this experiment, the amount of loaded FC was sufficient to saturate the entire air–water interface. However, the FC surface film was barely visible by micro-BAM (most likely because of its disordered state), but still very rigid and collapsible. The shape of mixed lipid aggregates formed from FC and meibum taken in a 2:8 (wt/wt) ratio differed significantly from those formed from pure meibum (Figs. 1, 7).
When FC and meibum were mixed in an 8:2 (wt/wt) ratio, an interesting phenomenon was observed (Fig. 9F): instead of net-like structures characteristic of pure meibum, large numbers of mobile, droplet-like aggregates formed. Considering the amphiphilic nature of FC and Cer, it is reasonable to assume that the cores of the aggregates that they form with meibum are composed of hydrophobic meibum lipids, while their shells are composed of much more polar FC or Cer. Thus, their structure may resemble that of large mixed micelles. Such interactions between FC and meibomian lipids might hamper, or even prevent, the normal spreading and respreading of meibum on the TF in vivo, and did increase hysteresis in the mixed FC/meibum films in vitro.
A much higher hysteresis ΔΔG measured for mixed MLF corroborated our micro-BAM observations, and reflected the deep structural rearrangements inside the mixed MLF, which were proportional to the Cer-to-meibum and FC-to-meibum ratios. The higher hysteresis meant that the mixed lipid aggregates assembled in tightly packed structures, which, once compressed, tended either to stay together longer or to dissociate at much lower surface pressures than pure meibomian lipids. This phenomenon can be a factor in vivo, measurably impeding the meibum's spreading–respreading behavior upon the blinking.
Thus, our findings suggest that the increased amounts of Cer and FC in meibum of, respectively, MGD and CPB patients reported in independent studies can have a disruptive effect on TF in general, and TFLL specifically, but only if their amounts reach sufficient levels. As we demonstrated in our study (Fig. 5), FC in meibum and tears can reach the threshold. Indicatively, in 2003, Shine et al. reported that after minocycline treatment the FC content of human meibum collected from patients with meibomian keratoconjunctivitis (meibomianitis) dropped from a very high 4.5% to a much more normal 0.3% of all detected lipids.20 Only limited information on the quantities of Cer in human normal and DE meibum currently is available,11 and gathering such information would necessitate a large clinical study, which was beyond the scope of this report.
It is important to mention that TF and TFLL also can be impacted by various exogenous factors that are not related directly to the changes in meibum as it is produced by MG. For example, adverse ocular disorders, such as chalazia, hordeolum, and various dermoids, are common lesions of the eyelid that affect a large proportion of the general population.41,42 In a recent study, more than 9000 cases of chalazia have been evaluated and found to be associated significantly with blepharitis (33% of all cases).43 Biochemically, the chalazion and the hordeolum are lipid-enriched lesions. No information on the exact lipid composition of hordeolum is available to the investigators. However, the lipid composition of chalazion44 is distinctively different from that of normal meibum, and its nodule is enriched with lipids, such as FFA, FC, and Cer. In fact, the levels of the latter three classes of lipids in studied chalazion samples were, respectively, 2.1% to 2.6%, 8.5% to 12.6%, and 4.2% to 11.6% of the total lipid pool of the lipids of chalazia.44 Therefore, one can speculate that spontaneous release, or forced extrusion, of these lipids onto the ocular surface may shift its lipid balance considerably. Inadvertent changes in the TF and TFLL also could be caused by FC, Cer, and their synthetic analogs used as drug-delivery vehicles (e.g., for topical steroids45), components of skin care formulations (e.g., EpiCeram; PuraCap Pharmaceutical, LLC, South Plainfield, NJ), and as cosmetics products (e.g., Cer-enriched capsules, creams, mascara, and eyeliners from various manufacturers), if applied on the eyelids or around the eye. Therefore, potentially adverse effects of FC and Cer on meibomian lipid films described in this study should be taken into account, especially in relation to dry eye disease.
Footnotes
Supported by an NIH Grant R01EY019480 (IAB), a core grant for vision research P30EY020799-03, and an unrestricted grant from the Research to Prevent Blindness Foundation, New York, New York.
Disclosure: J.C. Arciniega, None; E. Uchiyama, None; I.A. Butovich, None
References
- 1. Van Haeringen NJ. Clinical biochemistry of tears. Surv Ophthalmol. 1981; 26: 84–96 [DOI] [PubMed] [Google Scholar]
- 2. Brauninger GE, Shah DO, Kaufman HE. Direct physical demonstration of oily layer on tear film surface. Am J Ophthalmol. 1972; 73: 132–134 [DOI] [PubMed] [Google Scholar]
- 3. McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997; 95: 79–88, discussion 88–93 [PMC free article] [PubMed] [Google Scholar]
- 4. Andrews JS. Human tear film lipids. I. Composition of the principal non-polar component. Exp Eye Res. 1970; 10: 223–227 [DOI] [PubMed] [Google Scholar]
- 5. Tiffany JM. The lipid secretion of the meibomian glands. Adv Lipid Res. 1987; 22: 1–62 [DOI] [PubMed] [Google Scholar]
- 6. Joffre C, Souchier M, Gregoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008; 92: 116–119 [DOI] [PubMed] [Google Scholar]
- 7. McCulley JP, Shine WE. The lipid layer of tears: dependent on meibomian gland function. Exp Eye Res. 2004; 78: 361–365 [DOI] [PubMed] [Google Scholar]
- 8. Shine WE, McCulley JP. Association of meibum oleic acid with meibomian seborrhea. Cornea. 2000; 19: 72–74 [DOI] [PubMed] [Google Scholar]
- 9. Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996; 15: 340–346 [DOI] [PubMed] [Google Scholar]
- 10. Bron AJ, Tiffany JM. The meibomian glands and tear film lipids. Structure, function, and control. Adv Exp Med Biol. 1998; 438: 281–295 [DOI] [PubMed] [Google Scholar]
- 11. Lam SM, Tong L, Yong SS, et al. Meibum lipid composition in Asians with dry eye disease. PloS One. 2011; 6: e24339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nicolaides N, Santos EC, Smith RE, Jester JV. Meibomian gland dysfunction. III. Meibomian gland lipids. Invest Ophthalmol Vis Sci. 1989; 30: 946–951 [PubMed] [Google Scholar]
- 13. McCulley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003; 1: 97–106 [DOI] [PubMed] [Google Scholar]
- 14. McCulley JP, Sciallis GF. Meibomian keratoconjunctivitis. Am J Ophthalmol. 1977; 84: 788–793 [DOI] [PubMed] [Google Scholar]
- 15. Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM III, Smith RE. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981; 20: 522–536 [PubMed] [Google Scholar]
- 16. Butovich IA. The Meibomian puzzle: combining pieces together. Prog Retin Eye Res. 2009; 28: 483–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007; 42: 765–776 [DOI] [PubMed] [Google Scholar]
- 18. Jester JV, Nicolaides N, Kiss-Palvolgyi I, Smith RE. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Invest Ophthalmol Vis Sci. 1989; 30: 936–945 [PubMed] [Google Scholar]
- 19. Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991; 32: 2272–2280 [PubMed] [Google Scholar]
- 20. Shine W, McCulley JP, Pandya AG. Minocycline effect on meibomian gland lipids in meibomianitis patients. Exp Eye Res. 2003; 76: 417–420 [DOI] [PubMed] [Google Scholar]
- 21. Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci. 1986; 27: 486–491 [PubMed] [Google Scholar]
- 22. Arciniega JC, Nadji EJ, Butovich IA. Effects of free fatty acids on meibomian lipid films. Exp Eye Res. 2011; 93: 452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butovich IA. Lipidomic analysis of human meibum using HPLC-MSn. Methods Mol Biol. 2009; 579: 221–246 [DOI] [PubMed] [Google Scholar]
- 24. Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008; 49: 3779–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butovich IA, Lu H, McMahon A, Eule JC. Toward an animal model of the human tear film. Biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Invest Ophthalmol Vis Sci. 2012; 53: 6881–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butovich IA, Arciniega JC, Lu H, Molai M. Evaluation and quantitation of intact wax esters of human meibum by gas-liquid chromatography-ion trap mass spectrometry. Invest Ophthalmol Vis Sci. 2012; 53: 3766–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Butovich IA, Arciniega JC, Wojtowicz JC. Meibomian lipid films and the impact of temperature. Invest Ophthalmol Vis Sci. 2010; 51: 5508–5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009; 50: 501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Georgiev GA, Kutsarova E, Jordanova A, Krastev R, Lalchev Z. Interactions of Meibomian gland secretion with polar lipids in Langmuir monolayers. Coll Surf B Biointerfaces. 2010; 78: 317–327 [DOI] [PubMed] [Google Scholar]
- 30. Ong BL, Hodson SA, Wigham T, Miller F, Larke JR. Evidence for keratin proteins in normal and abnormal human meibomian fluids. Curr Eye Res. 1991; 10: 1113–1119 [DOI] [PubMed] [Google Scholar]
- 31. Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002; 21: S70–S74 [DOI] [PubMed] [Google Scholar]
- 32. Yoshida N, Sawada E, Imokawa G. A reconstructed human epidermal keratinization culture model to characterize ceramide metabolism in the stratum corneum. Arch Dermatol Res. 2012; 304: 563–577 [DOI] [PubMed] [Google Scholar]
- 33. Knop E, Knop N, Zhivov A, et al. The lid wiper and muco-cutaneous junction anatomy of the human eyelid margins: an in vivo confocal and histological study. J Anat. 2011; 218: 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Auw-Haedrich C, Reinhard T. Chronische blepharitis. Der Ophthalmologe. 2007; 104: 817–826 [DOI] [PubMed] [Google Scholar]
- 35. McCulley JP, Dougherty JM. Bacterial aspects of chronic blepharitis. Trans Ophthalmol Soc U K. 1986; 105 (pt 3): 314–318 [PubMed] [Google Scholar]
- 36. Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992; 11: 334–342 [DOI] [PubMed] [Google Scholar]
- 37. McMahon A, Butovich IA, Kedzierski W. Epidermal expression of an Elovl4 transgene rescues neonatal lethality of homozygous Stargardt disease-3 mice. J Lipid Res. 2011; 52: 1128–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McMahon A, Butovich IA, Mata NL, et al. Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Mol Vis. 2007; 13: 258–272 [PMC free article] [PubMed] [Google Scholar]
- 39. Cromie SR, Del Popolo MG, Ballone P. Amphiphilic character and aggregation properties of small cholesterol islands on water: a simulation study. J Phys Chem B. 2009; 113: 4674–4687 [DOI] [PubMed] [Google Scholar]
- 40. Slotte JP, Mattjus P. Visualization of lateral phases in cholesterol and phosphatidylcholine monolayers at the air/water interface – a comparative study with two different reporter molecules. BiochimBiophys Acta. 1995; 1254: 22–29 [DOI] [PubMed] [Google Scholar]
- 41. Ehrenhaus MP. Hordeolum. Available at: http://emedicine.medscape.com/article/1213080-overview#a0104. Accessed January 8, 2013. [Google Scholar]
- 42. Wessels IF. Chalazion. Available at: http://emedicine.medscape.com/article/1212709-overview#a0101. Accessed January 8, 2013. [Google Scholar]
- 43. Nemet AY, Vinker S, Kaiserman I. Associated morbidity of chalazia. Cornea. 2011; 30: 1376–1381 [DOI] [PubMed] [Google Scholar]
- 44. Nicolaides N, Flores A, Santos EC, Robin JB, Smith RE. The lipids of chalazia. Invest Ophthalmol Vis Sci. 1988; 29: 482–486 [PubMed] [Google Scholar]
- 45. Lee YB, Park HJ, Kwon MJ, Jeong SK, Cho SY. Beneficial effects of pseudoceramide-containing physiologic lipid mixture as vehicle for topical steroids. Eur J Dermatol. 2011; 21: 710–716 [DOI] [PubMed] [Google Scholar]


