Abstract
Purpose.
Although diabetic retinopathy (DR) is clinically diagnosed based on vascular pathology, diabetic patients with angiographically normal retinas have been found to exhibit subtle defects in vision. This has led to the theory that diabetes-associated metabolic abnormalities directly impair neural retinal function before the development of vasculopathy, thereby resulting in visual deficits. In this study, we sought to delineate the temporal relationship between retinal dysfunction and visual deficits in a rat model of Type 1 diabetes. Moreover, we investigated the relative contribution of retinal dysfunction versus diabetes-induced lens opacity, to the visual deficits found in early-stage DR.
Methods.
Pigmented Long Evans rats were rendered diabetic with streptozotocin (STZ). Control and diabetic rats were assessed across 12 weeks of hyperglycemia for visual function with optokinetic tracking weekly visual acuity and monthly contrast sensitivity, retinal function with dark-adapted electroretinograms (monthly electroretinograms [ERGs]), and cataract formation with slit lamp exam (biweekly).
Results.
Diabetic rats exhibited significantly reduced visual function and delayed ERG responses by 1 month post-STZ. Significant cataracts did not develop until 6 weeks post-STZ. Moreover, increases in lens opacity (r = −0.728) and ERG implicit times (r = −0.615 for rod-dominated response and r = −0.322 for rod/cone mixed response) showed significant correlations with reductions in visual acuity in diabetic rats.
Conclusions.
STZ-induced hyperglycemia reduces visual function, affecting both visual acuity and contrast sensitivity. The data suggest that visual defects found in early-stage DR may initially involve abnormalities of the neural retina and worsen with later development of cataracts.
STZ-induced diabetic rats have decreased visual function within weeks of hyperglycemic induction. Visual deficits were significantly correlated with retinal dysfunction and further reduced by formation of cataracts. These results provide additional support for neural defects in diabetic retinopathy.
Introduction
Diabetic retinopathy (DR) is a common complication of diabetes mellitus and a leading cause of blindness in working-age adults.1,2 Progressive vision loss after the diagnosis of DR has been associated with the severity of lesions in the retinal vasculature, such as hemorrhages, macular edema, and neovascularization.3 Unfortunately, by the time these vascular lesions are identified, vision loss is often advanced and irreversible. Interestingly, some studies have found that diabetic (DM) patients with angiographically normal retina experienced subtle visual dysfunction, including abnormal color vision and decreased contrast sensitivity.3–7 Because preventing vision loss is an important therapeutic goal for DM patients, detecting visual deficits at preclinical stages of DR may provide an early window for diagnosis and intervention.
To determine the temporal appearance of visual dysfunction in diabetes, it is important to establish to what degree animal models of diabetes replicate visual defects found in DM patients. An effective behavioral test of visual performance in animals is the assessment of optokinetic response, the ability of an animal to track moving stimuli by moving its head.8,9 Both visual acuity and contrast sensitivity can be measured by varying spatial frequency or contrast of the projected gratings. Assessment of optokinetic tracking (OKT) response with head movement has been reliably quantified in rodents8–11 and can readily distinguish mice with normal vision from those with retinal degeneration.12 A recent report using OKT to evaluate visual function of streptozotocin (STZ)-induced DM rats found reduction in visual acuity at 4 weeks post-STZ, which the authors attributed to reduced expression of visual cycle enzymes.13 However, it remains unclear how the changes in visual function correlate with the more commonly reported changes in electroretinogram (ERG), an indicator of diabetes-induced retinal dysfunction.
Since the observation of diminished and delayed oscillatory potentials in DM patients in the 1960s, alterations in ERG responses have been consistently found in both humans and animals with diabetes, even before the appearance of vascular lesions.14–25 Moreover, multiple reports have established that changes in the multifocal ERG are predictive of DR onset and progression of DR.14,26 Factors underlying ERG changes associated with DM may include neuronal dysfunction (such as decrease in synaptic proteins27 or changes in the levels of neurotransmitters28–30) and cell death in the neural retina.16,31–35 Although visual acuity deficits in early DR may be secondary to retinal dysfunction, the possibility exists that induced lens opacity may contribute to visual loss, as epidemiological studies have revealed increased prevalence of cataracts in DM patients when compared with the non-DM population.36 Thus, a better characterization of the roles of diabetes-associated cataract formation and retinal dysfunction on visual impairment is needed. Therefore, the purposes of this study were (1) to examine longitudinally changes in both visual acuity and contrast sensitivity in a Type 1 DM rat model, and (2) to evaluate relative contribution of cataracts and retinal dysfunction to early visual deficits in our model.
Methods
Animals and Experimental Design
Male Long Evans rats (200–225 g; Charles River, Wilmington, MA) were housed in shoe-box cages on a 12:12 light:dark cycle with chow and water provided ad libitum. All procedures were approved by the Atlanta Veterans Affairs Institutional Animal Care and Use Committee and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Hyperglycemia was induced with a single intravenous injection of STZ (100 mg/kg; Ferro Pfanstiehl Laboratories, Waukegan, IL) dissolved in citrate buffer (pH 4.0); control (CTRL) rats were injected with vehicle alone.37 Diabetes was defined as two successive daily blood glucose levels higher than 250 mg/dL (freestyle handheld blood glucose meter from tail-prick blood), which routinely occurred 2 to 3 days after STZ injection. Body weights and blood glucose were monitored two to three times per week. DM rats were treated with small pellets of sustained-release subcutaneous insulin (Linplant; Linshin Canada, Scarborough, ON, Canada) at a dose sufficient to prevent excessive weight loss and catabolic response but insufficient to control hyperglycemia.37 For our first set of animals (n = 6 per group), visual acuity threshold was assessed weekly, contrast sensitivity was measured monthly (±1 week), and cataract examination was evaluated biweekly for 12 weeks. A second set of CTRL and DM animals (n = 8 per group) underwent monthly ERG recordings to assess retinal function. We also examined their visual acuity weekly and lens clarity biweekly to ensure we had similar phenotypes as our first set of animals. As responses were similar for both right and left eyes, only the responses from the right eye for each animal are reported here.
Visual Function Tests
Visual function of each rat, without movement restriction, was tested using the virtual optokinetic system (OptoMotry system; Cerebral-Mechanics, Lethbridge, AB, Canada) as previously described.8 Briefly, the rat was placed on a platform at the center of a virtual-reality chamber composed of four computer monitors that display vertical sine wave gratings rotating at a speed of 12 deg/s. The experimenter monitored the rat in real time through a video camera positioned above the animal and noted the presence or absence of reflexive head movements (tracking) in response to the rotating gratings in the same direction. The experimenter also manually tracked the head of the rat to align the center of the virtual cylinder to the viewing position of the rat. For visual acuity assessment, the grating started at a spatial frequency of 0.042 cyc/deg with 100% contrast. The acuity threshold was determined automatically by the OKT software using a staircase paradigm based on observations of head-tracking movements. Similarly, the contrast sensitivity threshold was determined by reducing the contrast of the black and white gradients from 100% in a staircase paradigm until animal head-tracking movements were no longer observed. Contrast sensitivity was measured at the spatial frequency of 0.064 cyc/deg for the study. This was the spatial frequency that elicited the maximum sensitivity obtained from the rats at baseline when a contrast sensitivity curve was assessed across five spatial frequencies (0.031, 0.064, 0.092, 0.103, and 0.119 cyc/deg). The contrast sensitivity was calculated as a reciprocal of the Michelson contrast from the screen's luminance (i.e., [maximum + minimum] / [maximum − minimum]), as previously described.38
Cataract Examination and Scoring
After dilating the pupils of unanesthetized animals with 1% tropicamide, lens opacification was observed and graded by a researcher (blinded to the treatment condition) using slit lamp illumination on a cataract grade scale as described in Muranov et al.39 Briefly, the classifications were as follows: Grade 0, a clear lens; Grade 1, swollen fibers and subcapsular opacities; Grade 2, nuclear cataract in lens and swollen fibers in lens cortex; Grade 3, severe nuclear cataract with perinuclear area opacity in lens; Grade 4, total opacity of lens. To ensure equivalent assessment of the cataract severity for each observation, the slit lamp illumination was standardized to the same slit width and light intensity for all examinations.
Retinal Function Test with ERG
Rats were dark-adapted overnight and then prepared under dim red illumination as previously described.40 In brief, rats were anesthetized (ketamine [60 mg/kg] and xylazine [7.5 mg/kg]), pupils dilated (1% tropicamide), and the corneal surface anesthetized (0.5% tetracaine HCl). Using a custom made DTL fiber electrode, responses were recorded to flash stimuli presented in order of increasing luminance using a signal-averaging system (UTAS BigShot; LKC Technologies, Gaithersburg, MD). ERG stimuli consisted of a 12-step dark-adapted series (−3.4–2.1 log cd s/m2) to isolate rod-dominated and rod/cone mixed responses. After testing, rats received yohimbine (2.1 mg/kg) to reverse the effects of xylazine and prevent corneal ulcers.41
Amplitudes and implicit times were measured for both a- and b-waves. Oscillatory potentials (OPs) were digitally filtered using the ERG system software (75–500 Hz; EM Version 8.1.2, 2008; LKC Technologies). The amplitudes and implicit times of individual OP1 through OP4 were determined, but only OP2 and OP4 results are reported here.
Statistical Analysis
Statistical analysis was performed using statistical software (SigmaStat 3.5; Aspire Software International, Ashburn, VA). Two-way repeated-measures ANOVA was used to compare treatment groups across time points after STZ injection. Post hoc multiple comparisons were performed when appropriate using the Holm-Sidak method. All statistics reported are the two-way repeated-measures ANOVA interaction effect, unless otherwise noted. For correlation analysis, relationship between visual acuity and ERG implicit time was determined using Pearson's product-moment correlation coefficient, whereas relationship between visual acuity and cataract score was determined using Spearman's rank correlation coefficient. All analyses were performed with significance set at P less than 0.05.
Results
Average weight and blood glucose levels for both CTRL and DM groups at baseline and by the end of the 12-week study are summarized in the Table. Although the two treatment groups were indistinguishable at baseline, DM rats were smaller than CTRL rats (post hoc analysis, P < 0.001) and had higher blood glucose levels (post hoc analysis, P < 0.001) at the 12-week time point. Moreover, DM rats were hyperglycemic (all values >250 mg/dL) throughout the 12-week study (data not shown).
Table. .
Average Weight and BG Levels of the Two Treatment Groups (± SEM) for the Two Sets of Experiments
|
Set |
Treatment Group |
n |
Baseline |
12 wk post-STZ |
||
|
Weight, g |
BG, mg/dL |
Weight, g |
BG, mg/dL |
|||
| 1 | CTRL | 6 | 340 ± 15 | 129 ± 6 | 587 ± 23 | 120 ± 6 |
| DM | 6 | 319 ± 16 | 128 ± 1 | 392 ± 15 | 557 ± 22 | |
| 2 | CTRL | 8 | 211 ± 5 | 144 ± 8 | 578 ± 18 | 119 ± 2 |
| DM | 8 | 226 ± 5 | 131 ± 3 | 382 ± 13 | 538 ± 32 | |
In both sets of experiments, both treatment groups gained significant weight by the end of the study (Main Duration Effect: P < 0.001), but DM rats were significantly smaller than CTRL rats and had significantly higher BG levels (P < 0.001). BG, blood glucose; n, sample size.
Effects of Hyperglycemia on Visual Function
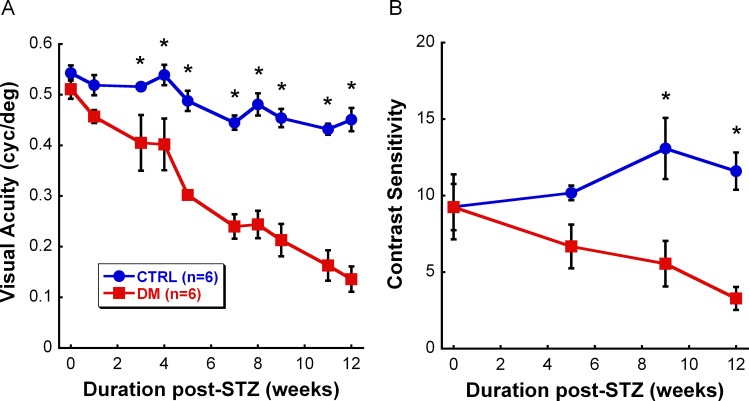
To assess the effects of sustained hyperglycemia on visual function, we measured visual acuity weekly and contrast sensitivity monthly. Although CTRL and DM groups had similar visual acuity thresholds at baseline to those reported previously in Long Evans rats,8 DM rats had a significant reduction in their visual acuity as early as 3 weeks post-STZ compared with CTRL rats (Fig. 1A: F9,119 = 6.802, P < 0.001). The deficit in visual acuity thresholds worsened with duration of hyperglycemia, starting with 22% reduction at 3 weeks post-STZ and increasing to 70% reduction at 12 weeks post-STZ. Additionally, DM rats showed a significant decrease in contrast sensitivity after 9 weeks post-STZ (Fig. 1B: F3,47 = 4.426, P = 0.011). Similarly, the decline in contrast sensitivity increased with hyperglycemic duration, starting with 34% reduction at 5 weeks post-STZ and reaching 72% reduction by the end of the study.
Figure 1.
Reduced visual function due to hyperglycemia over the 12-week study. (A) Significantly reduced visual acuity (22%) was observed in DM rats (red, n = 6) starting at 3 weeks post-STZ (P < 0.001) when compared with CTRL rats (blue, n = 6), and continued to decrease with disease duration. (B) Contrast sensitivity at 0.064 cyc/deg spatial frequency was reduced in DM rats (red, n = 6) starting at 9 weeks post-STZ (P = 0.011) when compared with CTRL rats (blue, n = 6), and worsened with disease progression. Although the deficit seemed to appear at 5 weeks post-STZ, the change was not statistically significant. Data shown are mean ± SEM. Asterisks represent significant post hoc comparisons, P < 0.05.
Effects of Cataract on Visual Function
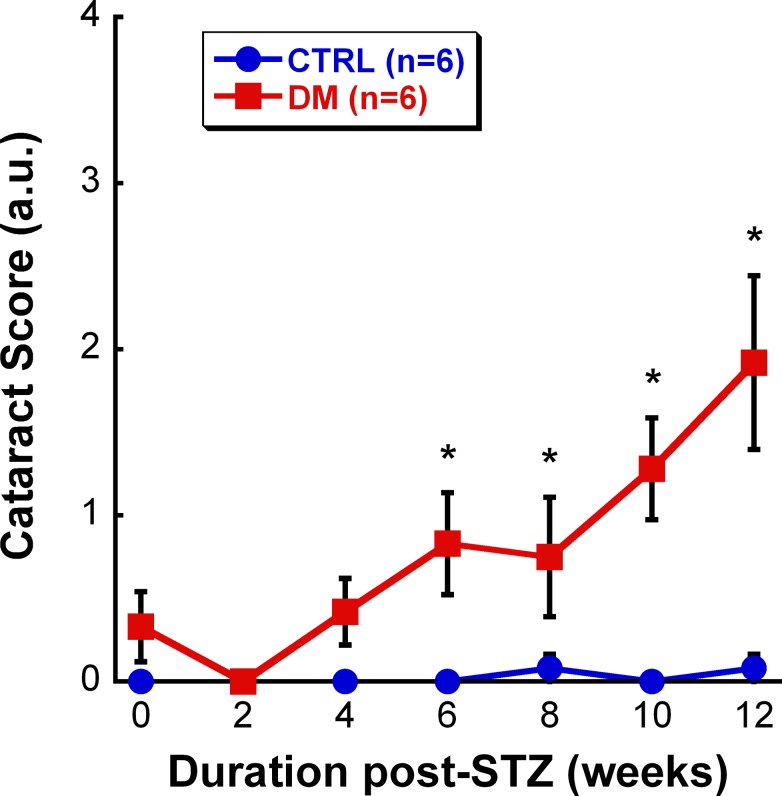
To evaluate the possibility of early cataract formation contributing to the diminished vision, each rat was examined for lens opacity biweekly. Although some DM rats developed mild cataracts starting at 4 weeks, the average cataract score of the DM group was not significantly greater than the CTRL group until 6 weeks post-STZ (Fig. 2: F8,107 = 7.136, P < 0.001). The clarity of the lens also worsened with the duration of hyperglycemia, gradually increasing from a score of 0.83 at 6 weeks post-STZ to a score of 1.92 at 12 weeks post-STZ.
Figure 2.
Cataract scores for CTRL (blue, n = 6) and DM (red, n = 6) groups over time. At 6 weeks post-STZ, DM animals had significantly higher cataract scores in comparison with CTRL rats (P < 0.001). Moreover, the cataracts worsened over the course of the study. Data shown are mean ± SEM. Asterisks represent significant post hoc comparisons, P < 0.05.
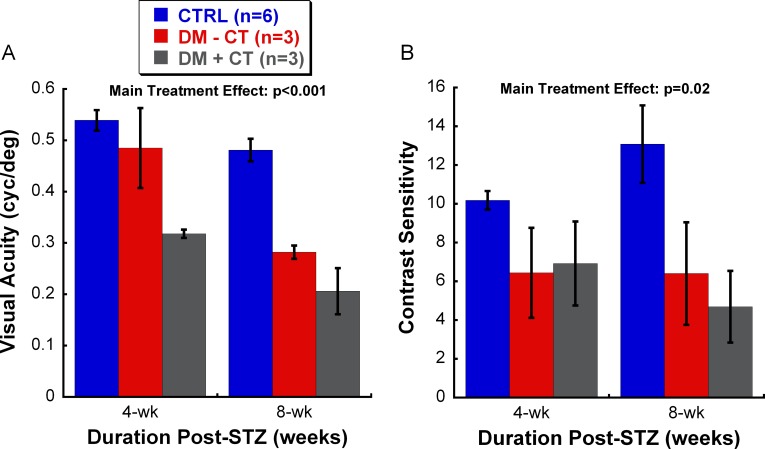
To determine the relative contribution of cataracts to visual deficits of the DM rats, we stratified the DM animals based on the presence or absence of cataracts at 4 and 8 weeks post-STZ. No stratification was done at 12 weeks post-STZ, as all DM rats developed significant cataracts by that time point. Figure 3A shows that the three groups had significantly different visual acuity thresholds (Main Treatment Effect: F2,23 = 49.186, P < 0.001). DM rats with any form of cataracts had the worst acuity (post hoc analysis, P < 0.001). More importantly, DM rats without cataracts also had significantly lower visual acuity than CTRL animals (post hoc analysis, P < 0.001), but higher than those with cataracts (post hoc analysis, P < 0.01). Figure 3B shows that the three groups also had significantly different contrast sensitivity levels (Main Treatment Effect: F2,23 = 6.263, P = 0.02). CTRL rats had the highest sensitivity when compared with the two stratified DM groups (post hoc analysis, P < 0.05). Interestingly, cataract development in DM rats did not seem to further reduce contrast sensitivity, as there was no significant difference in the sensitivity between DM rats that did develop cataracts and those that did not (post hoc analysis, P = 0.788).
Figure 3.
Stratification of visual acuity (A) and contrast sensitivity (B) of DM animals based on the presence or absence of cataracts. For visual acuity (A), a significant difference between the groups was found (Main Treatment Effect: P < 0.001), such that visual acuity decreased with the presence of hyperglycemia (DM-CT group) and was further reduced by the addition of cataracts (DM+CT group). These results indicate that other factor(s) besides optical opacities due to diabetes contribute to the reduction of visual function. For contrast sensitivity (B), CTRL rats have the highest thresholds among the three groups examined (Main Treatment Effect: P = 0.02). We did not observe a further reduction in contrast sensitivity of DM rats due to cataract formation. Data shown are mean ± SEM. CT, presence of cataracts.
Retinal Dysfunction as Potential Contributor to Visual Deficit
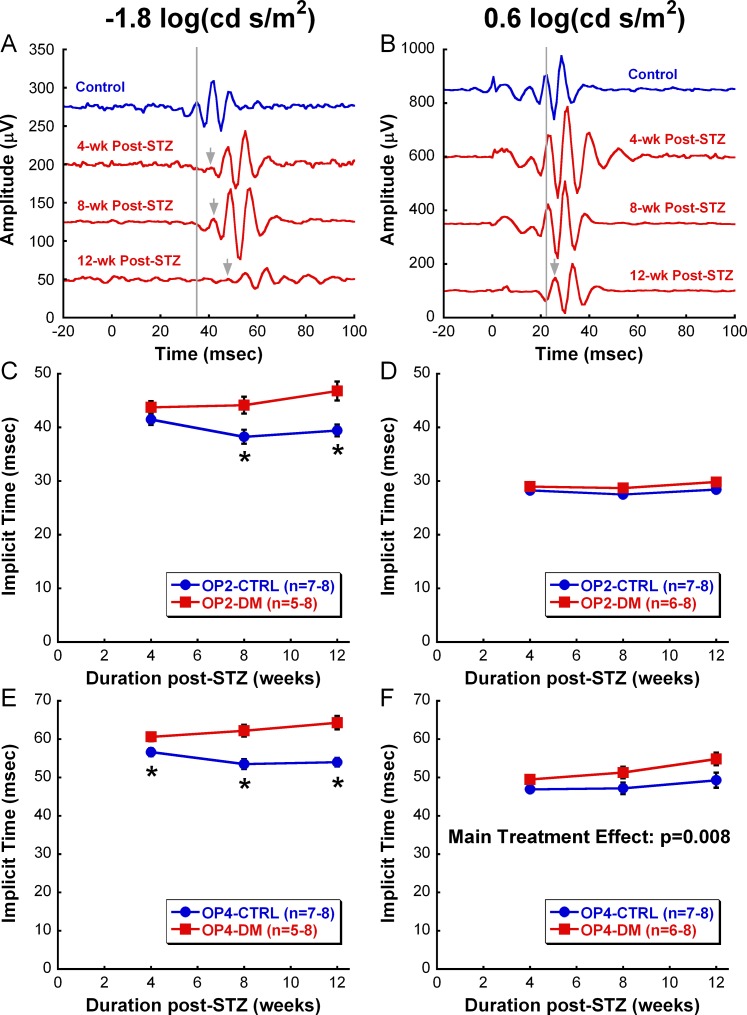
ERG responses, cataract formation, and visual acuity values were measured in a different set of CTRL and DM animals to further correlate retinal dysfunction to early visual deficits in DM rats. DM rats in this group also showed a significant reduction in their visual acuity starting at 1 month post-STZ and developed significant cataracts by 6 weeks post-STZ (data not shown). Serial ERGs obtained in this second set of rats revealed that both amplitudes and implicit times of a- and b-waves did not differ consistently between CTRL and DM animals over the experimental period (data not shown). However, consistent with previous ERG studies, DM animals displayed robustly delayed OP responses starting at 4 weeks post-STZ (Fig. 4).20,24 Figure 4 shows a summary of OP2 and OP4 implicit times from 4 to 12 weeks after hyperglycemia at representative dim (−1.8 log cd s/m2) and bright (0.6 log cd s/m2) flash stimuli. Significant delays in OP2 (Fig. 4C) and OP4 (Fig. 4E) implicit times were found under dim stimulus stimulation, as early as 8 weeks post-STZ for OP2 (F2,42 = 5.417, P = 0.012) and 4 weeks post-STZ for OP4 (F2,42 = 3.809, P = 0.037). Moreover, the deficit worsened significantly with the duration of hyperglycemia, especially for OP4, increasing from 7% delay in comparison with the CTRL group at 4 weeks post-STZ to 19% delay at 12 weeks post-STZ. In contrast, we observed no difference between CTRL and DM responses for OP2 in response to bright stimuli (Fig. 4D) and OP4 revealed a nonprogressive delay (on average 8%) due to DM treatment (Fig. 4F, Main Treatment Effect: F1,43 = 9.060, P = 0.008).
Figure 4.
Retinal dysfunction due to diabetes. (A, B) Representative OP waveforms to a dim-flash ([A], −1.8 log cd s/m2) or bright-flash ([B], 0.6 log cd s/m2) stimulus from a CTRL rat (blue) at 4-week time point and a DM rat (red) at 4-week, 8-week, and 12-week time points. The gray lines indicate the peak of OP1 in the CTRL rat, whereas the gray arrows indicate the peak of OP1 in the diabetic rat when delayed. (C, D) Average OP2 implicit times (± SEM) in response to dim flash (C) and bright flash (D) over the 12-week study. As early as 8 weeks post-STZ, OP2 of the DM group shows a significant delay in comparison with that of the CTRL group in response to only dim stimulus (P = 0.012). (E, F) Average OP4 implicit times (± SEM) in response to dim flash (E) and bright flash (F) over the 12-week study. OP4 of DM group in response to dim flash displays a significant delay at 4 weeks post-STZ (P = 0.037). In contrast, OP4 elicited with bright flash shows only a main treatment effect between CTRL and DM groups (Main Treatment Effect: P = 0.008). Data shown are mean ± SEM. Asterisks represent significant post hoc comparisons, P < 0.05.
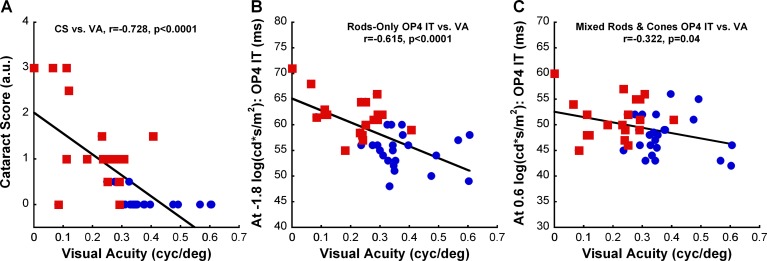
Figure 5 presents correlation analyses of the visual acuity of each animal against its corresponding cataract score and OP4 responses (elicited with either dim or bright flash) over the course of the study. Regardless of treatment conditions, cataract severity correlated strongly with visual acuities (Fig. 5A: Spearman Rank Order, r = −0.728, P < 0.0001). More interestingly, changes in visual acuity also correlated significantly with OP4 latency (Figs. 5B, 5C), whether collected from rod-dominated responses (Pearson Product, r = −0.615, P < 0.0001) or mixed rod/cone responses (Pearson Product, r = −0.322, P = 0.04). However, it is important to note that only DM rats, not CTRL rats, maintained the correlation between OP4 implicit times and visual acuities when we separated animals based on their disease state (data not shown). Moreover, it is interesting to note the segregation of the two treatment groups (CTRL versus DM) on all the scattered plots, demonstrating the effects of hyperglycemia on cataract formation, retinal function, and OKT responses (Fig. 5).
Figure 5.
Scatter plots of visual acuity value against corresponding (A) cataract score, (B) dim-flash OP4 latency, and (C) bright-flash OP4 latency for CTRL (n = 7–8) and DM (n = 5–8) rats at 4 weeks, 8 weeks, and 12 weeks postinjection. As expected, the visual acuities of our animals significantly correlate to cataract scores (Spearman Rank Order, P < 0.0001). More interestingly, changes in visual acuity also significantly correlated with OP4 implicit times elicited from rod-dominated response (Pearson Product, P < 0.0001) and mixed rod/cone response (Pearson Product, P = 0.04) conditions. The blue circle symbols represent data points for CTRL animals and the red square symbols represent data points for DM animals. CS, cataract score; IT, implicit time; r, correlation coefficient; VA, visual acuity.
Discussion
The present study detected abnormalities in visual acuity and contrast sensitivity in STZ-induced DM rats before expected onset of diabetes-associated retinal vascular lesions (Fig. 6), such as vascular leakage, pericyte dropout, and acellular capillaries (based on published studies).33,42,43 Some of this early visual loss can be attributed to the development of cataracts; however, significant decreases in visual acuity and contrast sensitivity were also found in DM rats with no signs of cataracts, suggesting that other factor(s) contributed to decreased visual function. We hypothesize that early visual deficits may result from retinal dysfunction due to STZ-induced hyperglycemia or hypoinsulinemia. Supporting this hypothesis, we found that DM rats have delayed ERG responses, specifically in OP implicit times (indicative of inner retinal dysfunction), at 4 weeks post-STZ, which was the time point when the DM rats first displayed visual deficits. Similar to our previous findings, the ERG abnormalities were most prominent and consistent under scotopic condition, suggesting rod pathways are most susceptible to diabetic insults (Kim MH, et al. IOVS 2008;49:ARVO E-Abstract 2212). In addition, according to our correlation analyses, visual acuity declines among DM rats were associated with ERG changes elicited by both dim and bright flashes. Interestingly, we also observed that early scotopic ERG changes were associated with diminished scotopic OKT responses, with scotopic visual function declining 1 week earlier than photopic visual function (data not shown). Collectively, the results of this study suggest that visual deficits in the early-stage DR rat model are initially related to retinal dysfunction, then subsequently worsen with the formation of cataracts.
Figure 6.
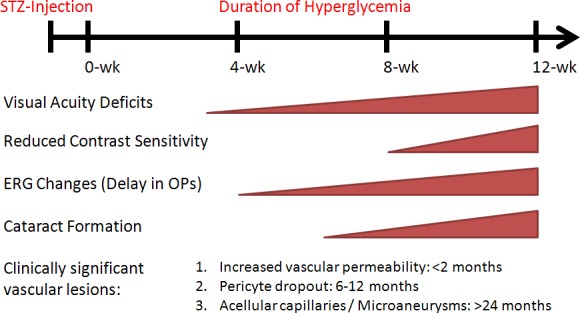
Chronological summary of the functional deficits found in our study. The onsets of clinically significant vascular lesions listed were estimates based on published reports on an STZ-induced diabetic rat model.33,42,43
Cataracts and Visual Dysfunction in Early-Stage DR
The effects of cataracts on early visual loss in diabetes have been observed in insulin-dependent diabetic (IDDM) patients without clinically diagnosed retinopathy.7,44 In these studies, the authors noted that color discrimination is abnormal in uncomplicated IDDM patients before the onset of vascular retinopathy. More interestingly, although IDDM patients with increased lens optical density had the worst visual deficit, IDDM patients with clear lenses also had abnormal color discrimination when compared with age-matched control subjects, suggesting underlying retinal dysfunction. Although diabetes-induced cataracts and their effects on visual function are well-documented,45 findings from this study reinforce the importance of other underlying factor(s) in contributing to the early visual deficits in an STZ-induced DM rat model. Further studies are needed to determine the exact contribution of ocular opacities on visual function, independent of diabetes-induced neuronal defects.
Current OKT Findings on Animal Models of Diabetes
A report by Kirwin et al.13 similarly found decreased visual acuity in STZ-induced DM rats at 4 weeks post-STZ. Kirwin et al.13 performed microarray analysis over several time points post-STZ and revealed that expression levels of several genes encoding visual cycle proteins, such as retinal pigment epithelium-specific protein 65 kDa, were downregulated. They concluded that OKT deficits in their animals were most likely due to direct hyperglycemic insult on the neural retina. Further supporting the hypothesis of a neuronal pathology in early stages of DR, we found similar onsets of inner retinal dysfunction, as measured with ERG alterations, and decreasing visual function. Interestingly, Kirwin et al.13 also observed some rats with cataracts at 6 weeks post-STZ; although contrary to our results, they did not find that cataracts significantly affected visual acuity. It is possible that the impact of cataracts on visual acuity may have reached significance if these DM rats had been examined at later time points.
A more recent study that examined both visual acuity and contrast sensitivity in a different Type 1 DM model, Ins2Akita mice, showed reduction in both aspects of vision after developing diabetes for 4 to 5 months.46 Similar to our study, the authors also found progressive worsening of visual dysfunction with duration of hyperglycemia. Although the authors did not conduct further functional or mechanistic experiments, they also hypothesized retinal pathology as a major contributor for the early visual deficits in Ins2Akita mice. The finding of visual deficits in Ins2Akita mice has been replicated in a separate study that examined the changes in visual function and ocular blood flow in this diabetic mouse model.47
Retinal Origins of Visual Defects in Diabetes
OKT is a reflex response that does not rely on the visual cortex,8 but rather depends on the accessory optic system (AOS).48 The AOS originates from the retina, with direction-selective and velocity-selective ganglion cells projecting to various nuclei in the midbrain region.9,48,49 The goal of AOS is to detect slip of the visual world on the retina, which then triggers corrective eye and head movements to stabilize the images.48 Therefore, it is not surprising that defects in OKT response occur at a similar time point as OP abnormalities, as retinal ganglion cells and amacrine cells are potential generators of OPs.22,50
The observed OP changes in our study also agree with other published studies on the effects of diabetes on inner retina. From a molecular biology standpoint, diabetes has been shown to alter the levels and receptors of several inner retinal neurotransmitters (most notably dopamine and gamma-amino-butyric acid [GABA]), as early as 4 weeks post-STZ.30,51,52 Cellularly, increased cell death of amacrine and ganglion cells has been observed within weeks of STZ injection.28,33 Functionally, other ERG studies have reported dysfunction in amacrine- and ganglion cell-dominated OPs and scotopic threshold response (STR) at 4 weeks post-STZ.5,21,22,24,53,54 Among these pathological changes, disruption of the retinal dopamingeric system is a highly plausible underlying factor that can lead to both ERG changes and OKT defects, as retinal dopamine has been shown to directly modulate OP generation50 and visual function.55 It is intriguing that other studies have detected a-wave losses as the major feature of DR neuronal dysfunction, although the difference in the ERG findings may be due to difference in animal strains, extent of glycemic control (i.e., frequency and dosage of insulin treatment), duration of hyperglycemia, or stimulation protocol.16,56
Aside from direct hyperglycemic insult on the retinal neurons, it is possible that retinal dysfunction may be secondary to hypoxia due to vascular dysfunction. Numerous studies have found defective vascular function at early stages of DR.42,57–59 More importantly, a recent report has not only demonstrated hypoxia in diabetic rat retina (at the retinal ganglion cell layer) at 4 weeks post-STZ, but also detected corresponding functional ganglion cell deficits with ERG (reduced STR amplitude).19 This hypothesis of hypoxia-induced retinal dysfunction leading to visual defects in diabetes was also supported in an earlier study in which breathing 100% oxygen improved contrast sensitivity of DM patients with minimal retinopathy.60
Taken together with these reports, our findings provide evidence of early inner retinal dysfunction due to diabetes, either directly or indirectly, which can then result in defects in different aspects of vision, including visual acuity and contrast sensitivity. However, it is important to recognize the possibility that defects in OKT response could also be due to disturbances in postretinal connections, such as downstream components of AOS.
Future Implications
It is intriguing that we were able to detect substantial reduction in OKT responses in our DM rats following such a short duration of hyperglycemia, since visual acuity determined with acuity eye charts as a diagnostic and clinical trial end point in humans has been insensitive to early-stage DR.3,5 This suggests that certain aspects of vision are selectively affected in early-stage DR (e.g., contrast sensitivity, scotopic vision, or optokinetic response), and may serve as better diagnostic and screening tools. Furthermore, the present study provides a temporal relationship of the observed visual defects in STZ-induced DM rats with corresponding ERG alterations and cataract development. Further research is warranted to establish the causal relationships between these pathologies and determine the best way to detect the pathologies in diabetic patients in which detection can be confounded by other factors, such as aging and other ocular diseases. Nonetheless, STZ-induced DM rats can serve as a valuable model to investigate the underlying mechanisms for the visual dysfunction in early-stage DR, in hope of revealing a potential therapeutic target to ameliorate such deficits. Moreover, the reproducibility of the visual deficits in this model can allow us to reliably test the efficacy of future treatment options to prevent or delay vision loss in DR. Last, as DR is a multifaceted disease, it is important to study DR with a multidisciplinary approach. Therefore, future studies to elucidate the underlying causes of vision loss should consider how diabetes differentially affects the neuronal and vascular tissues of the retina, and ultimately vision.
Footnotes
Supported by National Institutes of Health (NIH) Grant P30 EY006360, a Veterans Affairs Research Career Scientist Award and Departmental Award from Research to Prevent Blindness (MTP), a Veterans Affairs Merit Award, a Juvenile Diabetes Research Foundation (JDRF) Research Grant, a JDRF Innovative Award, a Children's Healthcare of Atlanta Research Center award, and NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK07601-01 (PMT).
Disclosure: M.H. Aung, None; M.K. Kim, None; D.E. Olson, None; P.M. Thule, None; M.T. Pardue, None
References
- 1. Klein BEK. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007; 14: 179–183 [DOI] [PubMed] [Google Scholar]
- 2. Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003; 290: 2057–2060 [DOI] [PubMed] [Google Scholar]
- 3. Jackson GR, Barber AJ. Visual dysfunction associated with diabetic retinopathy. Curr Diab Rep. 2010; 10: 380–384 [DOI] [PubMed] [Google Scholar]
- 4. Greenstein V, Sarter B, Hood D, Noble K, Carr R. Hue discrimination and S cone pathway sensitivity in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1990; 31: 1008–1014 [PubMed] [Google Scholar]
- 5. Ghirlanda G, Di Leo MA, Caputo S, Cercone S, Greco AV. From functional to microvascular abnormalities in early diabetic retinopathy. Diabetes Metab Rev. 1997; 13: 15–35 [DOI] [PubMed] [Google Scholar]
- 6. Kawasaki K, Yonemura K, Yokogawa Y, Saito N, Kawakita S. Correlation between ERG oscillatory potential and psychophysical contrast sensitivity in diabetes. Doc Ophthalmol. 1986; 64: 209–215 [DOI] [PubMed] [Google Scholar]
- 7. Hardy KJ, Lipton J, Scase MO, Foster DH, Scarpello JH. Detection of colour vision abnormalities in uncomplicated type 1 diabetic patients with angiographically normal retinas. Br J Ophthalmol. 1992; 76: 461–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Douglas RM, Alam NM, Silver BD, McGill TJ, Tschetter WW, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005; 22: 677–684 [DOI] [PubMed] [Google Scholar]
- 9. Thomas BB, Seiler MJ, Sadda SR, Coffey PJ, Aramant RB. Optokinetic test to evaluate visual acuity of each eye independently. J Neurosci Methods. 2004; 138: 7–13 [DOI] [PubMed] [Google Scholar]
- 10. Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res. 2000; 40: 2201–2209 [DOI] [PubMed] [Google Scholar]
- 11. Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004; 45: 4611–4616 [DOI] [PubMed] [Google Scholar]
- 12. Thaung C, Arnold K, Jackson IJ, Coffey PJ. Presence of visual head tracking differentiates normal sighted from retinal degenerate mice. Neurosci Lett. 2002; 325: 21–24 [DOI] [PubMed] [Google Scholar]
- 13. Kirwin SJ, Kanaly ST, Hansen CR, Cairns BJ, Ren M, Edelman JL. Retinal gene expression and visually evoked behavior in diabetic Long Evans rats. Invest Ophthalmol Vis Sci. 2011; 52: 7654–7663 [DOI] [PubMed] [Google Scholar]
- 14. Bearse MA Jr, Adams AJ, Han Y, et al. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006; 25: 425–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bresnick GH, Palta M. Oscillatory potential amplitudes. Relation to severity of diabetic retinopathy. Arch Ophthalmol. 1987; 105: 929–933 [DOI] [PubMed] [Google Scholar]
- 16. Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007; 13: 2699–2712 [DOI] [PubMed] [Google Scholar]
- 17. Fortune B, Schneck ME, Adams AJ. Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999; 40: 2638–2651 [PubMed] [Google Scholar]
- 18. Holopigian K, Seiple W, Lorenzo M, Carr R. A comparison of photopic and scotopic electroretinographic changes in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992; 33: 2773–2780 [PubMed] [Google Scholar]
- 19. Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011; 52: 9316–9326 [DOI] [PubMed] [Google Scholar]
- 20. Li Q, Zemel E, Miller B, Perlman I. Early retinal damage in experimental diabetes: electroretinographical and morphological observations. Exp Eye Res. 2002; 74: 615–625 [DOI] [PubMed] [Google Scholar]
- 21. Wolff BE, Bearse MA, Schneck ME, Barez S, Adams AJ., Multifocal VEP. (mfVEP) reveals abnormal neuronal delays in diabetes. Doc Ophthalmol. 2010; 121: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shirao Y, Kawasaki K. Electrical responses from diabetic retina. Prog Retin Eye Res. 1998; 1759–76 [DOI] [PubMed] [Google Scholar]
- 23. Lecleire-Collet A, Audo I, Aout M, et al. Evaluation of retinal function and flicker light-induced retinal vascular response in normotensive patients with diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 2861–2867 [DOI] [PubMed] [Google Scholar]
- 24. Kohzaki K, Vingrys AJ, Bui BV. Early inner retinal dysfunction in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2008; 49: 3595–3604 [DOI] [PubMed] [Google Scholar]
- 25. Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D. Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol. 2006; 50: 367–373 [DOI] [PubMed] [Google Scholar]
- 26. Harrison WW, Bearse MA, Ng JS, et al. Multifocal electroretinograms predict onset of diabetic retinopathy in adult patients with diabetes. Invest Ophthalmol Vis Sci. 2011; 52: 772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. VanGuilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur J Neurosci. 2008; 28: 1–11 [DOI] [PubMed] [Google Scholar]
- 28. Gastinger MJ, Singh RSJ, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006; 47: 3143–3150 [DOI] [PubMed] [Google Scholar]
- 29. Nishimura C, Kuriyama K. Alterations in the retinal dopaminergic neuronal system in rats with streptozotocin-induced diabetes. J Neurochem. 1985; 45: 448–455 [DOI] [PubMed] [Google Scholar]
- 30. Northington FK, Hamill RW, Banerjee SP. Dopamine-stimulated adenylate cyclase and tyrosine hydroxylase in diabetic rat retina. Brain Res. 1985; 337: 151–154 [DOI] [PubMed] [Google Scholar]
- 31. Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27: 283–290 [DOI] [PubMed] [Google Scholar]
- 32. Martin PM, Roon P, Ells V, et al. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004; 45: 3330–3336 [DOI] [PubMed] [Google Scholar]
- 33. Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 1156–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fletcher EL, Downie LE, Hatzopoulos K, et al. The significance of neuronal and glial cell changes in the rat retina during oxygen-induced retinopathy. Doc Ophthalmol. 2010; 120: 67–86 [DOI] [PubMed] [Google Scholar]
- 35. Villarroel M, Ciudin A, Hernández C, Simó R. Neurodegeneration: an early event of diabetic retinopathy. World J Diabetes. 2010; 1: 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Obrosova IG, Chung SSM, Kador PF. Diabetic cataracts: mechanisms and management. Diabetes Metabol Res Rev. 2010; 26: 172–180 [DOI] [PubMed] [Google Scholar]
- 37. Thulé PM, Campbell AG, Kleinhenz DJ, et al. Hepatic insulin gene therapy prevents deterioration of vascular function and improves adipocytokine profile in STZ-diabetic rats. Am J Physiol Endocrinol Metab. 2006; 290: E114–E122 [DOI] [PubMed] [Google Scholar]
- 38. Prusky GT, Alam NM, Douglas RM. Enhancement of vision by monocular deprivation in adult mice. J Neurosci. 2006; 26: 11554–11561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muranov K, Poliansky N, Winkler R, Rieger G, Schmut O, Horwath-Winter J. Protection by iodide of lens from selenite-induced cataract. Graefes Arch Clin Exp Ophthalmol. 2004; 242: 146–151 [DOI] [PubMed] [Google Scholar]
- 40. Ciavatta VT, Kim M, Wong P, et al. Retinal expression of Fgf2 in RCS rats with subretinal microphotodiode array. Invest Ophthalmol Vis Sci. 2009; 50: 4523–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turner PV, Albassam MA. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med. 2005; 55: 175–182 [PubMed] [Google Scholar]
- 42. Curtis TM, Gardiner TA, Stitt AW. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond). 2009; 23: 1496–1508 [DOI] [PubMed] [Google Scholar]
- 43. Lorenzi M, Gerhardinger C. Early cellular and molecular changes induced by diabetes in the retina. Diabetologia. 2001; 44: 791–804 [DOI] [PubMed] [Google Scholar]
- 44. Hardy KJ, Scarpello JH, Foster DH, Moreland JD. Effect of diabetes associated increases in lens optical density on colour discrimination in insulin dependent diabetes. Br J Ophthalmol. 1994; 78: 754–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bron AJ, Cheng H. Cataract and retinopathy: screening for treatable retinopathy. Clin Endocrinol Metab. 1986; 15: 971–999 [DOI] [PubMed] [Google Scholar]
- 46. Akimov NP, Rentería RC. Spatial frequency threshold and contrast sensitivity of an optomotor behavior are impaired in the Ins2Akita mouse model of diabetes. Behav Brain Res. 2012; 226: 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Muir ER, Rentería RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012; 53: 6488–6494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schiller PH. Parallel information processing channels created in the retina. Proc Natl Acad Sci U S A. 2010; 107: 17087–17094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giolli RA, Blanks RHI, Lui F. The accessory optic system: basic organization with an update on connectivity, neurochemistry, and function. Prog Brain Res. 2006; 151: 407–440 [DOI] [PubMed] [Google Scholar]
- 50. Wachtmeister L. Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res. 1998; 17: 485–521 [DOI] [PubMed] [Google Scholar]
- 51. Ramsey DJ, Ripps H, Qian H. Streptozotocin-induced diabetes modulates GABA receptor activity of rat retinal neurons. Exp Eye Res. 2007; 85: 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Santiago AR, Gaspar JM, Baptista FI, et al. Diabetes changes the levels of ionotropic glutamate receptors in the rat retina. Mol Vis. 2009; 15: 1620–1630 [PMC free article] [PubMed] [Google Scholar]
- 53. Matsubara H, Kuze M, Sasoh M, Ma N, Furuta M, Uji Y. Time-dependent course of electroretinograms in the spontaneous diabetic Goto-Kakizaki rat. Jpn J Ophthalmol. 2006; 50: 211–216 [DOI] [PubMed] [Google Scholar]
- 54. Ramsey DJ, Ripps H, Qian H. An electrophysiological study of retinal function in the diabetic female rat. Invest Ophthalmol Vis Sci. 2006; 47: 5116–5124 [DOI] [PubMed] [Google Scholar]
- 55. Jackson CR, Ruan G-X, Aseem F, et al. Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci. 2012; 32: 9359–9368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Phipps JA, Yee P, Fletcher EL, Vingrys AJ. Rod photoreceptor dysfunction in diabetes: activation, deactivation, and dark adaptation. Invest Ophthalmol Vis Sci. 2006; 47: 3187–3194 [DOI] [PubMed] [Google Scholar]
- 57. Clermont AC, Bursell S-E. Retinal blood flow in diabetes. Microcirculation. 2007; 14: 49–61 [DOI] [PubMed] [Google Scholar]
- 58. Ciulla TA, Harris A, Latkany P, et al. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002; 80: 468–477 [DOI] [PubMed] [Google Scholar]
- 59. Wang Z, Yadav AS, Leskova W, Harris NR. Inhibition of 20-HETE attenuates diabetes-induced decreases in retinal hemodynamics. Exp Eye Res. 2011; 93: 108–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harris A, Arend O, Danis RP, Evans D, Wolf S, Martin BJ. Hyperoxia improves contrast sensitivity in early diabetic retinopathy. Br J Ophthalmol. 1996; 80: 209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]